Abstract
We have analyzed paraffin sections from 190 patients with histologically confirmed Hodgkin’s disease (HD) for the presence of Epstein-Barr virus (EBV) using in situ hybridization to detect the EBV-encoded Epstein-Barr virus early RNAs (EBERs) and immunohistochemistry to identify latent membrane protein-1 (LMP1) expression. EBV was present in the tumor cells in 51 HD cases (27%) and was mainly confined to the mixed cellularity and nodular sclerosis subtypes. There was no difference between EBV-positive and EBV-negative HD patients with regard to age, clinical stage, presentation, and the number of alternating chemotherapy cycles of ChIVPP and PABIOE received. The complete remission rate after study chemotherapy was 80% in EBV-positive patients versus 69% in EBV-negative patients (P = .05). The 2-year failure-free survival rate was significantly better for EBV-positive patients when compared with the EBV-negative HD group (P = .02). Although 2-year and 5-year overall survival rates were better for EBV-positive HD patients, the differences were not statistically significant (P = .18 andP = .40, respectively). In conclusion, the results confirm the favorable prognostic value of EBV in the tumor cells of HD patients and suggest important differences in response to chemotherapy between EBV-positive and EBV-negative patients.
THE EPSTEIN-BARR virus (EBV), a B-lymphotropic herpesvirus that infects the majority of the world’s adult population, has been linked to a number of human malignancies, including Burkitt’s lymphoma, nasopharyngeal carcinoma, and Hodgkin’s disease (HD). The observation that persons with a history of infectious mononucleosis have a twofold to threefold increased risk of HD,1 and the detection of elevated levels of antibodies to viral antigens in HD patients before or at the time of diagnosis2-4 provided indirect evidence for a causal role for EBV in HD.
Direct evidence for the association was supplied by the localization of EBV DNA to the malignant Hodgkin and Reed-Sternberg (HRS) cells of HD.5 However, differences in the percentage of EBV-positive HD cases detected in various studies mainly depended on the methods used. Southern blot analysis and in situ hybridization with the use of the BamHI W fragment as a probe yielded between 20% and 40% positive cases.6-8 The highest percentage of positive cases has been obtained using the polymerase chain reaction (PCR) method,9-11 although these studies also detect the presence of EBV infection of bystander reactive lymphocytes and therefore overestimate the EBV association in HD. Perhaps the most widely used and most reliable method is in situ hybridization that targets the highly abundant Epstein-Barr virus early RNAs (EBERs). Using this approach, a variety of studies have demonstrated the presence of latent EBV infection in HRS cells. However, the frequency with which EBV is demonstrated in HD tumors also shows geographical variability, with percentages of between 40% and 50% for North American and European cases,12-15 57% for HD in China,16 but much higher rates in developing countries such as Peru17 and Kenya.18-20 Furthermore, several investigators have demonstrated the clonality of EBV in HD tissue by hybridization with the viral terminal repeat sequences.6,21 These findings indicate clonal expansion of single EBV-infected cells and underline a possible etiological role of EBV in a proportion of HD cases. In addition, immunohistochemical analysis has demonstrated that the HRS cells of EBV-positive cases express high levels of the EBV-encoded latent membrane protein-1 (LMP1).22,23 These data are supported by transcriptional analysis on fresh biopsies of HD that show EBV nuclear antigen-1 (EBNA1) mRNA together with LMP1 and LMP2A and LMP2B transcripts.24
Although the association between HD and EBV is now established, a potentially more important question is whether the presence of EBV within HRS cells influences outcome for patients with HD. This question has been addressed in three previous studies resulting in contradictory findings.25-27 The first two studies, which comprised relatively small numbers of subjects, failed to identify any effect of EBV on survival.25,26 In contrast, the third study demonstrated a significant association between expression of LMP1 by HRS cells and improved survival in 140 HD patients.27 Also, one of the first two studies used PCR to detect EBV,25 an approach that cannot determine the location of EBV within HD tissues and does not rule out the possible detection of EBV in nonmalignant B lymphocytes.
Therefore, the present study was performed to further examine the prognostic significance of EBV infection in HD, with the use of a clinical trials database that contained detailed information on patient demography, response to chemotherapy, and survival.
MATERIALS AND METHODS
Patients
In January 1983, the United Kingdom Central Lymphoma Group (CLG) commenced a study of alternating ChIVPP/PABIOE chemotherapy in previously untreated patients with advanced HD who were unsuitable for treatment with radiotherapy alone. The results of this trial have been reported elsewhere.28 Of the total of 280 patients recruited to this study, paraffin wax-embedded tissue blocks were available for 225 cases.
Histological Review
Hematoxylin and eosin-stained slides were prepared and used to review and subtype all 225 cases according to the Rye classification system. A histological diagnosis of HD was confirmed in 190 of 225 cases. The remaining cases were either not considered to have HD or there was insufficient material remaining in the tissue block to allow a precise histological diagnosis to be made. Before the immunohistochemical and in situ hybridization assays, 4-μm paraffin sections were prepared, deparaffinized, and washed in Tris-buffered saline (TBS), pH 7.6.
Detection of Latent EBV Infection
In situ hybridization for the detection of EBERs was performed according to standard methodology29 and was used to detect the presence of latent EBV infection in all HD samples. Positive controls for EBER in situ hybridization included paraffin wax sections of lymphoblastoid cell lines (LCLs) grown as solid tumors in severe combined immunodeficiency (SCID) mice and a known EBER-positive HD case. U6 and sense control probes were also included in all runs, and their use has been previously described elsewhere.29 Immunohistochemistry for LMP1 was performed on selected cases to confirm the presence of EBV infection in HRS cells. The standard alkaline phosphatase antialkaline phosphatase (APAAP) method was used, and sections were microwave-pretreated, as previously described.30 Positive controls for LMP1 consisted of paraffin wax sections of LCLs grown as solid tumors in SCID mice. Negative controls consisted of consecutive test sections in which primary antibody was replaced with nonimmune serum of the same IgG subclass.
Statistical Methods
Comparison of subgroup with EBV status with those with no EBV status.
Data were collected on 280 HD patients in the trial. EBV status was determined in 190 (68%) of these patients. The aim of the initial analysis was to investigate if the group in whom EBV status had been investigated was representative of all patients entered in the trial.
χ2 tests (including a continuity correction for two-level variables) were used to compare the groups in terms of baseline patient characteristics including gender, prior treatment, histology, symptoms, clinical stage, and all sites of disease (except Waldeyer’s ring for which the Fisher’s exact test was used). Wilcoxon tests were used to compare the groups in terms of age and follow-up, and log-rank tests were used to compare the groups in terms of survival and failure-free survival. Only patients who did not receive previous treatment were included in the investigation of follow-up, overall survival, and failure-free survival.
Comparison of EBV-positive with EBV-negative trial patients.
The second part of the analysis was used only for those patients in whom EBV status was known and involved a comparison of EBV-negative and EBV-positive groups. A combination of χ2 tests, Fisher’s exact tests (both for categorical data), and Wilcoxon tests (continuous data) were used to compare these groups in terms of gender, age, prior treatment, histology, symptoms, clinical stage, sites of disease, and follow-up. Survival and failure-free survival were compared with the use of log-rank tests and Kaplan-Meier curves. Survival was calculated as the time from beginning chemotherapy to date of death from any cause or date of the last known follow-up. For those patients who responded to treatment, failure-free survival was calculated as the time from beginning chemotherapy to either the date of relapse, or in those patients with no previous documented relapse, either the date of death from any cause or date of the last known follow-up. For those patients who did not respond to chemotherapy, failure-free survival was recorded as zero.
RESULTS
Detection of Latent EBV Infection in HD Samples
Of the 190 HD patients, the presence of latent EBV infection, as detected by in situ hybridization for the EBERs, was identified in HRS cells in 51 (27%) cases (designated as the EBV-positive group). The remaining 139 (73%) cases did not show the presence of the EBERs within HRS cells and will subsequently be referred to as the EBV-negative group. Immunohistochemistry for the LMP1 protein gave similar results to EBER in situ hybridization.
Comparison of Subgroup With EBV Status
When the group with known EBV status (n = 190) was compared with the individuals with no EBV status (n = 90), there was found to be no significant difference in terms of any of the parameters studied with the exception of prior treatment (P = .03). Because those who had prior treatment were already excluded from any analysis of outcome variables, this difference is not relevant.
Comparison of EBV-Positive and EBV-Negative Patients
Patient characteristics.
The results showed that there was no significant difference between the EBV-negative and EBV-positive groups in terms of clinical stage or site(s) of disease at presentation, age, or presence of B symptoms (Table 1). However, a significantly greater proportion of EBV-positive patients were male compared with the EBV-negative group (P = .001). Even when the P values were adjusted, with the use of Bonferroni’s correction to account for multiple characteristics being tested, gender remained significant at the 5% level (P = .01).
Characteristics of 190 HD Patients With Known EBV Status
| Characteristic . | EBVPositive . | EBVNegative . | P Value . |
|---|---|---|---|
| Gender: male | 43 (84%) | 80 (58%) | .001 |
| Median age (inter-quartile range) | 33 (22-49) | 33 (23-45) | .55 |
| B symptoms: present | 16 (31%) | 53 (38%) | .49 |
| Clinical stage | |||
| 1 | 2 (4%) | 10 (7%) | .87 |
| 2 | 16 (33%) | 45 (33%) | |
| 3 | 19 (39%) | 48 (35%) | |
| 4 | 12 (24%) | 35 (25%) | |
| Histological type | |||
| Lymphocyte predominant | 2 (5%) | 11 (9%) | |
| Nodular sclerosis (NS) | 30 (68%) | 86 (69%) | 1.00 |
| Mixed cellularity | 10 (23%) | 17 (14%) | |
| Lymphocyte depleted (LD) | 1 (2%) | 5 (4%) | |
| NS + LD | 1 (2%) | 5 (4%) | |
| NK | 7 | 12 | |
| Enlarged lymph nodes | |||
| Cervical | 32 (63%) | 100 (72%) | .30 |
| Axillary | 11 (22%) | 49 (35%) | .11 |
| Inguinal | 7 (14%) | 23 (17%) | .80 |
| Abdominal | 9 (18%) | 25 (18%) | 1.00 |
| Waldeyer’s ring | 3 (2%) | 1 (1%) | 1.00 |
| Hepatomegaly | 4 (8%) | 14 (10%) | .78 |
| Splenomegaly | 13 (25%) | 22 (16%) | .20 |
| Total no. of patients (n) | 51 | 139 |
| Characteristic . | EBVPositive . | EBVNegative . | P Value . |
|---|---|---|---|
| Gender: male | 43 (84%) | 80 (58%) | .001 |
| Median age (inter-quartile range) | 33 (22-49) | 33 (23-45) | .55 |
| B symptoms: present | 16 (31%) | 53 (38%) | .49 |
| Clinical stage | |||
| 1 | 2 (4%) | 10 (7%) | .87 |
| 2 | 16 (33%) | 45 (33%) | |
| 3 | 19 (39%) | 48 (35%) | |
| 4 | 12 (24%) | 35 (25%) | |
| Histological type | |||
| Lymphocyte predominant | 2 (5%) | 11 (9%) | |
| Nodular sclerosis (NS) | 30 (68%) | 86 (69%) | 1.00 |
| Mixed cellularity | 10 (23%) | 17 (14%) | |
| Lymphocyte depleted (LD) | 1 (2%) | 5 (4%) | |
| NS + LD | 1 (2%) | 5 (4%) | |
| NK | 7 | 12 | |
| Enlarged lymph nodes | |||
| Cervical | 32 (63%) | 100 (72%) | .30 |
| Axillary | 11 (22%) | 49 (35%) | .11 |
| Inguinal | 7 (14%) | 23 (17%) | .80 |
| Abdominal | 9 (18%) | 25 (18%) | 1.00 |
| Waldeyer’s ring | 3 (2%) | 1 (1%) | 1.00 |
| Hepatomegaly | 4 (8%) | 14 (10%) | .78 |
| Splenomegaly | 13 (25%) | 22 (16%) | .20 |
| Total no. of patients (n) | 51 | 139 |
Abbreviation: NK, not known.
Treatment outcome.
Ten patients from the EBV-positive group and 19 from the EBV-negative group had received prior treatment, and these were eliminated from the analysis at this stage. Median follow-up for the 123 patients still alive in the eligible group was 86 months (interquartile range, 57 to 107 months). There were no significant differences in follow-up for the different EBV status groups (P = .99). χ2 tests also showed that there were no statistically significant differences between the groups in terms of the numbers of chemotherapy cycles received (P = .48 for ChIVPP and P = .69 for PABIOE).
When χ2 tests were used to compare groups in terms of response to treatment (Table2), it was shown that EBV-positive patients were more likely to achieve a complete response to chemotherapy than EBV-negative patients (P = .05).
Effect of EBV Infection of HRS Cells on Treatment Outcome and Survival in HD Patients
| . | EBVPositive . | EBVNegative . | P Value . |
|---|---|---|---|
| Side effects during therapy | |||
| Hematological toxicity | 18 (44%) | 57 (48%) | .79 |
| Infection | 17 (41%) | 60 (50%) | .42 |
| Neuropathy | 9 (22%) | 21 (18%) | .71 |
| Response | |||
| CR | 33 (80%) | 82 (69%) | .05 |
| PR | 4 (10%) | 31 (26%) | |
| Fail | 4 (10%) | 5 (4%) | |
| NK | 0 | 2 | |
| Survival | |||
| 2-yr survival rate | 90.2% | 81.7% | |
| Difference (95% CI) | 8.5% (−3% to 20%) | .18 | |
| 5-yr survival rate | 84.5% | 78.2% | |
| Difference (95% CI) | 6.3% (−7.9% to 20.5%) | .40 | |
| 2-yr failure-free survival | 90.2% | 74.2% | |
| Difference (95% CI) | 16% (−3.9%-28.1%) | .02 | |
| 5-yr failure-free survival | 81.5% | 68.5% | |
| Difference (95% CI) | 13% (−2.9% to 28.8%) | .13 |
| . | EBVPositive . | EBVNegative . | P Value . |
|---|---|---|---|
| Side effects during therapy | |||
| Hematological toxicity | 18 (44%) | 57 (48%) | .79 |
| Infection | 17 (41%) | 60 (50%) | .42 |
| Neuropathy | 9 (22%) | 21 (18%) | .71 |
| Response | |||
| CR | 33 (80%) | 82 (69%) | .05 |
| PR | 4 (10%) | 31 (26%) | |
| Fail | 4 (10%) | 5 (4%) | |
| NK | 0 | 2 | |
| Survival | |||
| 2-yr survival rate | 90.2% | 81.7% | |
| Difference (95% CI) | 8.5% (−3% to 20%) | .18 | |
| 5-yr survival rate | 84.5% | 78.2% | |
| Difference (95% CI) | 6.3% (−7.9% to 20.5%) | .40 | |
| 2-yr failure-free survival | 90.2% | 74.2% | |
| Difference (95% CI) | 16% (−3.9%-28.1%) | .02 | |
| 5-yr failure-free survival | 81.5% | 68.5% | |
| Difference (95% CI) | 13% (−2.9% to 28.8%) | .13 |
Abbreviations: CR, complete response; PR, partial response; NK, not known; CI, confidence interval.
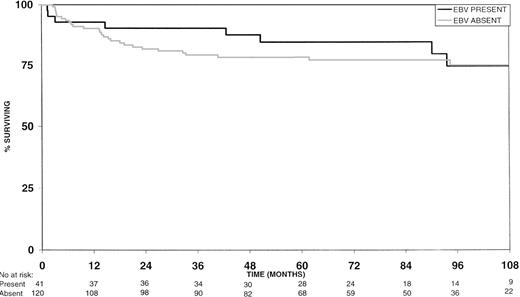
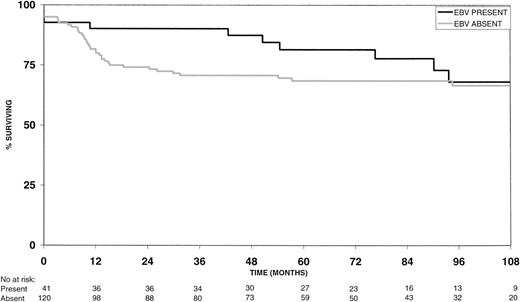
EBV-positive HD patients had longer 2-year and 5-year overall survival rates (Fig 1) than the EBV-negative group, but these differences were not statistically significant (P = .18 and P = .40, respectively; Table 2). However, 2-year failure-free survival rates were significantly better for EBV-positive versus EBV-negative HD patients (P = .02; Fig 2 and Table 2). Similarly, the 5-year failure-free survival rate was better for EBV-positive patients, but this difference was not statistically significant (P = .13; Fig 2 and Table 2).
DISCUSSION
There is accumulating evidence to suggest that EBV is not merely a silent passenger in HD but plays a key role in its pathogenesis. This is largely based on the demonstration of monoclonal EBV episomes in HD tumors,6,21 implying EBV infection as an initiating event and on the high level expression of the EBV-encoded LMP1 by HRS cells.22-24
However, few studies have focused on the potential prognostic significance of EBV in HD. On theoretical grounds, there are two opposing biological effects that could impact on prognosis. Firstly, by virtue of its well-documented effects promoting cell growth in vitro, the expression of LMP1 in the malignant cells of HD might be expected to exert a negative effect on survival for EBV-positive patients. LMP1 has been shown to have transforming effects in rodent fibroblast cell lines,31,32 enabling Rat-1 or NIH 3T3 cells to grow in medium supplemented with low serum.31 LMP1 also induces loss of contact inhibition in Rat-1 cells and causes both Rat-1 and BALB/c 3T3 cells to lose their anchorage dependence so that they clone with high efficiency in soft agar.31-33 Rat-1 cells expressing LMP1 are also tumorigenic in nude mice, whereas control Rat-1 cells are not.31
On the other hand, cytotoxic T lymphocyte (CTL) responses to LMP1 and LMP2, proteins that are consistently expressed by EBV-positive HRS cells, have been demonstrated in the blood of EBV-seropositive individuals.34-38 Furthermore, LMP2-specific CTLs have been identified and expanded from the peripheral blood of HD patients,39,40 suggesting that expression of LMP2 by HRS cells might stimulate an antineoplastic CTL response. Recent studies have also shown that the HD cell line, HDLM2, is able to process and present epitopes from LMP1 and LMP2 and is sensitive to lysis by EBV-specific CTLs.39 LMP1 expression may also increase immune recognition of infected cells in other ways. LMP1 has been shown to upregulate leukocyte function antigen 3 (LFA3) and intercellular adhesion molecule 1 (ICAM1) protein levels41-44 and major histocompatibility complex (MHC) class I and class II expression.45-47 Transfection of BL cells with LMP1 restores their ability to process both endogenous and exogenous antigens and present them to responder T cells.48,49 These observations are supported by the frequent detection of high level expression of human leukocyte antigen (HLA) class I molecules on HRS cells from EBV-positive, but not EBV-negative, HD tumors.50,51 The apparent downregulation of HLA class I molecules on EBV-negative HRS cells may also contribute to poorer survival for EBV-negative HD patients. HLA class I loss has previously been associated with poor prognosis in non-Hodgkin’s lymphomas and in other tumors.52
A previous study has reported a significant association between the detection of LMP1 in HRS cells and longer survival in a series of 140 HD patients.27 Therefore, both the present study and the Spanish study confirm the favorable prognostic effect of EBV expression in HD. The discrepancy regarding complete remission rates could be because of the different chemotherapy regimens administered: MOPP/ABVD in the Spanish study versus ChIVPP/PABIOE in the present study. Also, the present study excluded children, whereas the Spanish study included both children and adults. Clearly, further study on a larger sample of patients or meta-analysis of existing studies is indicated, not only to increase the power to detect differences in survival between EBV-positive and EBV-negative groups, but also to account for differences in outcome based on subtype association.
The finding that EBV-positive patients were more likely to achieve a complete response to chemotherapy than were EBV-negative patients, suggests that the malignant cells of EBV-positive HD may be more sensitive to the chemotherapy agents or that the residual EBV-positive cells after cytoreduction by chemotherapy might be targets for immune cytolysis. Several recent data indirectly support the hypothesis that the sensitivity of HRS cells to chemotherapy-induced apoptosis is higher in EBV-positive cells. Expression of the anti-apoptotic gene, Bcl-2, by HRS cells has been shown to predict poor clinical outcome in HD patients,53 whereas recent studies have shown that fewer HRS cells of EBV-positive HD express the Bcl-2 gene than do EBV-negative cases.54 Although it is possible that protection from chemotherapy-induced apoptosis in EBV-infected HRS cells may be mediated by other members of the bcl-2 family, including Bcl-x,55 more work is required to establish any effect of levels of expression of these genes on response to chemotherapy.
The demonstration of EBV in the tumors of HD patients may have important implications for other treatment strategies. In particular, there is growing interest in the potential development of immunotherapy approaches that use EBV-specific CTLs to target the EBV-infected malignant cell population. Such an approach has already been successful in the treatment of EBV-associated lymphoproliferative disease where adoptive transfer of donor EBV-specific CTLs has resulted in the eradication of tumors.56,57 The development of this form of therapy for HD may be particularly relevant to certain patients with recurrent or refractory disease, in whom conventional forms of treatment have produced long-term disease-free survival rates of less than 30%.58
ACKNOWLEDGMENT
The authors are grateful to Dr M. Cullen of the United Kingdom Central Lymphoma Group for access to patient data used in this study.
Supported by the Cancer Research Campaign.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul G. Murray, PhD, Biomedical Research Laboratories, School of Health Sciences, University of Wolverhampton, Wolverhampton, WV1 1DJ, UK; e-mail:p.g.murray@wlv.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal