We report a pair of identical twins with concordant acute lymphoblastic leukemia (ALL). Unusually, their diagnoses were spaced 9 years apart at ages 5 and 14. Leukemic cells in both twins had aTEL-AML1 rearrangement, which was characterized at the DNA level by an adaptation of a long distance polymerase chain reaction (PCR) method. The genomic fusion sequence was identical in the two leukemias, indicative of a single cell origin in one fetus, in utero. At the time twin 1 was diagnosed (aged 5 years), the bone marrow of twin 2 was hematologically normal. However, retrospective scrutiny of the DNA from an archived slide with clonotypic TEL-AML1 primers showed that the presumptive preleukemic clone was present and disseminated 9 years before a clinical diagnosis. These data provide novel insight into the natural history of childhood leukemia and suggest that consequent to a prenatal initiation of a leukemic clone, most probably by TEL-AML fusion itself, the latency of ALL can be both extremely variable and protracted. This, in turn, is likely to reflect the timing of critical secondary events.
PEDIATRIC ACUTE lymphoblastic leukemia (ALL) is a biologically and clinically diverse cancer. The striking age-associated peak incidence of disease in developed societies at 2 to 5 years of age is characterized as B-cell precursor or common (c) ALL1,2 and within this group are molecular subgroups with hyperdiploidy or karyotypically cryptic t(12;21), TEL-AML1(ETV6-CBFA2) gene fusions.3,4 The etiology of ALL is unknown. Recent evidence suggests exposure to ionizing radiation or electromagnetic fields (EMF) are not major factors.5,6 Epidemiologic studies, principally from the UK, have implicated an abnormal response to common infection(s).7,8 Key issues are the time frame of ALL development and whether the consistent molecular abnormalities observed are the product of primary or secondary etiological events. For infants with acute leukemia and MLL gene fusions, there is now compelling evidence for a prenatal initiation in utero. These data derive from studies on identical twins9-11 and, more directly, by retrospective detection using polymerase chain reaction (PCR) of clonotypic genomic MLL-AF4 fusion sequences in neonatal blood spots (Guthrie cards) of patients.12
The question of whether the more common form of childhood B-cell precursor leukemia (cALL) can also be initiated in utero is much less certain,13 although some mathematical modelling accords with a prenatal origin.14 While some epidemiologic studies provide further support for an origin of infant acute leukemia during pregnancy,15,16 there is sparse evidence for such an association in typical childhood ALL other than longstanding data indicating a small, but significant, increased risk (≈1.4×) from diagnostic radiation exposure in pregnancy.17,18 Changes in diagnostic procedures have effectively removed this risk. There is some biological evidence compatible with an in utero origin for cALL. For patients less than 3 years of age, the lack of N region nucleotides at the VDJ junctions of rearranged IGH alleles of leukemic blast cells in most cases is considered to reflect the minimal function of terminal deoxynucleotidyl transferase (TdT) in fetal B lymphopoiesis.19,20 Recent data from identical twin studies in ALL provides indirect, but convincing, evidence for an in utero origin in at least some cases. We described a pair of twins aged 3 years 6 months and 4 years 10 months at diagnosis (with cALL) who shared a nonconstitutive, but identical or clonotypic TEL-AML1 fusion, at the DNA level as well as an identical rearranged IGH allele.21 As with previous studies on other leukemias in twins,9,10,22these data were interpreted as reflecting an in utero single-cell origin of the TELAML1 fusion followed by prenatal dissemination of the clonal progeny from one twin to the other via intraplacental anastomoses.23 24
We now report another pair of identical twins with ALL that confirm the fetal origins of the TEL-AML1 gene, but that are especially informative in the context of postnatal latency for ALL.
MATERIALS AND METHODS
Patients’ obstetric and clinical history.
Female identical twins 244 and 2850 (Dutch Childhood Leukemia Study Group) were born on April 9, 1968. They were diagnosed with ALL aged 5 years 2 months (twin 244) and 13 years 11 months (twin 2850) at the Beatrix Children’s Hospital. Twin 2850 had CD10+ B lineage ALL (L2, common ALL) with normal cytogenetics; twin 244 was not immunophenotyped or karyotyped, but morphologically and histochemically was classified as ALL. The main hematologic parameters were as follows: twin 244, white blood cell (WBC) count 6.6 × 109/L, 21% blasts, bone marrow 98% blasts. Twin 2850, WBC 4.8 × 109/L, 35% blasts, bone marrow 94% blasts. Twin 244 was treated on protocol DCLSG ALL-2 and has remained in complete remission. Twin 2850 was treated using protocol DCLSG ALL-5, relapsed after 20 months, and died in second remission of infection 9 months later.
Frozen bone marrow cells (94% blasts) taken at diagnosis were available on twin 2850 and were found to be suitable for DNA, but not mRNA extraction. For twin 244, the only diagnostic material available was one bone marrow smear stained with Sudan Black and another stained with periodic-acid Schiff (PAS). A stained bone marrow smear was also available from twin 2850, which was made at the time of the diagnosis of her twin, ie, 9 years before she herself was diagnosed. This smear was at the time judged to be hematologically normal. At the time of study, therefore, the slides available on the twins had been kept for 15 years. The twins were identical in appearance and had the same blood and HLA groups. The mother was 22 years old at the time of birth and received no diagnostic radiation during pregnancy. The twins at birth had no congenital malformations, and there was no family history of leukemia or related conditions. There is no record of whether the placenta was monochorionic or dichorionic.
Preparation of DNA.
DNA was prepared from the bone marrow of twin 2850 essentially as described in Ford et al.25 DNA was prepared from PAS-stained bone marrow smears using TaKaRa Dexpat (BioWhitaker UK, Wokingham, UK) exactly as described by the manufacturer before being diluted further for PCR.
DNA was also isolated from an additional bone marrow slide prepared 8 years and 9 months before the onset of clinically diagnosed leukemia from twin 2850. Half of the contents of the slide was scraped into an eppendorf tube and treated with sodium dodecyl sulfate (SDS)-proteinase K and extracted with phenol twice. DNA was ethanol precipitated and dissolved in 20 μL of TE (10 mmol/L Tris-HCl, l pH 8.0, 1 mmol/L EDTA). DNA concentration was determined by GeneQuant (Pharmacia, St Albans, UK). Three microliters was used for subsequent PCR analysis.
Southern blot analysis of TEL rearrangement.
Long-distance inverse PCR (LDI-PCR).
With the AML1 gene still not completely sequenced, there is a need for a rapid method for cloning and sequencing of TEL-AML1fusion regions. Therefore, we have adapted an LDI-PCR method for this purpose. A similar approach has been used previously for rapid cloning of IGH gene rearrangements.27 DNA from twin 2850 was subjected to LDI-PCR analysis for genomic TEL-AML1 fusion by the methodology described by Willis et al.27 Briefly, 0.5 μg of twin 2850 DNA was digested with 5 U HindIII overnight in parallel with a normal control. Digested DNA was extracted by an equal volume of phenol followed by ether. DNA was diluted to 0.5 mL, then subjected to treatment with 5 U T4 DNA ligase overnight. The dilute nature of the DNA favors intramolecular ligation (circularization) of DNA fragments rather than intermolecular ligation. Ligated DNA was then purified in Qiagen spin columns and dissolved in 40 μL of TE. The circularized fragments were subjected LDI-PCR using primers inv3A and inv3B (Table 1, Fig 1A). Six picomoles of each primer was added to 1 μL DNA in 60 mmol/L Tris-SO4 (pH9.1), 18 mmol/L (NH4)2SO4, 1.7 mmol/L MgSO4, 200 μmol/L each deoxynucleotidyl triphosphate (dNTP), and 0.5 μL Elongase enzyme (GIBCO-BRL, Paisely, UK) in a 50-μL reaction. Tubes were cycled 35 times, 30 seconds at 94°C, 30 seconds at 66°C, and 18 minutes at 68°C. A total of 1 μL of this reaction was then subjected to a nested reaction using primers inv3A-1 and inv3B-1 using the same PCR conditions.
Primers Used in the Present Study
| Name . | Sequence 5′ to 3′ . |
|---|---|
| inv3A | ACCCTCCAGCTATAGTACTCATCAG |
| inv3B | AGGCACTGTTCTGCATTCTTGATGG |
| inv3A-1 | TTTATATTGCGGCCGCTCAGTTTGACATTCAGCAGCACCTTC |
| inv3B-1 | TTTATATTGCGGCCGCCTCAGAGAGGTATAAGACACAGTC |
| T28AMLA | TTACCTGCCTAGCAGAGAG |
| T28TELB | CATAACAGGACCCGGTGTGTGAT |
| T28TELB-1 | CCTTCCTGTGTCCATGTGTTCTCAT |
| T28TELA | CTACAAGTTGAGATCTATTGAGGTC |
| T28AMLB | CACCTCTTTTAAGCTCCGTGTGTG |
| T28AMLB-1 | TGTAGGAACATTAGAATTCAAGG |
| Name . | Sequence 5′ to 3′ . |
|---|---|
| inv3A | ACCCTCCAGCTATAGTACTCATCAG |
| inv3B | AGGCACTGTTCTGCATTCTTGATGG |
| inv3A-1 | TTTATATTGCGGCCGCTCAGTTTGACATTCAGCAGCACCTTC |
| inv3B-1 | TTTATATTGCGGCCGCCTCAGAGAGGTATAAGACACAGTC |
| T28AMLA | TTACCTGCCTAGCAGAGAG |
| T28TELB | CATAACAGGACCCGGTGTGTGAT |
| T28TELB-1 | CCTTCCTGTGTCCATGTGTTCTCAT |
| T28TELA | CTACAAGTTGAGATCTATTGAGGTC |
| T28AMLB | CACCTCTTTTAAGCTCCGTGTGTG |
| T28AMLB-1 | TGTAGGAACATTAGAATTCAAGG |
Using NotI sites present in the nested primers, PCR products from the second reaction were cloned into the NotI site of pBluescript (Stratagene, Amsterdam, The Netherlands) and sequenced with forward and reverse universal primers (automated sequencing on an ABI 373a sequencer). Additional sequencing primers were used as needed to sequence the entire HindIII fragment. On identification of the translocation breakpoint, additional primers were synthesized to amplify both the TEL-AML1 and the reciprocalAML1-TEL translocation from both twins. Primers T28TELA and T28AMLB were used for the former and T28AMLA and T28TELB for the latter (Table 1). A total of 10 ng of twin 2850 DNA and 1 μL of a 1:100 dilution (in TE buffer) of twin 244 DNA was used for PCR (20 pmol each primer, 50 mmol/L KCl, 10 mmol/L Tris-HCl, 2.5 pmol each dNTP, and 2.5 U of Taq polymerase [Perkin Elmer, Warrington, UK]), 35 cycles 94°C for 1 minute, 64°C for 1 minute, and 72°C for 1 minute followed by 10 minutes at 72°C). A heminested reaction for both translocations was then performed using the same sense primers (T28AMLA, T28TELA), but a proximal antisense primer (T28AMLB-1, T28TELB-1) for the two separate reactions. PCR products were purified by agarose gel electrophoresis and directly sequenced with the PCR primers.
RESULTS
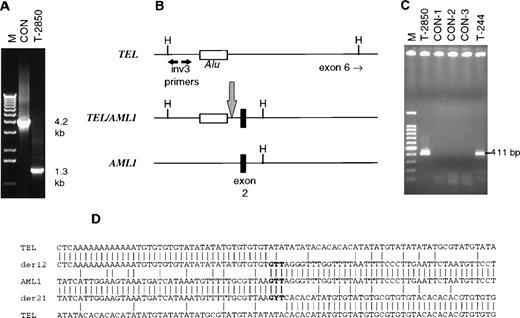
Twin 2850 was identified as having a TEL rearrangement in intron 5 by Southern blotting (data not shown). LDI-PCR was applied to various TEL intron 6 restriction fragments, and a product differing in size from the normal genomic product was identified using a HindIII digest and inv3 primers (Fig 1A). This product was cloned and sequenced, yielding TEL sequence on one side and non-TEL on the other. Using a BLAST sequence similarity search, it was discovered that the breakpoint was located just before exon 2 ofAML1 (Fig 1B). To confirm the TEL-AML1 translocation, primers T28TELA and T28AMLB were used to amplify a 488-bp amplicon in twin 2850 (data not shown). This product was sequenced and found to be identical to the LDI-PCR product. Using one of the stained slides from twin 244, we were unable to amplify this product even using the heminested reaction (T28TELA and T28AMLB-1, which yields a 419-bp product); the size of the amplicon could not be reduced due to the presence of an Alu sequence in TEL immediately preceding the breakpoint (Fig 1B). Therefore, we attempted to amplify the AML1-TEL reciprocal fusion product from twin 244 slide DNA.
Analysis of TEL/AML1 translocations in concordant twin leukemias. (A) Long-distance inverse PCR analysis of an intron 5TEL HindIII fragment of normal control (CON) and leukemic (T-2850) DNA. The expected band size is 4.2 kb, patient T-2850 exhibited a 1.3-kb band. (B) Schematic of the normal TEL andAML1 genes along with the TEL/AML1 translocation in T-2850. The inv3 primers anneal to the 5′ end of the 4.2-kbHindIII fragment as shown; the translocation brought aHindIII site of AML1 closer to the inv3 primer sites than the normal HindIII site in TEL. The location of anAlu repeat sequence in TEL is shown, as well as exon 2 of AML1. A large arrow indicates the translocation breakpoint. (C) PCR analysis of the reciprocal AML1/TEL translocation in the twins. The 411-bp heminested PCR product was amplified in both twins, but not in control DNA samples. A total of 10 ng of T-2850 and 1 μL of a 1:100 dilution of DNA isolated from the T-244 slide was amplified, along with 50 ng of control DNA samples. CON-1 is DNA from a normal healthy individual, CON-2 and CON-3 are DNA samples from other pediatric leukemia patients that also had TEL/AML1translocations by RT-PCR. (D) Sequence surrounding the translocations in the twins. Normal TEL is shown at top and bottom andAML1 in the center. TEL/AML1 is indicated by der 12 andAML1/TEL by der 21. The duplicated “GTT” at the breakpoint site is shown in bold, a Y is shown at the center nucleotide in the der 21 sequence to indicate the T → C mutation in T-244. The bottom sequence of TEL is shifted 44 nucleotides to the left to indicate a 47-bp deletion on translocation.
Analysis of TEL/AML1 translocations in concordant twin leukemias. (A) Long-distance inverse PCR analysis of an intron 5TEL HindIII fragment of normal control (CON) and leukemic (T-2850) DNA. The expected band size is 4.2 kb, patient T-2850 exhibited a 1.3-kb band. (B) Schematic of the normal TEL andAML1 genes along with the TEL/AML1 translocation in T-2850. The inv3 primers anneal to the 5′ end of the 4.2-kbHindIII fragment as shown; the translocation brought aHindIII site of AML1 closer to the inv3 primer sites than the normal HindIII site in TEL. The location of anAlu repeat sequence in TEL is shown, as well as exon 2 of AML1. A large arrow indicates the translocation breakpoint. (C) PCR analysis of the reciprocal AML1/TEL translocation in the twins. The 411-bp heminested PCR product was amplified in both twins, but not in control DNA samples. A total of 10 ng of T-2850 and 1 μL of a 1:100 dilution of DNA isolated from the T-244 slide was amplified, along with 50 ng of control DNA samples. CON-1 is DNA from a normal healthy individual, CON-2 and CON-3 are DNA samples from other pediatric leukemia patients that also had TEL/AML1translocations by RT-PCR. (D) Sequence surrounding the translocations in the twins. Normal TEL is shown at top and bottom andAML1 in the center. TEL/AML1 is indicated by der 12 andAML1/TEL by der 21. The duplicated “GTT” at the breakpoint site is shown in bold, a Y is shown at the center nucleotide in the der 21 sequence to indicate the T → C mutation in T-244. The bottom sequence of TEL is shifted 44 nucleotides to the left to indicate a 47-bp deletion on translocation.
PCR using primers T28AMLA and T28TELB, followed by a heminested reaction with T28AMLA and T28TELB-1, yielded a 411-bp product in both twin 2850 and 244, and not in normal or leukemic control DNA (Fig 1C). On sequencing these products, it was noted that there was an apparent 117-bp deletion in TEL, as well as several point mutations when compared with the database sequence of TEL (Genbank HSU61375). However, on PCR amplifying and sequencing the TEL genomic sequence of this region from DNA samples from several individuals, we found that the majority had the variant sequence also found in the twins (data not shown). This region is therefore polymorphic. When comparing both the TEL-AML1 sequence and the reciprocalAML1-TEL, there was a 47-bp loss of TEL sequence in the process of translocation. In contrast, the sequence “GTT,” a part of the germ line AML1, was duplicated and present in both translocations of twin 2850. Twin 244 had the identical sequence of theAML1-TEL as twin 2850, but interestingly, had a T → C point mutation at the center thymidine of the repeated “GTT” (Fig1D). This is unlikely to be a PCR artefact, as we have been able to amplify and sequence it in independent repeat PCR reactions. This mutation, probably occurring during clonal evolution, is also a useful indicator of lack of contamination between the twin samples.
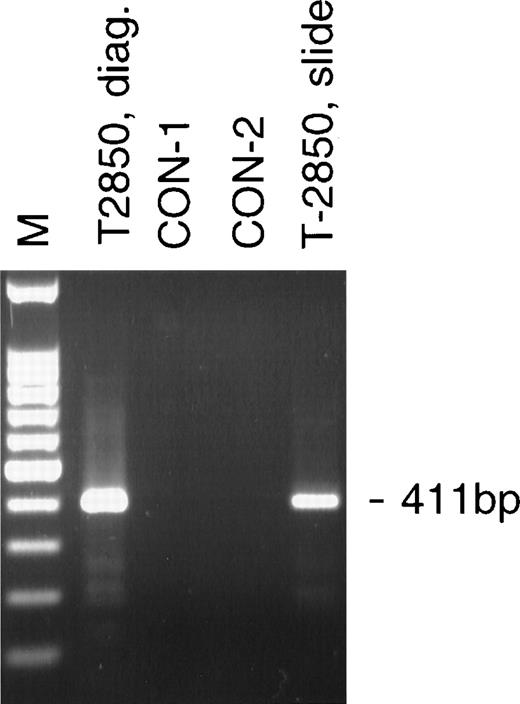
A bone marrow aspirate was taken and a stained smear prepared from twin 2850 at the same time that twin 244 was diagnosed with leukemia, ie, when the twins were 5 years old. This was considered hematologically normal. A total of 27 ng of DNA was isolated from half of this slide. The DNA was analyzed by PCR using the heminested primer set to theAML-1-TEL translocation as used on the diagnostic slide from twin 244 (Fig 1C). DNA from the bone marrow slide was positive for the translocation (Fig 2), thus demonstrating the detection of the leukemic clone more than 8 years before clinical disease. The sequence was identical to that found in twin 2850 in DNA isolated at the time of diagnosis.
Analysis of the AML-1-TEL translocation in a bone marrow smear 8 years and 9 months before diagnosis of leukemia. The 411-bp heminested AML-1-TEL PCR product was amplified from 10 ng of diagnostic DNA from T-2850 (T-2850, diag.) and 4 ng of DNA isolated from a stored bone marrow smear prepared 8 and a half years before diagnosis (T-2850, slide). Lanes containing 50 ng of DNA from normal controls (CON-1, CON-2) were negative.
Analysis of the AML-1-TEL translocation in a bone marrow smear 8 years and 9 months before diagnosis of leukemia. The 411-bp heminested AML-1-TEL PCR product was amplified from 10 ng of diagnostic DNA from T-2850 (T-2850, diag.) and 4 ng of DNA isolated from a stored bone marrow smear prepared 8 and a half years before diagnosis (T-2850, slide). Lanes containing 50 ng of DNA from normal controls (CON-1, CON-2) were negative.
DISCUSSION
TEL-AML1 translocations are characterized at the genomic level by breaks in a restricted region of TEL (intron 5) and in a very large, and currently unsized, region of AML1 (intron 1 or 2). We took advantage of this by cloning the translocation breakpoint using inverse primers from the TEL side. Inverse PCR has been used previously for identifying sequences flanking transposon insertion sites,28 exon/intron border sequences, and for identification of IGHJ fusion partners in leukemia/lymphoma.27 We will report a more complete description of the technique as applied to the cloning ofTEL-AML1 translocations elsewhere (JW and MG, submitted).
The TEL-AML1 gene fusion, in common with other hybrid recombinations in leukemia, involves intronic DNA breaks that are clustered, but diverse.29,30 As a consequence, each patient and each leukemic clone has a unique or clonotypic genomic fusion sequence. TEL-AML1 and other gene fusions are acquired, nonconstitutive abnormalities and so the finding that leukemic cells from identical twins share the same clonotypic sequence is most plausibly interpreted as reflecting a single cell origin of the double leukemia and a shared clone. As in previous molecular studies with leukemic cells in identical twins, there is only one likely basis for a shared clone and that is with a fetal origin in one twin followed by prenatal intraplacental metastasis of clonal progeny from that twin to the other. A common leukemic clone identified by unique genotype has now been recorded for five infant twin pairs with ALL (four pairs) or AML (one pair) and MLL gene fusions,9-11 a twin pair of children with T-ALL/T-non-Hodgkin’s lymphoma (NHL),22 and one previous pair of twins with ALL and a TEL-AML1 fusion.21 The present data confirm in an additional twin pair that the TEL-AML1 can originate in utero. These data further reinforce the point thatTEL-AML1 fusion can be a very early molecular event and quite possibly represents the first or initiating mutation in B-cell precursor ALL. Moreover, because there is no reason to suppose that this can only occur in the context of a twin pregnancy, we conclude that this common molecular abnormality must also arise prenatally in at least some nontwin children with ALL.
The concordance rate for ALL in noninfant twins is not known, but we calculate from our twin survey in the UK over the past 10 years that it may be around 5% or 1 in 20 (unpublished observations, December 1998). Ninety-five percent discordance therefore requires an explanation and there are two different possibilities to be considered: first, that 95% of ALL in children, twin and nontwin, are initiated postnatally; second, that ALL in children is usually initiated prenatally, but some secondary postnatal event is required for which twins are more likely than not to be discordant. Retrospective scrutiny of neonatal blood spots (Guthrie cards) of cALL patients12 may resolve this issue.
The present twin pair provides new and unexpected insight into the time frames that can be required for critical sequential events in the common subtype of childhood ALL. The data clearly suggest that after an in utero initiation, the subsequent latency of the clinical development of leukemia can be very protracted, 14 years in the case of one of these twins. Moreover, in the latter case, we were able to retrospectively identify the presence of the clonotypicTEL-AML1 genomic fusion sequence in a hematologically normal bone marrow smear made 9 years before the subsequent diagnosis of ALL in this twin. This result suggests that the presumptive preleukemic clone may have been disseminated in the marrow. At the same time, this clone was clearly restrained in its net growth advantage and pathological impact for almost a decade. These data therefore provide some support for the suggestion13 that the natural history of pediatric ALL may parallel balanced chimerism in chronic myeloid leukemia (CML) evolving eventually to an acute leukemic blast crisis. Unfortunately, we were not able to quantify the number ofTEL-AML1 positive cells in the bone marrow at this time and cannot comment therefore on how widespread the dissemination of the preleukemic clone may have been or what degree of clonal advantage may have been accrued (over 5 years) from the initialTEL-AML1 fusion event. As we required two rounds (nested) of PCR to see a signal (compared with a single round for a control gene, GSTmu), we assume that only a small number of cells were present. With what little material remained (one half of a stained smear), we attempted to enumerate cells with the TEL-AML1fusion gene by two-color fluorescent in situ hybridization (FISH), but unfortunately, the aged stained smears do not provide interpretable signals with the TEL and AML1probes available (ie, compared with fresh material) (C. Harrison, J.L.W., and M.G., unpublished observations, August 1998).
Although documentation of a 14-year period of latency in childhood ALL is unprecedented, we have reported another twin pair with monoclonal T-cell malignancy diagnosed at age 9 and 11 years.22 Adult epithelial cancers are generally recognized as having an evolutionary time frame of decades, and this is deemed to reflect the time scale required for the accumulation of a complementary set of mutations and any associated exposures that facilitate these events. Leukemias, not having to escape the confines and restraints of fixed tissue architecture, may require fewer genetic events and therefore could evolve to malignancy over a shorter time frame. For children who develop acute leukemia as a consequence of a known genotoxic exposure, for example, with cancer therapy (chemotherapy or irradiation)31 or after the atomic bombs in Japan,18,32 the period of excess risk, reflecting variable latency, is mostly in the range of 1 to 10 years; the maximum risk and average latent period varying with type, pattern, and level of exposure (overall average ≈5 years). A 14-year postnatal latency for childhood ALL is therefore very protracted, but not incompatible with prior data. It does, however, raise the possibility hitherto not seriously considered that, even for older children with leukemia, the disease may have a prenatal origin. The great majority of ALL cases withTEL-AML1 fusion are between 2 and 10 years of age; only a few cases are recorded between 10 and 15 years of age33 or in adult ALL.34 This indicates that if, as we suggest,TEL-AML1 may be a common prenatal initiating event, then a 14-year latency, as in the twin case here, is unusual and represents the tail end of an age-associated risk.
The other twin in the current pair was diagnosed aged 5 years 2 months, 8 years 9 months before her twin sister. Such a diversity of age at diagnosis is unusual in concordant twin leukemias in children. Simultaneous diagnosis is the norm for infant twins with acute leukemia,9 although the second twin may have a diagnosis derived from hematologic and molecular evidence in the absence of clinical symptoms.35 We have now studied a total of 11 pairs of noninfant twin children with cALL, although not all have been molecularly characterized. Their average age at diagnosis is no different from that of nontwin children with cALL (≈3 to 4 years) and the average difference in the timing of onset of clinical symptoms is around 18 months (unpublished observations, December 1998). The striking difference in the postnatal latency period in the present twin pair most probably reflects that necessary secondary events required for the development of overt leukemia within the clone of preleukemic cells spawned prenatally were independently acquired at very different times. This situation could arise if such events occur entirely by chance or if they require promotion by particular patterns of exposure, such as infection, that can occur intermittently.8 36 Ongoing epidemiological case/control studies may shed some light on these possibilities.
ACKNOWLEDGMENT
We thank Dr T.G. Willis for discussions of the LDI-PCR method and Barbara Deverson for help in preparation of the manuscript.
Supported by the Leukaemia Research Fund.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Professor Mel Greaves, PhD, Leukaemia Research Fund Centre, Institute of Cancer Research, Chester Beatty Laboratories, 237 Fulham Rd, London SW3 6JB, UK; e-mail:m.greaves@icr.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal