To the Editor:
In their recent report, Gu et al1 have shown the expression of laminin-2, laminin-8, and laminin-10 in mouse and human bone marrow, which contain the common laminin β1 chain. The investigators, however, failed to detect the laminin β2 chain in rat bone marrow by immunoblotting and immunofluorescence using the monoclonal antibody D5.
It is generally accepted that the laminin β2 chain, originally described as a specific marker of neuromuscular junctions (s-laminin), has a wide tissue distribution including vascular and neuronal basal laminae.2 Moreover, the laminin β2 chain is frequently observed in embryonal basement membranes and there is a developmental switch with a loss of β2 chain containing laminin isoforms in adult terminally differentiated tissues. A reexpression of the laminin β2 chain was demonstrated in lesions associated with proliferation and dedifferentiation of adult tissues as well as in cell culture.3-5 We hypothesized that proliferation and differentiation of adult human hematopoietic cells in vivo and in vitro might also be associated with expression of the laminin β2 chain.
Therefore, human bone marrow aspirates (air-dried slides) as well as mononuclear cells from healthy donors (n = 5) were used to evaluate the laminin β2 chain expression. Mononuclear cells (2 × 105 nucleated cells on each spot) were attached to poly-L-lysine coated adhesions slides (Marienfeld, Bad Mergentheim, Germany) to evaluate matrix protein expression of hematopoietic cells. In addition, we used cytokine supported liquid cultures (stem cell factor [SCF], 10 ng/mL, Genzyme, Rüsselsheim, Germany; interleukin-3 [IL-3], 100 ng/mL, Novartis, Nürnberg, Germany; and thrombopoietin [TPO], 100 ng/mL, Genzyme) of purified CD34+cells6 7 as well as human long-term bone marrow cultures (LTBMC) as models for studying human hematopoiesis. Positively selected peripheral blood CD34+ cells (n = 5) were cultured up to 12 days in serum-free culture medium and mononuclear cells isolated from normal bone marrow (n = 5) were cultured up to 28 days in LTBMC medium (StemCell Technologies Inc, Vancouver, Canada). Lab-Tek tissue culture chamber slides (Nunc TC, Wiesbaden,Germany) were used to analyze the expression of laminin β2 chain by means of immunocytology (APAAP; Dako, Glostrup, Denmark; MoAb C4, dilution 1:1,000 [DSHB, Baltimore, MD]).
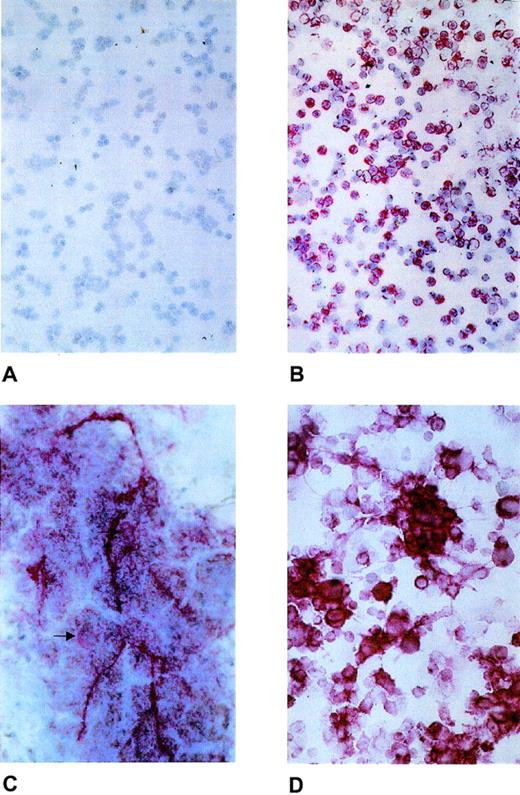
As shown in Fig 1, laminin β2 chain was visualized as pericellular staining of bone marrow cells (Fig 1B), as intracellular staining of megakaryocytes, and also as extracellular deposition (Fig 1C). The majority of the round cells and cell clusters of the ex vivo expanded cell population also showed laminin β2 chain expression (Fig 1D), which was further confirmed by LTBMC (data not shown). In addition, laminin β2 chain mRNA expression was investigated by reverse transcriptase-polymerase chain reaction (RT-PCR) according to Church and Aplin8 in positively selected peripheral blood CD34+ cells (Fig2).
Expression of laminin β2 chain in normal human bone marrow and ex vivo–expanded CD34+ peripheral blood progenitor cells. (A) Negative control with nonimmune mouse serum. (B) Laminin β2 chain expression as pericellular staining of bone marrow mononuclear cells. (C) Laminin β2 chain expression of normal megakaryocytes (arrow) and as extracellular deposition. (D) Laminin β2 chain expression in hematopoietic cells generated ex vivo from purified CD34+ peripheral blood progenitor cells.
Expression of laminin β2 chain in normal human bone marrow and ex vivo–expanded CD34+ peripheral blood progenitor cells. (A) Negative control with nonimmune mouse serum. (B) Laminin β2 chain expression as pericellular staining of bone marrow mononuclear cells. (C) Laminin β2 chain expression of normal megakaryocytes (arrow) and as extracellular deposition. (D) Laminin β2 chain expression in hematopoietic cells generated ex vivo from purified CD34+ peripheral blood progenitor cells.
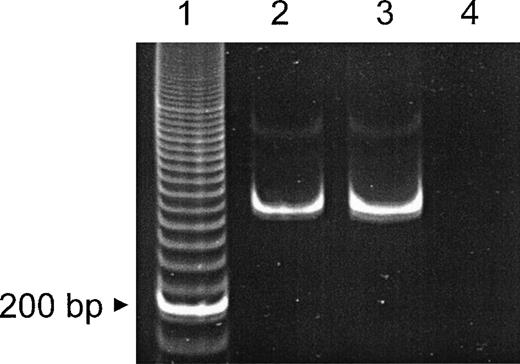
Detection of β2 laminin mRNA by RT-PCR. mRNA was obtained from MACS-purified normal CD34+ peripheral blood progenitor cells and an oral squamous cell carcinoma cell line (positive control). RT-PCR (40 cycles) of the laminin β2 chain subunit mRNA resulted in a 315-bp fragment. Lane 1, 20-bp ladder; lane 2, CD34+ cells; lane 3, oral squamous cell carcionoma cell line; lane 4, control (primers only).
Detection of β2 laminin mRNA by RT-PCR. mRNA was obtained from MACS-purified normal CD34+ peripheral blood progenitor cells and an oral squamous cell carcinoma cell line (positive control). RT-PCR (40 cycles) of the laminin β2 chain subunit mRNA resulted in a 315-bp fragment. Lane 1, 20-bp ladder; lane 2, CD34+ cells; lane 3, oral squamous cell carcionoma cell line; lane 4, control (primers only).
In summary, in contrast to the studies by Gu et al in rat bone marrow, we could clearly demonstrate β2 chain laminin expression in normal human bone marrow cells including CD34+ cells during cytokine-mediated ex vivo expansion of CD34+ peripheral blood progenitor cells as well as in normal human LTBMC. Therefore, β2 chain laminin can be considered as a structural growth factor in normal human hematopoiesis. The discrepancy to the findings of Gu et al should be interpreted as a further example that in addition to differentiation and maturation, species-dependent differences in laminin chain expression also may occur.9

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal