Abstract
T-cell depletion (TCD) of the donor marrow graft has been shown to reduce the severity of graft-versus-host disease (GVHD) in patients with chronic-phase (CP) chronic myelogenous leukemia (CML) undergoing HLA-identical sibling allogeneic marrow transplantation. However, there has been a corresponding reduction in the graft-versus-leukemia effect so that any decrease in GVHD-related mortality has been offset by an increased rate of disease relapse. Therapy of recurrent disease with donor leukocyte infusions (DLI) has been proven to be effective salvage therapy for the majority of patients who relapse after allogeneic BMT with CP CML. However, the overall impact of salvage DLI therapy on the survival of CP CML patients initially transplanted with TCD marrow grafts is not defined. To address this question, we have evaluated a clinical strategy of TCD followed by targeted adoptive immunotherapy with DLI in 25 CP CML patients undergoing allogeneic BMT from HLA-identical siblings. All patients received a standardized preparative regimen along with ex vivo TCD and posttransplant cyclosporine as GVHD prophylaxis. Durable engraftment was observed in all 25 patients. The incidence of grade II to IV acute GVHD was 8%. The cumulative incidence of transplant-related mortality (TRM) was 4%, and the 1-year probability of overall survival was 96%. The 3-year cumulative relapse incidence was 49%. All relapsed patients received DLI to reinduce remission. The total T-cell dose administered to these patients varied from 0.1 to 5.0 × 108 T cells/kg. Complete responses were observed in 12 of 14 patients, with 1 additional patient still too early to evaluate. Three patients died of GVHD after DLI, and 1 relapsed into blast crisis after a transient cytogenetic remission. Of the remaining 10 patients, 8 are in molecular remission, 1 is alive in relapse, and 1 is receiving DLI treatment. The median follow-up after infusion of surviving DLI patients in remission is 5.3 years. The probability of overall 5-year survival for the entire population is 80%, with a median follow-up of 6.4 years. We conclude that the clinical strategy of TCD followed by targeted adoptive immunotherapy with DLI for those patients with evidence of recurrent disease is a viable transplant strategy for CP CML, resulting in 80% survival and a low risk of acute GVHD and transplant-related mortality.
T-CELL DEPLETION (TCD) has been shown to reduce the severity of graft-versus-host disease (GVHD) in patients undergoing HLA-identical sibling allogeneic bone marrow transplantation (BMT) for chronic-phase (CP) chronic myelogenous leukemia (CML).1-3 However, removal of donor T cells from the marrow graft compromises the graft-versus-leukemia (GVL) effect that plays a primary role in preventing disease recurrence.4 5 This has resulted in increased relapse rates and an overall decrease in survival when compared with patients transplanted with unmodified grafts. The decreased survival is attributable to the fact that benefits derived from a reduction in GVHD-associated mortality due to TCD is more than offset by an increased rate of CML recurrence.
For many patients who relapse with CML after BMT, donor leukocyte infusions (DLI) are an effective salvage therapy.6-12Because of the higher relapse rate associated with TCD, the vast majority of patients treated with DLI have been previously transplanted with TCD marrow grafts. DLI is able to induce remissions in 70% to 80% of patients relapsing after a transplant for CP CML. Prior studies have shown that a majority of patients have sustained molecular remissions,7,8,10,12 indicating that these patients may in fact be cured of their disease. However, this therapy has not been without complications, because some patients develop fatal GVHD and/or marrow aplasia attributable to the infusion of immunocompetent donor T cells.6-12 Although these adverse effects have limited the efficacy of DLI, the fact that the majority of relapsed patients can achieve durable remissions has made this therapy a valuable adjunct to the transplant management of CP CML. However, the overall impact of DLI on the survival of CP CML patients initially transplanted with TCD marrow grafts is unknown.
In this report, we describe the efficacy of a clinical strategy of TCD marrow transplantation followed by targeted adoptive immunotherapy with DLI in patients who relapse posttransplant. The goals of this study were to define whether this approach would result in an overall survival rate comparable to that observed in recipients of unmodified marrow grafts. We also sought to determine the effect of this strategy on transplant-related mortality after initial BMT, which is the major obstacle preventing the application of allogeneic BMT to older patient populations.
MATERIALS AND METHODS
Patient population.
Twenty-five consecutive patients with first CP CML who received BMT from HLA-identical sibling donors between January 1, 1988 and June 30, 1998 at Froedert Memorial Lutheran Hospital or the Children’s Hospital of Wisconsin were evaluated. The patient demographic data are shown in Table 1. First chronic phase was defined using previously published criteria.13 Each recipient and donor candidate underwent serotyping for HLA-A and B alleles by standard microcytotoxicity assays. HLA-DR and DQ disparities were assessed by either microcytotoxicity assays or by oligonucleotide genotyping.14 15 Informed consent was obtained from each patient (or their guardians), and all treatment was administered under protocols approved by the Institutional Review Committees of the Medical College of Wisconsin.
Patient Characteristics
| Age (yr) | |
| Median | 37 |
| Range | 13-58 |
| Sex (M/F) | 15/10 |
| Diagnosis to BMT | |
| ≤1 yr | 23 |
| ≥1 yr | 2 |
| Median | 133 days |
| CMV serostatus | |
| Positive | 12 |
| Negative | 13 |
| Age (yr) | |
| Median | 37 |
| Range | 13-58 |
| Sex (M/F) | 15/10 |
| Diagnosis to BMT | |
| ≤1 yr | 23 |
| ≥1 yr | 2 |
| Median | 133 days |
| CMV serostatus | |
| Positive | 12 |
| Negative | 13 |
Preparative regimen, GVHD prophylaxis, and supportive care.
All patients were treated in laminar air flow or HEPA-filtered rooms. Pretransplant conditioning consisted of high-dose cytosine arabinoside (3 g/m2 × 6, days −7 to −4), cyclophosphamide (45 mg/kg × 2, days −6 and −5), and methylprednisolone (1 g/m 2 × 4, days −2 to 0) followed by fractionated total body irradiation to a total dose of either 13.32 or 14 Gy (days −2 to 0).16,17 GVHD prophylaxis consisted of ex vivo T-cell depletion with the αβ T-cell receptor antibody, T10 B9, and baby rabbit complement plus posttransplant cyclosporine.18 All patients received prophylactic antibiotics according to institutional guidelines. Cytomegalovirus (CMV)-seronegative patients received blood components from CMV-seronegative donors.
Assessment of engraftment, GVHD, and relapse.
The date of engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count (ANC) was ≥500/μL. Trilineage engraftment was documented by bone marrow examination in the majority of patients 3 to 4 weeks after transplant. Follow-up marrow studies were performed at 100 days, 6 months, 1 year, and at least yearly after transplantation, whenever possible, to evaluate engraftment and disease status. Durable engraftment was confirmed by cytogenetic analysis, restriction fragment length polymorphism (RFLP) studies, or analysis of variable number of tandem repeats (VNTRs) in blood or marrow samples to distinguish donor from recipient cells. Acute GVHD was graded as 0 to IV according to criteria of Glucksberg et al,19 whereas chronic GVHD was defined as none, limited, or extensive.20Patients who had evidence of engraftment were evaluable for acute GVHD, whereas patients who engrafted and also survived more than 100 days were evaluable for chronic GVHD. Transplant-related mortality was defined as death resulting from any causes other than relapse. Complications such as fatal GVHD resulting from DLI were attributable to disease recurrence and therefore were not classified as transplant-related mortality. Relapse was defined by either morphologic evidence of CML in the peripheral blood, marrow, or extramedullary sites or by the recurrence and sustained presence of the Philadelphia chromosome on cytogenetic analysis. Patients whose sole evidence of disease was positivity for the bcr/abl RNA transcipt by the polymerase chain reaction (PCR) were not classified as having relapsed. Acute GVHD occurring in patients treated with DLI was operationally defined as that occurring within 100 days of infusion, whereas chronic GVHD was defined as that present after day 100 from infusion.
DLI therapy.
Patients with evidence of relapse were treated with DLI to reinduce remission. Patients were taken off all immunosuppressive or cytotoxic agents (eg, prednisone, cyclosporine, hydroxyurea, or interferon) before the administration of leukocyte infusions. In no case did discontinuation of immunosuppression result in a clinical antileukemic effect or GVHD. Leukocyte collections from the original bone marrow donors were performed using a Cobe spectra apheresis system (Cobe Laboratories, Inc, Lakewood, CO), as previously described.7Patients requiring multiple infusions received them every other day immediately after collection from the donor. For donor/recipient pairs who were ABO-compatible, leukocytes were infused directly into the patient without further processing. Conversely, in patients who were ABO-incompatible with the donor, Ficoll-hypaque density gradient centrifugation was performed to remove red blood cells. No GVHD prophylaxis was administered to any of the patients. The first 13 patients were infused with naı̈ve unactivated donor T cells. The most recent patient received activated donor T cells that had been retrovirally transduced with the thymidine kinase gene.21The percentage of lymphocytes in each apheresis product was determined using an STKR Coulter Counter (Coulter Corp, Hialeah, FL). The percentage of CD3+ T cells was calculated by flow cytometric analysis. The total T-cell dose administered to patients was calculated using the following formula: (total nucleated cell dose) × (% lymphocytes) × (% CD3+ T cells). The first 9 patients treated with DLI received a total of approximately 2.5 to 5.0 × 108 T cells/kg (vide infra). Subsequent patients received a lower dose of 0.1 to 1.0 × 108 T cells/kg based on a study suggesting that this dose might reduce GVHD without compromising antileukemic efficacy.22 Peripheral blood and bone marrow specimens were obtained from all patients at least monthly for the first 3 months and then at 2-month intervals for the next 4 months to assess disease status. Patients who received DLI were classified as having attained complete remission if they had documented cytogenetic remission by marrow examination at any time after the administration of DLI. Patients were defined as being in continuous complete remission if the most recent marrow examination failed to show the presence of the Philadelphia chromosome.
PCR assay for detection of minimal residual disease.
Total cellular RNA was prepared from peripheral blood, peripheral blood buffy coats, or peripheral blood mononuclear cells isolated by Ficoll-hypaque, bone marrow, or cultured B cells (negative control) and K562 (Philadelphia chromosome positive) cells. Peripheral blood and marrow cells were used interchangeably based on previous data demonstrating that both are equally sensitive for detecting residual disease in CML patients.23 Cells were either viably frozen or directly added to 4 mol/L guanidinium isothiocyanate. RNA was prepared from 3 to 5 million cells. The conditions used for extraction of RNA and PCR amplification have been previously detailed.17,24 All assays were performed in duplicate, beginning with independent RNA isolations. Mock RNA preparations were included in every assay as negative controls. The criteria used for defining positive assay results have been previously published.17 Assay sensitivity was monitored through the addition and detection of 0.05 ng RNA from the Ph+ cell line K562, mixed within a replicate assay for each unknown sample. This quantity of RNA represents nucleic acid isolated from approximately five K562 cells, which requires two rounds of nested primer PCR for detection. Whenever possible, assays were performed at 6-month intervals.
Statistical analysis.
Endpoints were calculated at the date of last contact with the date of latest follow-up being November 15, 1998. The median duration of follow-up since BMT for the patient population was 77 months (range, 5 to 117 months). Cumulative actuarial probabilities of overall survival and acute and chronic GVHD were calculated using the Kaplan-Meier method.25 Cumulative incidence curves for relapse and transplant-related mortality were computed as described by Pepe and Motomi.26 Data for these two variables are presented as the value ± the standard error. Patients were censored at the time of death in the analysis of GVHD and relapse. Confidence limits for the Kaplan-Meier estimate were based on the arcsine transformation.27
RESULTS
Engraftment.
The mean bone marrow inoculum administered after TCD was 8.96 × 107 nucleated cells/kg (range, 1.43 to 19.3 × 107/kg). Limiting dilution assays were performed in the last 15 patients in the cohort to determine the degree of TCD. In these patients, the median log TCD was 1.90 (range, 1.15 to 3.26) and the median T-cell dose was 3.4 × 105 T cells/kg (range, 0.044 to 31.5 × 105 T cells/kg). Sixteen patients were treated with either granulocyte-macrophage colony-stimulating factor (GM-CSF) or granulocyte colony-stimulating factor (G-CSF) to accelerate myeloid engraftment after BMT. The median times to an ANC ≥500/μL and ≥1,000/μL for 3 consecutive days was 17 days (range, 11 to 29 days) and 21 days (range, 12 to 40 days), respectively. The median time to a platelet count ≥20,000 was 25 days (range, 13 to 159 days). All patients had at least one marrow or peripheral blood sample within the first 4 months posttransplant examined for the extent of donor chimerism. Thirteen patients had greater than 90% donor cells by either VNTR or RFLP analysis (median, 98%; range, 90% to 100%). Nine recipients had complete donor chimerism on cytogenetic analysis using either sex mismatching or informative polymorphisms to distinguish donor from recipient cells. In 3 patients, chimerism studies were uninformative, but these patients had sustained hematopoietic recovery. No patient had evidence of marrow graft rejection.
GVHD.
All 25 patients were evaluable for the development of both acute and chronic GVHD after BMT. The probability of developing grade II-IV acute GVHD was 8% (95% confidence interval [CI], 2.3% to 28%). No patient developed grade III or grade IV acute GVHD. Limited chronic GVHD occurred in 7 patients, whereas extensive chronic GVHD developed in 3 patients. The overall probability of developing any chronic GVHD and extensive chronic GVHD was 43% (95% CI, 29.9% to 60%) and 13% (95% CI, 4.8% to 33.6%), respectively. Surviving patients not treated with DLI were tapered off immunosuppressive therapy at a median of 6 months (range, 2.5 to 24 months) posttransplant.
Treatment of relapsed patients with DLI.
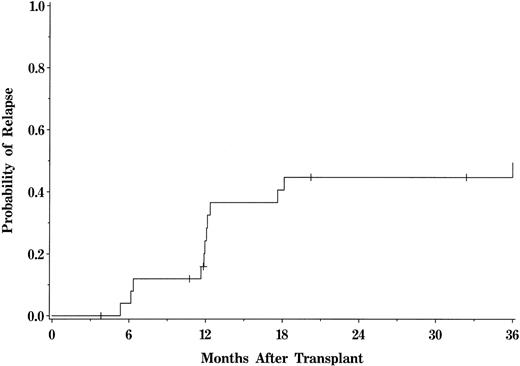
The 3-year cumulative incidence of hematologic or sustained cytogenetic relapse for the entire cohort was 49% (95% CI, 38.6% to 61.3%; Fig 1). Fourteen relapses occurred in patients transplanted with HLA-identical sibling marrow grafts. For those patients who relapsed, the median time from BMT to relapse was 368 days (range, 187 to 1,494 days). Five patients who relapsed had a history of both acute and chronic GVHD, 2 patients had acute GVHD only, and 2 had chronic GVHD only. Five patients had no history of GVHD. All 14 patients with relapse subsequently received DLI to reinduce remission (Table 2). The median time from relapse to DLI was 110 days (range, 45 to 2,022 days). Eight recipients were in hematologic relapse and 6 were in cytogenetic relapse at the time of DLI. None of the patients treated with DLI had GVHD at the time of infusion or was on immunosuppressive therapy. Six patients developed acute GVHD, including 3 with grade I, 1 with grade II, and 2 with grade IV. Three of these patients died of GVHD and 2 others progressed to extensive chronic GVHD. Five patients with no or grade I acute GVHD also subsequently developed extensive chronic GVHD. Marrow aplasia was observed in 1 patient who required several marrow boosts from the original donor before effective hematopoietic function was restored.
Actuarial probability of relapse for the entire patient population. Tick marks represent patients currently alive in remission.
Actuarial probability of relapse for the entire patient population. Tick marks represent patients currently alive in remission.
Clinical Characteristics of DLI Patients
| UPN . | BMT To Relapse (d) . | Relapse to DLI (d) . | Status at Time of DLI . | T-Cell Dose (×108/kg) . | Acute/Chronic GVHD . | Response to DLI . | Current Clinical Status . |
|---|---|---|---|---|---|---|---|
| 251 | 361 | 867 | HR | 2.9 | 0/E | CR | CCR, d3484 |
| 335 | 187 | 1,023 | HR | 3.4 | 0/E | CR | CCR, d2881 |
| 350 | 1,095 | 63 | Cy Rel | 2.6 | 0/E | CR | CCR, d2985 |
| 352 | 383 | 2,022 | HR | 1.1 | I/0 | NR | Alive, relapse, d3037 |
| 357 | 363 | 230 | HR | 2.7 | I/0 | CR | Dead, GVHD |
| 372 | 376 | 140 | HR | 2.7 | 0/0 | CR | CCR, d2546 |
| 487 | 1,494 | 115 | Cy Rel | 0.1 | IV/NE | CR | Dead, GVHD |
| 506 | 369 | 65 | HR | 2.8 | II/E | CR | CCR, d2106 |
| 563 | 162 | 67 | HR | 5.2 | 0/0 | CR | CCR, d1823 |
| 593 | 551 | 71 | Cy Rel | 2.5 | IV/NE | CR* | Dead, GVHD |
| 602 | 193 | 129 | HR | 2.9 | 0/E | CR | Dead, Blast Crisis |
| 732 | 536 | 78 | Cy Rel | 0.1 | 0/0 | CR | CCR, d1371 |
| 755 | 367 | 45 | Cy Rel | 0.1 | I/E | CR | CCR, d1195 |
| 927 | 354 | 105 | Cy Rel | 0.1 | 0/NE | NE | Alive, d508 |
| UPN . | BMT To Relapse (d) . | Relapse to DLI (d) . | Status at Time of DLI . | T-Cell Dose (×108/kg) . | Acute/Chronic GVHD . | Response to DLI . | Current Clinical Status . |
|---|---|---|---|---|---|---|---|
| 251 | 361 | 867 | HR | 2.9 | 0/E | CR | CCR, d3484 |
| 335 | 187 | 1,023 | HR | 3.4 | 0/E | CR | CCR, d2881 |
| 350 | 1,095 | 63 | Cy Rel | 2.6 | 0/E | CR | CCR, d2985 |
| 352 | 383 | 2,022 | HR | 1.1 | I/0 | NR | Alive, relapse, d3037 |
| 357 | 363 | 230 | HR | 2.7 | I/0 | CR | Dead, GVHD |
| 372 | 376 | 140 | HR | 2.7 | 0/0 | CR | CCR, d2546 |
| 487 | 1,494 | 115 | Cy Rel | 0.1 | IV/NE | CR | Dead, GVHD |
| 506 | 369 | 65 | HR | 2.8 | II/E | CR | CCR, d2106 |
| 563 | 162 | 67 | HR | 5.2 | 0/0 | CR | CCR, d1823 |
| 593 | 551 | 71 | Cy Rel | 2.5 | IV/NE | CR* | Dead, GVHD |
| 602 | 193 | 129 | HR | 2.9 | 0/E | CR | Dead, Blast Crisis |
| 732 | 536 | 78 | Cy Rel | 0.1 | 0/0 | CR | CCR, d1371 |
| 755 | 367 | 45 | Cy Rel | 0.1 | I/E | CR | CCR, d1195 |
| 927 | 354 | 105 | Cy Rel | 0.1 | 0/NE | NE | Alive, d508 |
Abbreviations: CR, complete remission; CCR, continuing complete remission; NR, no response; NE, not evaluable; HR, hematologic relapse; Cy Rel, cytogenetic relapse.
No morphologic evidence of leukemia in bone marrow at autopsy; no cytogenetic testing performed.
Twelve of 14 patients achieved cytogenetic remission at a median of 3 months (range, 1 to 8 months) after DLI. One of these 12 patients relapsed 2 months later and eventually died of blast crisis CML. The 3 patients who died from GVHD were either in hematologic or cytogenetic remission at the time of death. Two still had molecular evidence of disease; 1 was in molecular remission (UPN 357). One patient has failed to respond to DLI and is currently alive in relapse 7 months after DLI. The last patient is too early to evaluate. Nine of 10 surviving patients treated with DLI are off all immunosuppressive medications; 1 continues to have moderate oral GVHD 6 years after DLI and is on treatment with topical steroids. The median follow-up after infusion of surviving DLI patients in remission (n = 8) is 5.3 years (range, 2.2 to 6.7 years).
Remission status as assessed by PCR.
Serial PCR assays were performed in all 25 patients to more stringently define the remission status of recipients after TCD allogeneic BMT with or without adjunctive DLI. A total of 267 RNA samples (19 pretransplantation and 248 posttransplantation) were evaluable using previously published criteria.17 Pretransplantation samples were obtained in 19 patients and all were positive for the bcr/abl RNA transcript. In 6 patients, no pretransplant sample was available. One hundred fifty-eight of 248 posttransplant samples were collected from the bone marrow and 90 from the peripheral blood. Positive assays were observed in 44 of 90 (49%) of peripheral blood samples and in 93 of 158 (59%) of bone marrow samples. The average number of posttransplant samples assayed per patient was 9.9 (range, 2 to 17).
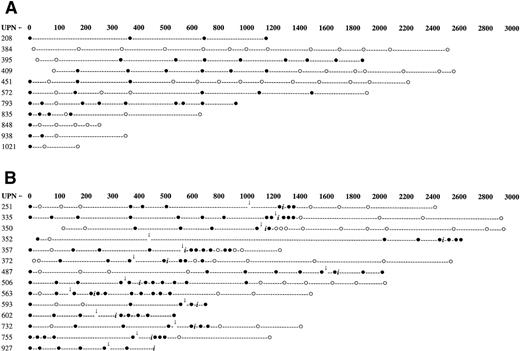
Serial molecular assays performed in patients who did not require treatment with DLI for relapsed disease are shown in Fig 2A, whereas those conducted in the 14 patients who required DLI are shown in Fig 2B. Nine of 11 cytogenetic responders to DLI also achieved molecular remission. The remaining 2 patients died of GVHD before molecular remission could be documented. No relapses have been observed in patients achieving molecular remission. Eleven patients who have not received DLI are currently alive in cytogenetic remission. Eight of these 11 patients are also in molecular remission at the time of last analysis, whereas 3 patients are positive for the bcr/abl RNA transcript. The bcr/abl signal was detectable only after two rounds of PCR amplification in these patients, indicative of a leukemic burden of between 1 in 103 and 1 in 106 cells,24 which is below the level of detection of cytogenetic analysis. When all patients in the study are combined, 15 of 20 (75%) surviving patients are currently in molecular remission.
Serial PCR analysis for patients who received HLA-identical sibling marrow grafts for the treatment of CP CML. (A) Patients who have not relapsed and therefore have not been treated with DLI. (B) Patients who were treated with DLI for relapsed disease. (•) Patients testing positive for the presence of the bcr/abl RNA transcript. (○) Patients testing negative for the presence of the bcr/abl RNA transcript. Arrows indicate time of relapse posttransplant. (i) Denotes the time of first donor leukocyte infusion.
Serial PCR analysis for patients who received HLA-identical sibling marrow grafts for the treatment of CP CML. (A) Patients who have not relapsed and therefore have not been treated with DLI. (B) Patients who were treated with DLI for relapsed disease. (•) Patients testing positive for the presence of the bcr/abl RNA transcript. (○) Patients testing negative for the presence of the bcr/abl RNA transcript. Arrows indicate time of relapse posttransplant. (i) Denotes the time of first donor leukocyte infusion.
Transplant-related mortality and survival.
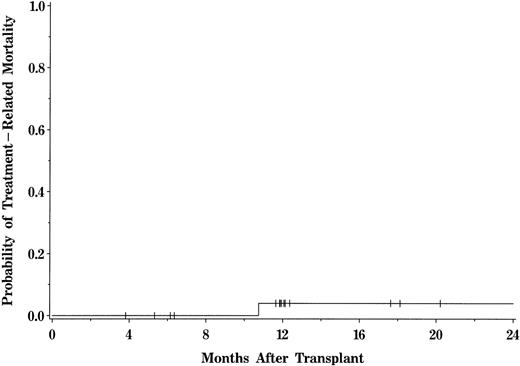
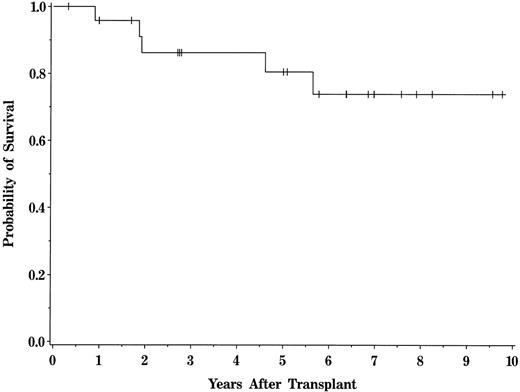
One patient died from transplant-related toxicity. This death was attributable to a gastrointestinal hemorrhage (UPN 848) in a patient who was receiving immunosuppressive therapy for GVHD at the time of demise. Overall transplant-related mortality after BMT was 4% (95% CI, 1% to 24%) for the entire patient population (Fig 3). No deaths attributable to relapse or DLI therapy occurred within the first year of transplant, resulting in a 1-year probability of survival of 96%. The probability of overall survival for the entire population at 5 years is 80% (95% CI, 58.9% to 91.5%), with a median follow-up of 6.4 years (range, 0.4 to 9.8 years; Fig 4). The median Karnofsky score of surviving patients is 100 (range, 70 to 100).
Actuarial probability of transplant-related mortality for patients transplanted with HLA-identical sibling marrow grafts.
Actuarial probability of transplant-related mortality for patients transplanted with HLA-identical sibling marrow grafts.
Actuarial probability of survival for the entire patient population. Tick marks represent patients currently alive.
Actuarial probability of survival for the entire patient population. Tick marks represent patients currently alive.
DISCUSSION
Allogeneic BMT using HLA-identical sibling donors has proven to be effective therapy for patients with CP CML, with long-term survival rates of 50% to 80%.1-3,28,29 In most clinical series, patients have been transplanted with unmodified marrow grafts due to the concern about the increased relapse rate associated with TCD that has counterbalanced any salutory effects from a reduction in GVHD. In that regard, data reported to the International Bone Marrow Transplant Registry indicate that, of a total of 3,141 CP CML patients undergoing HLA-identical sibling marrow grafts between 1992 and 1997, 94% were transplanted with non-TCD grafts (M. Horowitz, personal communication, December 1998). The 1-year transplant-related mortality in this population was 26%, indicating that a substantial number of patients die of this complication. Studies from single institutions and cooperative groups have reported 1-year mortality rates of between 10% and 40% in the same patient population.29-31 The divergent problems inherent in each transplant approach prompted us to consider an alternative strategy whereby TCD could be used to decrease transplant-related mortality and DLI would then be used as needed to address the problem of increased relapse. This approach viewed the initial transplant as one element in a multistep strategy designed to achieve engraftment without collateral life-threatening toxicity from GVHD and then to target adjunctive immunotherapy to those patients who had evidence of recurrent disease.
The current study demonstrated that use of this clinical strategy resulted in durable engraftment in all patients with only modest toxicity from GVHD. Transplant-related mortality occurred in only 1 of 25 recipients. The probability of survival at 1 year was 96%, with only one death observed within the first 6 months posttransplant, indicating that the regimen was well tolerated in a cohort in which approximately 50% of patients were greater than 40 years of age. As expected, relapse was substantial, with a probability of disease recurrence of 49% at 3 years. However, approximately 60% of relapsed patients have achieved durable remissions with DLI therapy, and all surviving remitters remain in molecular remission with a median follow-up of 5.3 years. The resulting overall 5-year survival rate of 80% therefore compares favorably to results using unmodified marrow grafts.
Schattenberg et al32 have reported similar results with this approach, although in their study, not all relapsed patients were treated with DLI and several additional recipients received non-TCD second marrow transplants for graft failure. The potential role of DLI was therefore not evaluable in all patients. Moreover, the median follow-up of more than 6 years in the present study is substantially longer and provides more conclusive proof of the durability of both DLI-induced remissions and remissions in patients not requiring salvage immunotherapy.
The major problem with the use of DLI in this clinical setting is the toxicity attributable to the infusion of donor leukocytes, specifically that due to GVHD and marrow aplasia, which has limited the overall efficacy of this therapy. Both of these complications have contributed to and resulted in the death of patients who were otherwise in remission. Improvements in overall survival using this clinical strategy are therefore contingent upon alternative approaches designed to reduce the severity of these specific problems. With respect to marrow aplasia, studies indicate that the incidence of this complication is inversely proportional to the extent of residual donor hematopoiesis.33 Prior studies7 10 support the premise that treating patients in early relapse when there is still evidence of donor hematopoiesis essentially eliminates this complication. In CML, this goal can usually be accomplished, because relapse can often be detected before it is clinically evident and patients have reverted back to complete recipient chimerism by using cytogenetic and molecular assays.
The most significant complication of DLI therapy was GVHD, which was the cause of death in 3 of the patients in the present study and has been reported to be fatal in 10% to 20% of patients.6-12Although conventional pharmacologic approaches are successful in the majority of patients with GVHD after DLI, not all respond to this approach. The optimal strategy for the amelioration of GVHD in DLI patients has yet to be defined, although several alternative approaches are currently being explored that offer the potential to reduce GVHD-related toxicity. Mackinnon et al22 have shown that the use of limiting T-cell doses (1 × 107/kg) may be superior to the administration of higher T-cell doses for the treatment of CML in cytogenetic or molecular relapse. In their study, 8 patients treated at this cell dose achieved remission, whereas only 1 had evidence of GVHD. This strategy appears to be dependent on treatment in early (cytogenetic/molecular v hematologic) relapse and will require confirmatory studies to ascertain whether GVHD can indeed be abrogated without compromising antileukemic reactivity. An alternative strategy is the incorporation of a suicide gene into T cells in an effort to more precisely modulate GVH/GVL reactivity. The gene that is currently being used in clinical trials is the herpes simplex thymidine kinase gene, which allows for the selective elimination of transduced donor T cells after ganciclovir administration.34 Finally, the use of specific T-cell subsets (eg, CD4+ T cells) as opposed to unfractionated DLI is an approach that may have utility in increasing the therapeutic index.35 36 Given that a majority of patients who die of GVHD after DLI are in remission at the time of death, the ability to mitigate GVHD-related toxicity offers the potential to improve overall survival in this patient population.
However, there are several potential disadvantages with this clinical strategy that need to be considered. One pertains to the possibility that a patient who is transplanted in the CP will subsequently relapse with accelerated phase or blast crisis disease. In such instances, DLI has been shown to be much less effective; therefore, these patients might not be salvageable with immunotherapy. Although reported,37 this was not observed in this series and appears to be a very uncommon occurrence, because the vast majority of such patients relapse in the CP of their disease.28,38 A second concern pertains to the responsiveness of relapsed patients to DLI therapy even when they are treated in the early phase of their disease. Prior studies have shown that 70% to 80% of patients who relapse into CP CML respond to treatment, but a minority either fail to respond or relapse after initial therapy.39 The reason that some patients do not respond to DLI is unknown, but appears to be due to an inability of donor T cells to recognize target antigens on the leukemia cell surface and eradicate disease. Two patients in the current study who were in hematological relapse ultimately relapsed after a transient DLI-induced remission. A potential solution to this problem is to administer DLI when patients are in cytogenetic relapse. Studies have shown that CML patients treated in cytogenetic or molecular relapse have a significantly better response rate than patients treated in hematologic relapse,40 41 presumably due to the presence of a minimal disease burden. The administration of DLI in cytogenetic relapse might be advantageous from the standpoint that this approach would avoid treating patients in molecular relapse who, as observed in this study, may not manifest any further disease progression for a prolonged period of time. Patients could then still be captured for treatment before there were any overt clinical signs of recurrence.
In summary, this study demonstrates that ex vivo TCD of the donor marrow graft to reduce GVHD followed by adjunctive immunotherapy with DLI for the treatment of disease relapse is a viable strategy for the therapy of CP CML with HLA-identical sibling donors. Transplant-related mortality is low with this approach and survival rates are comparable or superior to those observed in recipients of unmodified marrow grafts. This strategy may be particularly beneficial in older recipients, who comprise the majority of patients with CML and are at greatest risk from complications of GVHD and transplant-related mortality. Another advantage of this approach is that patients who are not destined to relapse are not exposed to the same degree of GVHD risk that derives from a non-TCD graft. We conclude that the use of TCD in this patient population should be reassessed in light of the emerging clinical approaches that are currently being explored for the therapy of relapsed disease posttransplant. These refinements offer the potential to augment the therapeutic index of posttransplant immunotherapy with the goal of further improving survival in these patients and even extending the age of viable candidates for allogeneic BMT.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to William R. Drobyski, MD, Bone Marrow Transplant Program, Froedert Memorial Lutheran Hospital, 9200 W Wisconsin Ave, Milwaukee, WI 53226.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal