Neutrophil accumulation at sites of inflammation is mediated by specific groups of cell adhesion molecules including the β2 (CD18) integrins on leukocytes and the selectins (P- and E-selectin on the endothelium and L-selectin on the leukocyte). This is supported by studies of patients with leukocyte adhesion deficiency syndromes whose leukocytes are genetically deficient in the expression of β2 integrins or selectin carbohydrate ligands (eg, sialyl-Lewisx). However, inherited deficiency or dysfunction of endothelial cell adhesion molecules involved in leukocyte recruitment has not been previously described. In this report we describe a child with recurrent infections and clinical evidence of impaired pus formation reminiscent of a leukocyte adhesion deficiency syndrome, but whose neutrophils were functionally normal and expressed normal levels of CD18, L-selectin, and sialyl-Lewisx. In contrast, immunohistochemical staining of inflamed tissue from the patient showed the absence of E-selectin from the endothelium, although E-selectin mRNA was present. However, E-selectin protein was expressed as significantly elevated levels of circulating soluble E-selectin were detected, the molecular size of which was consistent with a proteolytically cleaved form of E-selectin. Gene sequencing failed to show evidence of a secreted mutant variant. These data represent, to our knowledge, the first description of a potentially inherited dysfunction of an endothelial cell adhesion molecule involved in leukocyte recruitment and provide additional human evidence of the importance of endothelial selectins in the inflammatory response.
THE RECRUITMENT of neutrophils out of the circulation, and across the vascular endothelium into extravascular sites of infection or injury, involves a cascade of events mediated by soluble inflammatory mediators and specific groups of cell adhesion proteins.1-5 The process begins with the leukocyte rolling along the surface of the endothelium, a step that has been classically described as mediated by the selectins (L-selectin on the neutrophil and P- and E-selectin on the endothelium) and their carbohydrate-rich counterligands. As the neutrophil rolls, L-selectin is shed and its β2 (CD18) integrins are activated by at least one of a variety of chemokines and chemoattractants that associate with the endothelial surface.6 Leukocyte activation results in arrest of rolling and firm adhesion of the leukocyte to the endothelium, events that result from the binding of the β2 integrins expressed on the leukocytes to the immunoglobulin (Ig) superfamily members, ICAM-1 and ICAM-2, expressed on the endothelium. Once the leukocyte has adhered to the endothelium, it migrates over the luminal surface, locates an endothelial intercellular junction and then “squeezes” between endothelial cells to enter the extravascular space. Although not well defined, there is evidence to suggest that the β2 integrins, ICAM-1, and PECAM-1 participate in this final step of transendothelial migration.1
Evidence supporting this paradigm has come from investigations of patients with leukocyte adhesion deficiency (LAD) syndromes who are genetically deficient in the expression of β2 integrins (LAD type 1)7 or selectin carbohydrate ligands such as sialyl-Lewisx (LAD type 2)8,9 on the surface of their leukocytes. Affected patients suffer from severe recurrent bacterial infections, typically without pus formation, despite markedly elevated circulating neutrophil counts. Although these patients are phenotypically similar, in vivo studies of chemoattractant-induced neutrophil recruitment have shown that the neutrophils from the 2 groups are functionally different. Specifically, neutrophils from patients suffering from LAD type 1 showed normal rolling behavior but failed to adhere or emigrate across vascular endothelium in response to chemotatic stimulation under flow or static conditions.7 In contrast, neutrophils from patients with LAD type 2 rolled poorly and failed to stick to the vasculature during flow, but were observed to adhere or emigrate under static conditions.8,9 Recently, a novel syndrome designated LAD-1/variant (or LAD-1b) has been described with clinical features consistent with classic LAD type 1 but with normal β2 integrin levels. In the patient reported, leukocyte activation did not trigger the conformational changes required for converting β2 integrins to an activated high-avidity ligand-binding state.10
Although deficiencies of leukocyte adhesion proteins involved leukocyte emigration have been described as outlined above, to date inherited deficiency or dysfunction of endothelial cell adhesion molecules involved in leukocyte recruitment have not been previously reported. In this report, we describe a patient with recurrent infections and clinical evidence of impaired pus formation suggestive of a LAD syndrome but whose neutrophils were functionally normal and expressed normal levels of CD18, L-selectin, and sialyl-Lewisx(sLex). However, analysis of inflamed tissue from this patient showed markedly decreased endothelial surface expression of E-selectin in conjunction with increased levels of circulating soluble E-selectin. Based on these data, we speculate that in this patient there may be upregulation of proteolytic activity at the surface of the vascular endothelium that results in markedly accelerated release of E-selectin from the endothelial surface. This represents, to our knowledge, the first description of a potentially inherited dysfunction of an endothelial cell adhesion molecule involved in leukocyte recruitment and provides further human evidence of the role of endothelial selectins in the accumulation of leukocytes at inflammatory sites.
MATERIALS AND METHODS
Patient history.
The patient presented to us at 10 years of age after a long history of bacterial infections. She developed omphalitis withPseudomonas species at 5 weeks of age. In the first year of life, she additionally had mastoiditis and adenitis both requiring surgical drainage, as well as otitis media multiple times. Between 2 and 5 years of age she was intermittently on prophylactic antibiotics. In that period she had cervical adenitis twice and an uncomplicated varicella infection. Between the ages of 5 and 10 years, she was on constant antibiotic prophylaxis. During that time she developed septic arthritis twice, multiple episodes of impetigo, periorbital cellulitis,Pseudomonas cellulitis, and sepsis twice (Haemophilus influenza and Escherichia coli, respectively).
The patient’s family history is remarkable only for a previous sibling died at 32 weeks of gestation of a staphylococcal infection of the fetus, amniotic fluid, and placenta. The patient also has 2 half-sisters who are completely well. There is no history of recurrent infections in either parent or more distant relatives.
At 10 years of age, she developed a rapidly progressive gangrene withClostridium septicum involving her right lower extremity. After surgical debridement, she was left with an infected, nonhealing ulcer that failed to respond to prolonged intravenous antibiotics and a muscle flap procedure. Biopsy of the ulcer showed very modest numbers of neutrophils in small nests of cells. Eventually, a below-the-knee amputation of her right leg was performed. She has had a mild neutropenia (absolute neutrophil count [ANC], 400 to 600 cells/μL) since birth, with appropriate increases in response to infection. Cyclic neutropenia was not shown. Following her amputation, she was treated with granulocyte colony-stimulating factor to maintain an ANC of approximately 7,000 cells/μL and trimethoprim-sulfamethoxazole prophylaxis. Despite these interventions, in the 2 years since her amputation she has continued to have multiple bouts of infection including episodes of cellulitis (4 times), pyelonephritis (3 times), and dental abscesses (2 times).
Although she is capable of generating pus and inflammatory infiltrates, clinically the magnitude is much less than what is typically seen, particularly in the early stages of an infection. For example, at 11 years of age she developed a Klebsiella pyelonephritis with flank pain, fever and greater than 200,000 colonies of pure K pneunomiae, and no white blood cells on the initial urinalysis. However, repeat urinalysis 20 hours later subsequently showed a modest number of white blood cells with decreasing bacterial counts. Furthermore, her skin and dental abscesses have always been associated with fever and pain, but typically only very modest swelling and/or fluctuance have been observed with these processes.
An immunologic evaluation11 12 was performed during a period of good health when she was 10 years old (Table1). Standard immunologic evaluations of neutrophil function were normal as were standard laboratory evaluations of antibody production and function. T-cell function was intact with decreased proliferative responses to diphtheria and tetanus that were not considered to be clinically significant. CD4 counts were mildly reduced. Neutrophil chemotaxis was performed according to standard protocols using 2.5 × 107 neutrophils per assay. A control was run simultaneously and migration toward 10−5mol/L f-met-leu-Phe was measured. Random migration toward buffer was subtracted. Neutrophil killing was tested by incubating 3 × 106 neutrophils/mL with log-phase growth S aureus. Aliquots were removed at 0, 30, 60, 90, 120 minutes and plated onto blood agar plates according to standard protocols. Colony counts were generated the next day. Results within 50% to 150% of the normal control are considered normal. Proliferative responses to phytohemagglutin, pokeweed mitogen, and Con A were measured in triplicate cultures of 3 dilutions of stimulus obtained 72 hours after stimulation. Responses to recall antigens (diphtheria, candida, and tetanus) were also measured in triplicate cultures with 2 dilutions of each stimulus. These cultures were obtained at 7 days. Lymphocyte subset analyses and proliferative responses were determined in the Clinical Immunology Laboratory at The Children’s Hospital of Philadelphia. A bone marrow biopsy performed during a period of health showed normal cellularity for her age. The myeloid to erythroid ratio was 2:1. All lineages were present. HIV serological evaluations were negative. Informed consent was obtained for all studies described.
Summary of Immunological Evaluation
| Assay . | Patient Result . | Control Result or Normal Range . |
|---|---|---|
| Dichloroflourescein | 93.8% stimulation | 95.7% stimulation |
| MFI 1.7 | MFI 1.19 | |
| Neutrophil Chemotaxis | 4.83 mm | 4.17 mm |
| Neutrophil S aureus killing assay | 85% killing at 2 hours | 80% killing at 2 hours |
| IgG | 1,310 mg/dL | 726-1,085 mg/dL |
| IgA | 165 mg/dL | 70-229 mg/dL |
| IgM | 155 mg/dL | 35-72 mg/dL |
| Phytohemagglutin stimulation | 61,392 cpm | 75,797 cpm |
| Pokeweed mitogen stimulation | 25,015 cpm | 43,455 cpm |
| Concanavalin A stimulation | 34,818 cpm | 51,830 cpm |
| Diphtheria stimulation | 1,198 cpm | 21,890 cpm |
| Candida stimulation | 10,043 cpm | 7,938 cpm |
| Tetanus stimulation | 749 cpm | 20,738 cpm |
| CD4 | 645 cells/μL | 700-1,100 cells/μL |
| CD8 | 879 cells/μL | 600-900 cells/μL |
| CD3 | 1,698 cells/μL | 1,400-2,000 cells/μL |
| CD19 | 337 cells/μL | 300-500 cells/μL |
| CD16/CD56 | 71 cells/μL | 200-300 cells/μL |
| Assay . | Patient Result . | Control Result or Normal Range . |
|---|---|---|
| Dichloroflourescein | 93.8% stimulation | 95.7% stimulation |
| MFI 1.7 | MFI 1.19 | |
| Neutrophil Chemotaxis | 4.83 mm | 4.17 mm |
| Neutrophil S aureus killing assay | 85% killing at 2 hours | 80% killing at 2 hours |
| IgG | 1,310 mg/dL | 726-1,085 mg/dL |
| IgA | 165 mg/dL | 70-229 mg/dL |
| IgM | 155 mg/dL | 35-72 mg/dL |
| Phytohemagglutin stimulation | 61,392 cpm | 75,797 cpm |
| Pokeweed mitogen stimulation | 25,015 cpm | 43,455 cpm |
| Concanavalin A stimulation | 34,818 cpm | 51,830 cpm |
| Diphtheria stimulation | 1,198 cpm | 21,890 cpm |
| Candida stimulation | 10,043 cpm | 7,938 cpm |
| Tetanus stimulation | 749 cpm | 20,738 cpm |
| CD4 | 645 cells/μL | 700-1,100 cells/μL |
| CD8 | 879 cells/μL | 600-900 cells/μL |
| CD3 | 1,698 cells/μL | 1,400-2,000 cells/μL |
| CD19 | 337 cells/μL | 300-500 cells/μL |
| CD16/CD56 | 71 cells/μL | 200-300 cells/μL |
Abbreviation: MFI, mean fluorescence intensity.
Neutrophil isolation and labeling.
Human neutrophils (PMNs) were isolated from EDTA anticoagulated blood by density gradient centrifugation using MonoPoly Resolving Medium (Flow/ICN; Biomedical, Aurora, OH).13 Isolated PMNs were washed once with Hanks’ balanced salt solution (HBSS) without calcium and magnesium (JRH Biosciences, Lanexa, KS) and resuspended in buffer as indicated.
Binding of labeled antibodies.
PMNs were resuspended in HBSS with calcium to a concentration of 1 × 107 cell/mL and transferred to a 96-well plate (0.1 mL/well). PMNs were primed with buffer or 2 ng/mL tumor necrosis factor-α (TNF-α; Genzyme, Cambridge, MA) for 45 minutes at 37°C and then activated for 30 minutes at 37°C with either buffer or FMLP (Sigma, St Louis, MO) at 10 μmol/L. PMNs were washed once with 200 μL HBSS with calcium and incubated with 50 μL of 5 μg/mL of125I-labeled anti–L-selectin (Immunotech, Westbrook, ME), anti-CD11b (AMAC, Westbrook, ME), anti-CD18 (clone CLB54; Centocor, Malvern, PA) or anti-PECAM-1 (Centocor) for 30 minutes at room temperature. PMNs were washed twice with 100 μL HBSS with calcium, solubilized with 100 μL 1N NaOH and counted in a gamma counter.
Fluorescence-activated cell sorting (FACS) analysis.
Human neutrophils were treated with mouse anti-human sLex(clone 2H5; PharMingen, San Diego, CA) and mouse anti-human PSGL-1 (clone 3E2.25.5; Immunotech, Westbrook, ME) for 1 hour at 4°C. The primary antibody was then removed, the cells washed with phosphate-buffered saline (PBS) and a 1:200 dilution of fluorescein isothiocyanate–labeled goat anti-mouse (Cappell Laboratories, West Chester, PA) was added for 30 minutes at 4°C. After washing in PBS, flow cytometry was performed using an Ortho Cytofluorograph 50H cell sorter equipped with a 2150 data handling system (Ortho Instruments, Westwood, MA).
Neutrophil adhesion to selectin IgG coated plates.
P-selectin–IgG and E-selectin–IgG fusion proteins (10 μg/mL in HBSS), produced as previously described,14 were captured on anti-human IgG Fc specific (Jackson Immuno Research, West Grove, PA) coated 96-wells. Plates were blocked with 5 mg/mL human serum albumin in HBSS before use. PMNs were fluorescently labeled with BCECF (Molecular Probes, Eugene, OH) as previously described12 and diluted to 2 × 106 cells/mL in HBSS with calcium. PMNs (100 μL per well) were added to the selectin fusion protein coated plates and incubated for 30 minutes at room temperature. Unbound cells were removed by two 150 μL washes with HBSS. Specificity of this assay has been previously shown with selectin-specific blocking antibodies.14
Neutrophil endothelial adhesion.
Human umbilical vein endothelial cells (HUVEC) (Cell Systems, Kirkland, WA), passage 4 were grown to confluence in 96-well tissue culture plates. Where indicated, cells were stimulated with 2 ng/mL TNF-α in HUVEC media. PMNs were isolated and resuspended in 5 mL HBSS without calcium and radioactively labeled with 0.5 mCi 111Indium (DuPont/NEN, Boston, MA) in the presence of 0.4 mmol/L tropolone (Sigma) for 15 minutes at 37°C. PMNs were washed twice with HBSS without calcium and resuspended to 2 × 106 cells/mL in HBSS with calcium. PMNs (100 μL per well) were added to HUVEC plates and phorbol myristate acetate (PMA) (Sigma) at 1 μg/mL final or fMLP 10 μmol/L final were added if indicated. Plates were incubated 37°C for 30 minutes. Unbound PMNs were removed with 2 × 150 μL washes with HBSS. Cells were solubilized with 100 μL 1N NaOH and counted in a gamma counter. The number of PMNs bound was calculated using the specific activity of the cells.
Neutrophil transendothelial migration.
HUVEC at passage 4 were seeded onto 24-well fibronectin-coated 3.0 μm Transwell inserts (Becton Dickinson, Bedford, MA) and grown to confluence. HUVECs were stimulated on the basolateral side with 2 ng/mL TNF-α for 4 hours. The inserts and underlying wells were rinsed to remove TNF-α and fresh media was added to the top and bottom chambers. 111Indium-labeled PMNs (see above) were resuspended to 1 × 107 cells/mL and 100 μL added to the top chamber of the transwell. Plates were incubated for 90 minutes at 37°C and PMNs that had traversed into the bottom chamber were counted in a gamma counter. The number of PMNs that had transmigrated was calculated using the specific activity of the cells.
Neutrophil chemotaxis.
The chemotaxis assay was performed similarly to the transmigration assay, but the HUVEC monolayer was not activated with TNF-α. PMNs were added to the top of the HUVEC transwells and fMLP (10 μmol/L) was added to the bottom chambers. PMNs responding to the chemotactic stimulus and migrating to the lower chamber were quantified as described.
Short-term skin organ culture.
Skin organ culture was adapted from previously described methods.15 Patient skin acquired through punch biopsy and neonatal foreskin from elective circumcisions were immediately placed in RPMI 1640 medium supplemented with 10% fetal calf serum, 1% penicillin-streptomycin and 10 mmol/L L-glutamine. Skin specimens were cut into smaller pieces (2 × 2 mm in size), placed in 6-well culture plates, partially submerged in 0.5 mL of media with dermal side down and epidermis uncovered and incubated overnight for 18 to 24 hours at 37°C in 5% CO2-humidified air. This overnight incubation allowed for resolution of any changes in endothelial cell adhesion molecule expression because of the physical trauma of obtaining and preparing the skin samples. On the following day the original media was replaced with fresh media without or with TNF-α (12,000 U/mL; Boehringer Mannheim, Indianapolis, IN) and incubated for 8 hours at 37°C in 5% CO2-humidified air. In separate experiments these were determined to be sufficient induce ICAM-1 and E-selectin expression in adult and neonatal control skin. After this incubation the skin explants were snap frozen in O.C.T. embedding compound (Tissue-Tek/Sakura, Torrance, CA) and processed for immunohistochemical staining.
Immunohistochemical staining.
Surgically removed tissue and explants from skin organ cultures were snap frozen in O.C.T. compound and subjected to immunohistochemical staining with light counterstaining with hematoxylin as previously described.16 Antibodies used17 were 1) anti-human PECAM-1 (Immunotech, Westbrook, ME); 2) anti–E-selectin antibodies and P-selectin antibodies (Becton Dickinson Advanced Cellular Biology, San Jose, CA); 3) anti–E-selectin antibodies HEL 3/2 (provided by Drs Dale Cummings and Tim Ahern of Genetics Institute, Cambridge, MA) and ES1 (provided by Rodger McEver, University of Oklahoma, Oklahoma City, OK); and 4) anti–ICAM-1 and anti–Mac-1 (PharMingen). Antibody concentrations used for staining ranged from 1 to 10 μg/mL.
Reverse transcription-polymerase chain reaction (RT-PCR), single-strand conformation polymorphism (SSCP) analysis, and gene sequencing.
Total RNA was extracted from surgical skin tissue,18incubated at 65°C for 10 minutes and then used to synthesize cDNA. cDNA was obtained by RT using an oligo d(T)16, M-MLV Reverse Transcriptase (Perkin-Elmer-Cetus, Norwalk, CT). To amplify the lectin-binding and epidermal growth factor–like domains,19reverse transcribed cDNA was subjected to PCR using a sense primer (5′-CACAGATCTGGTCTTACAACACCTCCA-3′) and an antisense primer (5′-TTTACACGTTGGCTTCTCGTT-3′). For the SSCP analysis, cDNA from surgical tissue was amplified using overlapping primers (available on request) to generate PCR products of approximately 400 bp in the presence of 35S. These products were run on a nondenaturing polyacrylamide gel to screen for mutations.20 21 Two coding region polymorphisms/mutations were identified using this strategy. Based on this, the patient’s E-selectin gene was fully sequenced in both directions from PCR products specific for each exon amplified from genomic template (available on request). Mutations were confirmed using separate DNA preparations from the patient and her parents. Amplification protocols for each primer pair are available on request.
Measurement of soluble E-selectin.
The concentration of soluble E-selectin present in patient and control serum was determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available assay kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions.
Immunoblot analysis.
The molecular species of soluble E-selectin present in patient and control serum was determined by immunoblot analysis with the anti–E-selectin monoclonal antibody, 1.2B6 (Immunotech) using previously described procedures.22
RESULTS
Expression of leukocyte cell adhesion molecules.
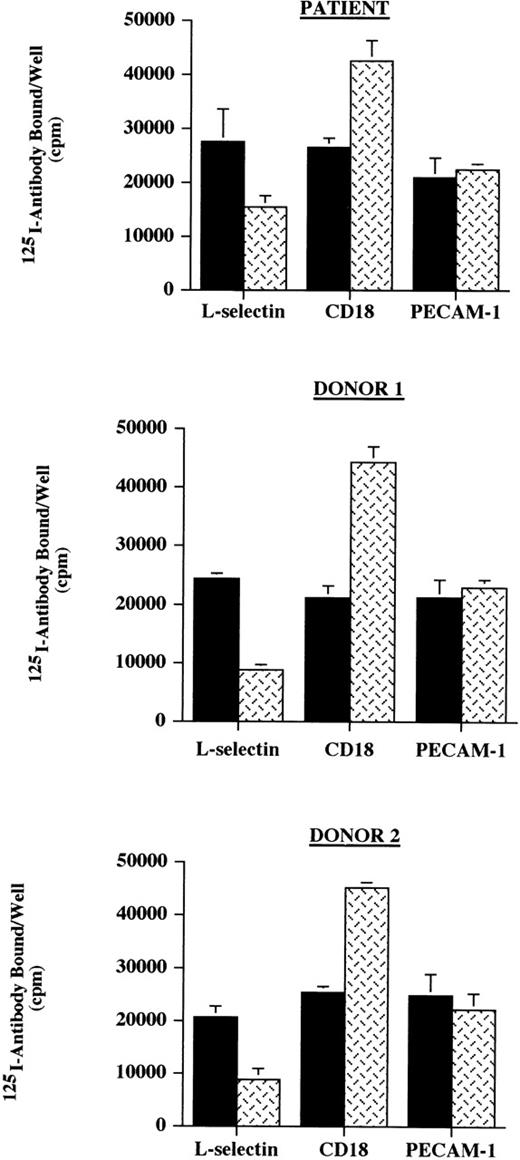
Because the clinical features of this patient were suggestive of a leukocyte adhesion deficiency, the surface expression of cell adhesion molecules on neutrophils known to be involved in leukocyte emigration was investigated. The expression L-selectin, CD18, and PECAM-1 on the patient’s neutrophils was initially assessed and was noted to be comparable with the expression found on neutrophils from normal control donors (Fig 1). Stimulation with inflammatory mediators (TNF-α and fMLP) decreased the expression of L-selectin and increased the surface level of CD18 as has been previously reported,23 suggesting that for neutrophils, these proteins are regulated normally in this patient.
Neutrophil binding of 125I-labeled antibodies. Neutrophils (1 × 106) from the patient and 2 normal donors, unactivated (control, ▪) or activated with the combination of TNF- and fMLP (TNF/fMLP, ), were incubated with125I-labeled antibodies against L-selectin, CD18, and PECAM-1 in a 96-well plate. The expression of these cell adhesion molecules was assessed by the binding of labeled antibody as determined by the total counts per well of cells. The expression of L-selectin, CD18, and PECAM-1 on the patient’s neutrophils were comparable with the expression found on neutrophils from normal control donors and stimulation with TNF- and fMLP decreased the expression of L-selectin and increased the surface level of CD18 as has been previously reported. These data are representative of 3 experiments done in triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Neutrophil binding of 125I-labeled antibodies. Neutrophils (1 × 106) from the patient and 2 normal donors, unactivated (control, ▪) or activated with the combination of TNF- and fMLP (TNF/fMLP, ), were incubated with125I-labeled antibodies against L-selectin, CD18, and PECAM-1 in a 96-well plate. The expression of these cell adhesion molecules was assessed by the binding of labeled antibody as determined by the total counts per well of cells. The expression of L-selectin, CD18, and PECAM-1 on the patient’s neutrophils were comparable with the expression found on neutrophils from normal control donors and stimulation with TNF- and fMLP decreased the expression of L-selectin and increased the surface level of CD18 as has been previously reported. These data are representative of 3 experiments done in triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Normal levels of sLex, the carbohydrate binding motif present on selectin ligands, and P-selectin glycoprotein ligand 1 (PSGL-1) were detected on neutrophils from the patient (Fig 2). To evaluate further the neutrophil ligands for endothelial selectins, neutrophils from the patient and normal donors were allowed to adhere to immobilized P- and E-selectin IgG chimeric proteins. No significant differences in neutrophil adhesion to immobilized endothelial selectin was noted between the patient and normal donors (Fig3), suggesting that the neutrophil ligands for P- and E-selectin are present and functional in the patient.
Neutrophil expression of sLex and PSGL-1. The surface expression of sLex (A and C) and PSGL-1 (B and D) on neutrophils from a normal donor (A and B) and the patient (C and D) was determined by FACS analysis. Filled and unfilled tracings represent, respectively, the background staining and staining for the targeted protein. For the normal donor and the patient comparable levels of expression of sLex and PSGL-1 were noted.
Neutrophil expression of sLex and PSGL-1. The surface expression of sLex (A and C) and PSGL-1 (B and D) on neutrophils from a normal donor (A and B) and the patient (C and D) was determined by FACS analysis. Filled and unfilled tracings represent, respectively, the background staining and staining for the targeted protein. For the normal donor and the patient comparable levels of expression of sLex and PSGL-1 were noted.
Neutrophil binding to immobilized selectin-IgG chimeric proteins. 111Indium-labeled neutrophils from the patient and 2 normal donors were added to uncoated wells (▪) or wells coated with P- () or E-selectin IgG (▩) chimeric proteins incubated at 37°C for 30 minutes and the number of adherent cells per well after washing was determined following incubation. No significant differences in neutrophil adhesion to immobilized endothelial selectin was noted between the patient and normal donors. These data are representative of 2 experiments done in triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Neutrophil binding to immobilized selectin-IgG chimeric proteins. 111Indium-labeled neutrophils from the patient and 2 normal donors were added to uncoated wells (▪) or wells coated with P- () or E-selectin IgG (▩) chimeric proteins incubated at 37°C for 30 minutes and the number of adherent cells per well after washing was determined following incubation. No significant differences in neutrophil adhesion to immobilized endothelial selectin was noted between the patient and normal donors. These data are representative of 2 experiments done in triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Neutrophil endothelial adhesion and transendothelial migration.
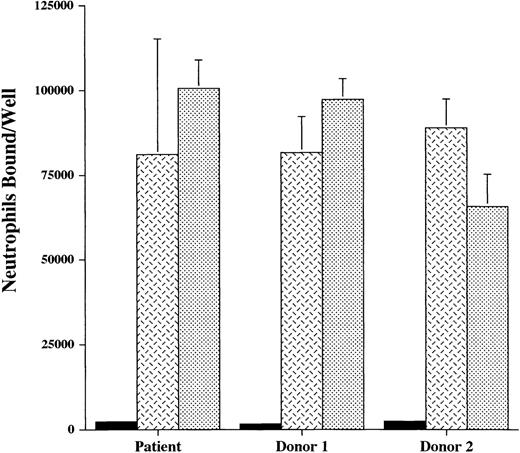
The finding of normal neutrophil expression of CD18 and selectin ligands did not exclude the possibility of functional disturbances in these molecules that may interfere with the ability of the patient’s neutrophils to interact with the endothelium. We therefore studied the adhesion of unactivated neutrophils to cytokine-stimulated endothelium, or activated neutrophils to unstimulated endothelium. In these studies, normal endothelial cells (HUVEC) were used. For the patient, adhesion of unactivated neutrophils to 4- and 24-hour TNF-α–stimulated endothelium, as well as adhesion of fMLP- or PMA-treated neutrophils to quiescent endothelium was intact (Fig 4). Adhesion stimulated by fMLP and PMA was predominately mediated by CD18 binding, as evidenced by the effectiveness of a blocking anti-CD18 antibody. Adhesion to TNF-α–stimulated endothelial cells was only partially blocked by anti-CD18, because E-selectin also mediates adhesion to these cells.
Neutrophil adhesion to endothelial cells. The adhesion of111Indium-labeled neutrophils from the patient and 2 normal donors, without (▪) or with () anti-CD18 antibody, to monolayers of normal endothelial cells (HUVEC) was studied in 2 ways. Neutrophils were activated with fMLP (10−6 mol/L) or PMA (2 μg/mL) and added to unstimulated endothelium or unstimulated neutrophils were added to endothelium stimulated with TNF- (2 ng/mL) for 4 hours or 24 hours. Neutrophils bound to the endothelium per well was determined. Control indicates the adhesion of unactivated neutrophils to unstimulated endothelial cells. In these multiple conditions, adhesion of the patient’s neutrophils to endothelium did not differ significantly from that of the normal donors. These data are representative of 3 experiments done in duplicate or triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Neutrophil adhesion to endothelial cells. The adhesion of111Indium-labeled neutrophils from the patient and 2 normal donors, without (▪) or with () anti-CD18 antibody, to monolayers of normal endothelial cells (HUVEC) was studied in 2 ways. Neutrophils were activated with fMLP (10−6 mol/L) or PMA (2 μg/mL) and added to unstimulated endothelium or unstimulated neutrophils were added to endothelium stimulated with TNF- (2 ng/mL) for 4 hours or 24 hours. Neutrophils bound to the endothelium per well was determined. Control indicates the adhesion of unactivated neutrophils to unstimulated endothelial cells. In these multiple conditions, adhesion of the patient’s neutrophils to endothelium did not differ significantly from that of the normal donors. These data are representative of 3 experiments done in duplicate or triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
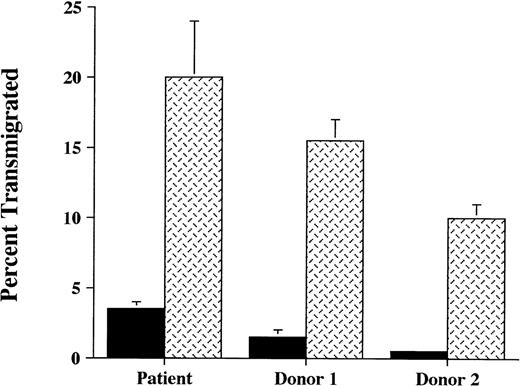
Neutrophil transendothelial migration was also investigated in a migration assay involving HUVEC grown on transwell inserts. In these in vitro studies, it was observed that neutrophil migration across TNF-α–stimulated HUVEC was preserved in this patient (Fig5). Neutrophil chemotaxis through HUVEC in response to fMLP was also intact (data not shown). Taken together, these studies suggest that the patient’s neutrophils are able to appropriately engage normal endothelium.
Neutrophil transendothelial migration. The ability of111Indium-labeled neutrophils from the patient and 2 normal donors to migrate across unstimulated endothelial cells (▪), or endothelial monolayers stimulated previously for 4 hours with TNF- (2 ng/mL) (), was studied. Normal endothelial cells (HUVEC) were used. After 90 minutes of incubation at 37°C, transendothelial migration of the patient’s neutrophils was equivalent to or exceeded that of normal donors. These data are representative of 3 experiments done in duplicate or triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Neutrophil transendothelial migration. The ability of111Indium-labeled neutrophils from the patient and 2 normal donors to migrate across unstimulated endothelial cells (▪), or endothelial monolayers stimulated previously for 4 hours with TNF- (2 ng/mL) (), was studied. Normal endothelial cells (HUVEC) were used. After 90 minutes of incubation at 37°C, transendothelial migration of the patient’s neutrophils was equivalent to or exceeded that of normal donors. These data are representative of 3 experiments done in duplicate or triplicate in which the patient was compared with 2 normal donors and in which different normal donors were used in each experiment.
Expression of endothelial cell adhesion molecules from inflamed tissues.
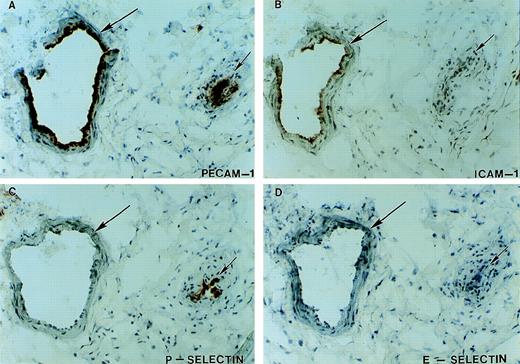
After the patient’s amputation (see Patient history), tissue from the necrotic muscle flap, the nonhealing ulcer and the surgical margin were obtained and processed for immunohistochemical staining. The availability of this tissue provided the opportunity to evaluate the expression of cell adhesion molecules on the endothelium of her vasculature known to be involved in leukocyte recruitment. Immunohistochemical staining of multiple (>6) tissue sections from three distinct sites were performed. The margins of the ulcer showed that although PECAM-1, ICAM-1, and P-selectin were expressed on the vasculature, no expression of E-selectin was detectable on the vessels (large or small) from inflamed tissue (Figs 6 and7). Staining with 3 different anti–E-selectin antibodies gave similar results. There was also patchy staining for VCAM-1 on her endothelium (data not shown). Figure 8 shows serial sections of tissue from the junction between necrotic and viable tissue. In this obviously inflamed tissue, as evidenced by the presence of Mac-1 positive leukocytes (neutrophils and monocytes) and intense neovascularization, no expression of E-selectin was noted on the vasculature. In contrast, staining of tissue from the margins of chronically infected nonhealing wounds/ulcers located on the lower extremities of 2 diabetics who were at risk for amputation showed the expression of E-selectin on vessels in these tissues (Fig9). Of note, although our patient appeared clinically to manifest less pus formation, the presence of extravascular leukocytes as indicated in Fig 7, suggests that her leukocytes still had some capacity to migrate into sites of infection or injury.
Expression of endothelial cell adhesion molecules in inflamed tissues. Serial tissue sections from visually and histologically inflamed ulcerated tissue removed at the time of the patient’s below-the-knee amputation were stained immunohistochemically with antibodies against PECAM-1 (A), ICAM-1 (B), P-selectin (C), and E-selectin (D). Two vessels (long and short arrows) are identified by staining with antibody against PECAM-1. Although ICAM-1 expression (B, long arrow) and P-selectin expression (C, short arrow) were detected on the vasculature, no expression of E-selectin was detected on these or other vessels in the surrounding tissue. Staining with 3 anti–E-selectin antibodies gave similar results.
Expression of endothelial cell adhesion molecules in inflamed tissues. Serial tissue sections from visually and histologically inflamed ulcerated tissue removed at the time of the patient’s below-the-knee amputation were stained immunohistochemically with antibodies against PECAM-1 (A), ICAM-1 (B), P-selectin (C), and E-selectin (D). Two vessels (long and short arrows) are identified by staining with antibody against PECAM-1. Although ICAM-1 expression (B, long arrow) and P-selectin expression (C, short arrow) were detected on the vasculature, no expression of E-selectin was detected on these or other vessels in the surrounding tissue. Staining with 3 anti–E-selectin antibodies gave similar results.
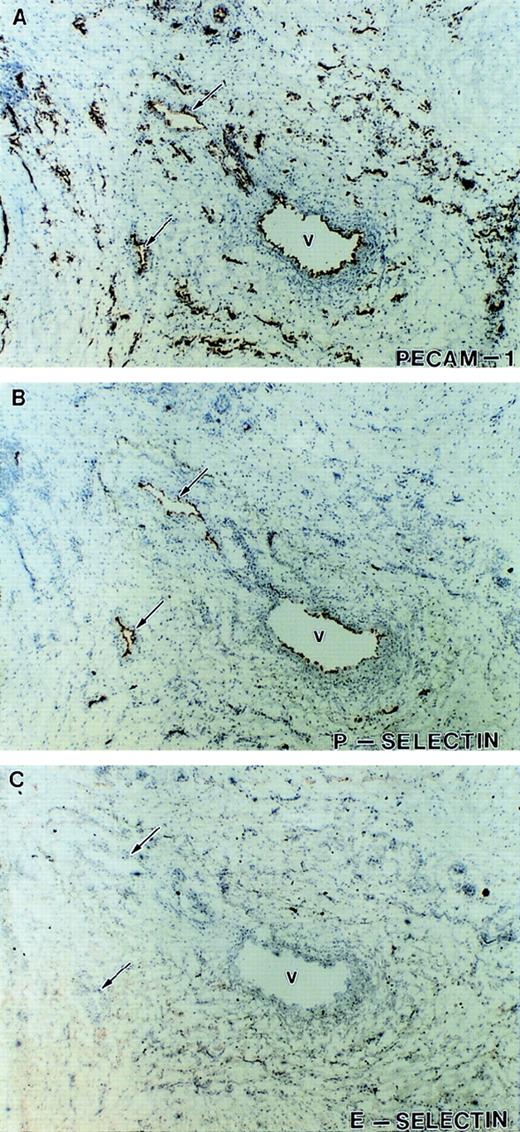
Expression of P-selectin and E-selectin in inflamed tissue. Immunohistochemical staining of serial tissue sections from another sample of visually and histologically inflamed ulcerated tissue different from that presented in Fig 5. Shown are sections stained with antibodies against PECAM-1 (A) to identify the vessels, P-selectin (B), and E-selectin (C). Although a subpopulation of the vasculature expressed P-selectin (V and arrows), no significant expression of E-selectin was detected on any of the vessels in this tissue.
Expression of P-selectin and E-selectin in inflamed tissue. Immunohistochemical staining of serial tissue sections from another sample of visually and histologically inflamed ulcerated tissue different from that presented in Fig 5. Shown are sections stained with antibodies against PECAM-1 (A) to identify the vessels, P-selectin (B), and E-selectin (C). Although a subpopulation of the vasculature expressed P-selectin (V and arrows), no significant expression of E-selectin was detected on any of the vessels in this tissue.
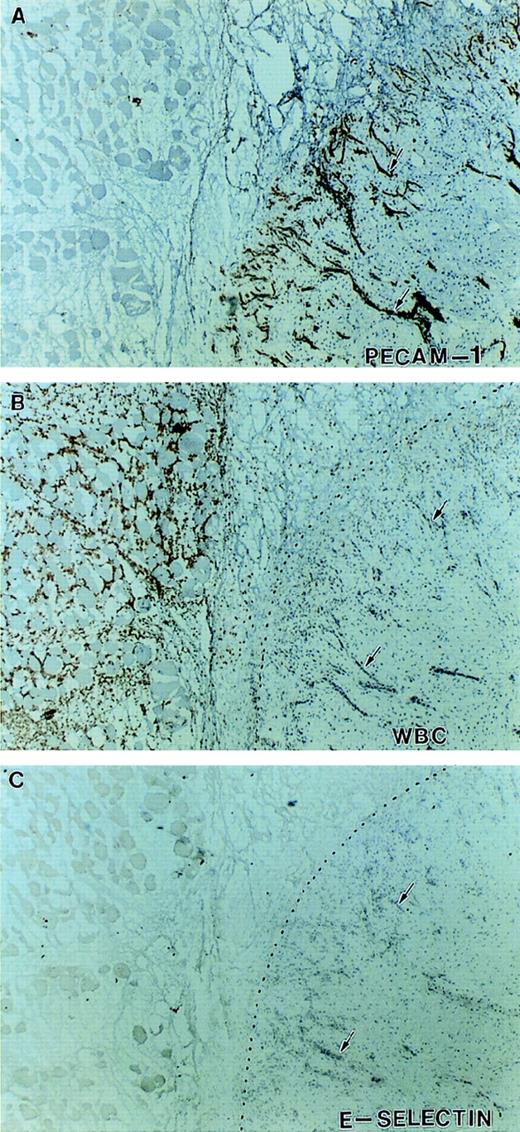
Expression of E-selectin in inflamed tissue. Immunohistochemical staining of serial sections at the junction between viable and necrotic tissue indicated by the dotted line. Sections were stained with antibodies against PECAM-1 (A), Mac-1 (B), and E-selectin (C). There is intense neovascularization at the interface of viable and necrotic tissue (A) and infiltration of Mac-1–positive leukocytes (neutrophils and monocytes) in the necrotic tissue (B). Despite this indication of active inflammation, no significant expression of E-selectin was detected on any of the vessels in this tissue (arrows).
Expression of E-selectin in inflamed tissue. Immunohistochemical staining of serial sections at the junction between viable and necrotic tissue indicated by the dotted line. Sections were stained with antibodies against PECAM-1 (A), Mac-1 (B), and E-selectin (C). There is intense neovascularization at the interface of viable and necrotic tissue (A) and infiltration of Mac-1–positive leukocytes (neutrophils and monocytes) in the necrotic tissue (B). Despite this indication of active inflammation, no significant expression of E-selectin was detected on any of the vessels in this tissue (arrows).
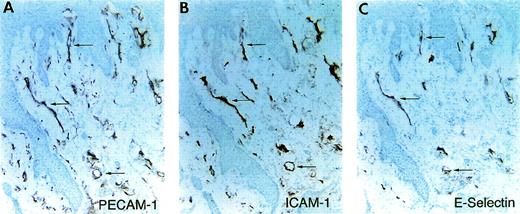
Expression of E-selectin in a chronic ulcer. Immunohistochemical staining of serial tissue sections of a sample of visually and histologically inflamed tissue from the margin a diabetic chronic nonhealing wound/ulcer. Sections stained with antibodies against PECAM-1 (A) to identify the vessels, ICAM-1 (B), and E-selectin (C). In this inflamed tissue, E-selectin was detected on a subpopulation of the vasculature (arrows).
Expression of E-selectin in a chronic ulcer. Immunohistochemical staining of serial tissue sections of a sample of visually and histologically inflamed tissue from the margin a diabetic chronic nonhealing wound/ulcer. Sections stained with antibodies against PECAM-1 (A) to identify the vessels, ICAM-1 (B), and E-selectin (C). In this inflamed tissue, E-selectin was detected on a subpopulation of the vasculature (arrows).
TNF-α–stimulation of organ skin cultures.
To evaluate further the observations noted above and to investigate the ability of her endothelium to upregulate E-selectin, short-term organ cultures of control and normal skin from the patient were incubated for 8 hours with TNF-α (12,000 U/mL). In unstimulated control and patient skin (data shown for control), 10% to 20% of the vessels expressed ICAM-1, although there was very little expression of E-selectin (Fig10). In control skin, but not the patient’s skin, TNF-α–stimulation increased the expression of E-selectin on the dermal vessels. This difference was not because of an inability of the patient’s skin to respond to TNF-α, because like control skin, cytokine-induced upregulation of ICAM-1 was observed in dermis and on the vasculature of the patient’s skin as previously described. These data, along with that from the in situ tissue staining, suggest that despite appropriate inflammatory stimuli there is an absence of endothelial surface expression of E-selectin on the endothelium of this patient.
Expression of E-selectin in TNF-–stimulated skin organ cultures. Immunohistochemical staining of skin organ cultures stimulated for 8 hours with TNF- (12,000 U/mL). Serial sections of unstimulated normal skin (A through C), stimulated normal skin (D through F) and stimulated skin from the patient (G through I) were stained with antibody against PECAM-1 (A, D, and G) to identify the vessels (arrows and arrowheads), ICAM-1 (B, E, and F) and E-selectin (C, F, and I). The epidermis (Epi) is indicated for the stimulated normal skin. In unstimulated normal skin there was some expression of ICAM-1 (B, arrowheads), although most of the vessels did not express it (B, double arrows), and negligible expression of E-selectin (C). A similar pattern was seen for unstimulated skin from the patient (data not shown). In stimulated normal skin, there was marked upregulation in the number of vessels expressing ICAM-1 (E) and a smaller but clearly significant increase in the number of vessels expressing E-selectin (F). In contrast to normal skin, stimulated skin from the patient showed upregulation of ICAM-1 on the vasculature (H) but no increased E-selectin expression (I). The asterisk indicates dermal interstitium in which there is also upregulate expression of ICAM-1 on the dermal stroma particularly evident in the sample of stimulated normal skin (E).
Expression of E-selectin in TNF-–stimulated skin organ cultures. Immunohistochemical staining of skin organ cultures stimulated for 8 hours with TNF- (12,000 U/mL). Serial sections of unstimulated normal skin (A through C), stimulated normal skin (D through F) and stimulated skin from the patient (G through I) were stained with antibody against PECAM-1 (A, D, and G) to identify the vessels (arrows and arrowheads), ICAM-1 (B, E, and F) and E-selectin (C, F, and I). The epidermis (Epi) is indicated for the stimulated normal skin. In unstimulated normal skin there was some expression of ICAM-1 (B, arrowheads), although most of the vessels did not express it (B, double arrows), and negligible expression of E-selectin (C). A similar pattern was seen for unstimulated skin from the patient (data not shown). In stimulated normal skin, there was marked upregulation in the number of vessels expressing ICAM-1 (E) and a smaller but clearly significant increase in the number of vessels expressing E-selectin (F). In contrast to normal skin, stimulated skin from the patient showed upregulation of ICAM-1 on the vasculature (H) but no increased E-selectin expression (I). The asterisk indicates dermal interstitium in which there is also upregulate expression of ICAM-1 on the dermal stroma particularly evident in the sample of stimulated normal skin (E).
Expression of E-selectin message.
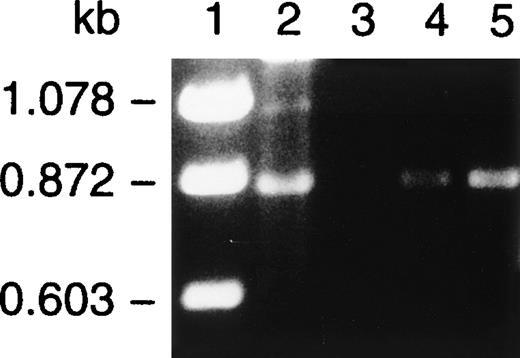
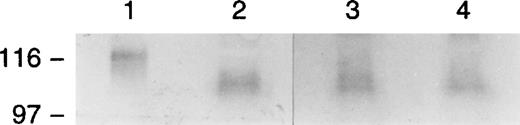
One possible explanation for the absence E-selectin on the endothelium, despite appropriate inflammatory stimuli, was an absence of E-selectin RNA transcripts. We therefore looked for the presence of E-selectin message in inflamed and normal tissues obtained at the time of her amputation. Total cellular RNA was isolated from ulcerated inflamed tissue and the surgical margin and subjected to RT-PCR using primers designed to amplify the lectin-binding and epidermal growth factor domains of the molecule. The products generated by RT-PCR are shown in Fig 11. PCR with these primers of E-selectin cDNA, (lane 2) yielded the expected product of 870 bp. An identical product was also identified in tissues samples from the abnormal, inflamed tissue (lanes 4 and 5), confirming that message for E-selectin was present in this patient.
RT-PCR of surgical tissue from the patient. RT-PCR using primers designed to amplify the lectin and epidermal growth-like domains was conducted on total cellular RNA isolated from surgical tissue from the patient that included normal tissue from the margins of the surgical amputation (lane 3) and ulcerated nonhealing inflamed tissue from two distinct sites (lanes 4 and 5). PCR with these primers of E-selectin cDNA (lane 2) yielded the expected product of 870 bp. An identical product was also identified in tissue samples from the abnormal, inflamed tissue (lanes 4 and 5) but was not readily detected in normal tissue at the surgical margin (lane 3).
RT-PCR of surgical tissue from the patient. RT-PCR using primers designed to amplify the lectin and epidermal growth-like domains was conducted on total cellular RNA isolated from surgical tissue from the patient that included normal tissue from the margins of the surgical amputation (lane 3) and ulcerated nonhealing inflamed tissue from two distinct sites (lanes 4 and 5). PCR with these primers of E-selectin cDNA (lane 2) yielded the expected product of 870 bp. An identical product was also identified in tissue samples from the abnormal, inflamed tissue (lanes 4 and 5) but was not readily detected in normal tissue at the surgical margin (lane 3).
Expression of E-selectin protein.
The observation that E-selectin was not detected on inflamed endothelium, despite the presence of E-selectin message, suggested for this patient that E-selectin was secreted or rapidly shed from the endothelial surface. To investigate this further, we measured by ELISA the level of soluble (s) E-selectin in sera from the patient along with age-matched controls. Two samples of sera from the patient were obtained when she was clinically stable without evidence of infection. As shown in Table 2, more sE-selectin was detected in sera from the patient compared with controls (186 ± 38 ng/mL v 90 ± 13 ng/mL). Levels of soluble ICAM-1 (sICAM-1) and tumor necrosis factor receptor-1 (sTNF-R1) were noted to be slightly elevated (sICAM-1, 312 ng/mL, normal range, 115 to 306 ng/mL; sTNF-R1, 2,245 pg/mL, normal range, 749 to 1,966 pg/mL). To determine the molecular form(s) of the sE-selectin present in her circulation, samples of the patient’s serum along with controls were transferred to nitrocellulose and immunoblotted with an anti–E-selectin polyclonal antibody (Fig12). A single molecular species was detected in the patient’s serum of ∼100 to 110 kD comparable in size with the form noted in control sera and appropriately smaller than the full-length species found in extracts from endothelial cells stimulated with TNF-α. This suggests that the molecular form of sE-selectin in this patient’s circulation results principally from the proteolytic cleavage of surface E-selectin.
Serum Soluble E-selectin Concentration
| Serum Sample . | sE-selectin (ng/mL) . |
|---|---|
| Patient (1) | 224 |
| Patient (2) | 148 |
| Mean | 186 ± 38 |
| Control (1) | 98 |
| Control (2) | 71 |
| Control (3) | 85 |
| Control (4) | 106 |
| Mean | 90 ± 13 |
| Serum Sample . | sE-selectin (ng/mL) . |
|---|---|
| Patient (1) | 224 |
| Patient (2) | 148 |
| Mean | 186 ± 38 |
| Control (1) | 98 |
| Control (2) | 71 |
| Control (3) | 85 |
| Control (4) | 106 |
| Mean | 90 ± 13 |
The concentration of sE-selectin was determined by ELISA in the patient sera from 2 separate samples and in 4 age-matched controls.
Western blot analysis of sera for E-selectin. TNF-–stimulated HUVEC extracts (lane 1) and sera from the patient (lane 2) and from 2 normal donor controls (lanes 3 and 4) were transferred to nitrocellulose and immunoblotted with an anti–E-selectin antibody. A single molecular species was detected in the patient’s serum of approximately 100 to 110 kD, comparable in size with the form noted in control sera and appropriately smaller than the full-length species found in extracts from endothelial cells stimulated with TNF-.
Western blot analysis of sera for E-selectin. TNF-–stimulated HUVEC extracts (lane 1) and sera from the patient (lane 2) and from 2 normal donor controls (lanes 3 and 4) were transferred to nitrocellulose and immunoblotted with an anti–E-selectin antibody. A single molecular species was detected in the patient’s serum of approximately 100 to 110 kD, comparable in size with the form noted in control sera and appropriately smaller than the full-length species found in extracts from endothelial cells stimulated with TNF-.
Sequencing of E-selectin gene.
SSCP analysis was initially used to screen for coding region mutations. PCR products containing the CR5 and cytoplasmic 2 domains both showed conformational changes. These PCR products were directly sequenced and 2 heterozygous mutations were identified. Based on these findings, the patient’s E-selectin gene was fully sequenced using exon-specific PCR products in both directions. Each PCR product included at least 50 bp from each flanking intron. The patient was found to be heterozygous for 4277C-T histidine to tyrosine missense mutation in the CR5 exon. She was also heterozygous for a 5822T-C mutation which does not result in any amino acid change in the cytoplasmic 2 exon. One further intronic mutation was identified in intron 10 just after the transmembrane exon at 5334T-C. 4277T and 5822C were previously identified in cDNA sequencing.19 These may represent common polymorphisms, although we were unable to identify either mutation in 30 controls. The intronic mutation is thus of uncertain significance. It does not lie within any known motif involved in splice signaling, although it could potentially affect the ability to form membrane bound E-selectin if it did alter splicing of the transmembrane domain exon.
DISCUSSION
At sites of acute injury and infection in the systemic circulation, the initial interaction of the intravascular leukocyte with the endothelium consists of the tethering and rolling of leukocytes along postcapillary venular endothelium.1-5 These early events are believed to be largely mediated by the interactions of selectins with their carbohydrate-rich protein ligands.24 Evidence for the importance of these endothelial selectins comes from patients with an inherited defect in fucose metabolism that results in an inability to synthesize the fucosylated tetrasaccharides (eg, sialyl-Lewisx [sLex]) that decorate selectin ligands and mediate ligand binding.8,9 As originally described, patients with this so-called leukocyte adhesion deficiency type 2 suffer from recurrent bacterial infections, typically without pus formation, despite markedly elevated circulating neutrophil counts. In vitro, the neutrophils of these patients are unable to adhere or roll on E-selectin, P-selectin, or cytokine or mediator-stimulated endothelial cells. Interestingly, for one of these patients, infections requiring hospitalization or intravenous antibiotics have not occurred over last 5 years, although periodontitis has been a chronic problem. This has occurred despite the fact that his neutrophils are still deficient in SLex and are functionally abnormal.25
Although our patient did experience recurrent infections with clinical evidence of reduced pus formation, the patients neutrophils were not deficient in sLex and adhered to cytokine-stimulated endothelial cells and recombinant P- and E-selectin proteins (Figs 2and 3). In addition, she did not show evidence for a deficiency or dysfunction of her β-2 integrins (Figs 1, 4 and 5). Our data, however, do not exclude the possibility of more subtle defects that might interfere with neutrophil rolling. Interestingly, unlike previously reported patients with LAD where neutrophilia is present, this patient showed a mild neutropenia with an ability to appropriately respond to infection. Increasing her neutrophil counts to normal values with G-CSF did not appear to decrease the occurrences of bacterial infections. This latter observation, coupled with the mild degree of neutropenia and her demonstrated ability to mount neutrophilia in response to infection, make it unlikely that the neutropenia alone is responsible for her severe, recurrent infections. The mechanism(s) responsible for this neutropenia are not clear, but we speculate that the processes which reduce the surface expression of endothelial E-selectin may also alter the expression of other adhesion receptors on leukocytes and the endothelium that are involved in release of leukocytes from the bone marrow.
Although our patient did not have evidence of a leukocyte adhesion deficiency, she did show a striking absence of E-selectin from the surface of the endothelium despite inflammatory stimuli (Figs 6 through8). These observations were supported by the finding that in skin organ cultures, TNF-α increased the vascular expression of E-selectin in normal, but not patient, skin (Fig 10). E-selectin message was found in inflamed tissues (Fig 11), and although E-selectin was not present on the endothelium, its message was translated as evidenced by the high levels of circulating sE-selectin (Table 2). As significant mutations in the E-selectin gene were not detected, the absence of endothelial E-selectin does not appear to be the result of a secreted alternatively spliced or mutational variant. This is supported by the finding that the molecular species of sE-selectin detected in the patient and control sera were of the same molecular weight (Fig 12). Of note, her serum did not inhibit the TNF-induced expression of E-selectin on HUVEC (data not shown), suggesting that proteolytic activity in her serum was not responsible for the loss of surface endothelial E-selectin. We therefore propose that there may be upregulation of proteolytic activity on the surface of the vascular endothelium of the tissues we studied that results in markedly accelerated release of E-selectin from the endothelial surface. As the entire vasculature was not surveyed, our data do not exclude the possibility that in specific organs, such as the lung and the gastrointestinal tract, the vasculature may be normal.
An important question is how might the loss of the endothelial surface expression of E-selectin with an accompanying increase in circulating sE-selectin increase the susceptibility to infection in this patient? There are a number of possibilities. First, given the role of selectins in the initial rolling phenomena, the loss of endothelial surface expression of E-selectin may compromise the ability of leukocytes to roll along the endothelium and therefore impair leukocyte emigration into sites of inflammation. However, mutant mice deficient in E-selectin do not show an increased incidence of spontaneous infections, and neutrophil recruitment in response chemical-induced peritonitis is impaired only if P-selectin is also simultaneously blocked.26 These and other studies with selectin-deficient mice27 28 suggest that with respect to the process of leukocyte recruitment, E- and P-selectin may serve redundant functions, and/or that in the setting of chronic loss each may be able to compensate for the persistent absence of the other. Consistent with this proposal is the demonstration in our patient of P-selectin endothelial expression and some extravascular accumulation of Mac-1–positive leukocytes in chronically inflamed tissues (Fig 8). While data from mutant mice are very instructive, its is possible (and probably likely) that the specific or relative functions of P- and E-selectin in humans differ from that of mice, and therefore care should be exercised in extrapolating from the murine studies.
Second, there is evidence that E-selectin may mediate other processes that are crucial to host defenses. This is suggested by studies of E-selectin–deficient mice subjected to systemic pneumococcal infection.29 After intraperitoneal inoculation with S pneumonie, mice deficient in E-selectin showed more prominent morbidity, substantially increased 10-day mortality, persistent bacteremia, and a higher bacterial load compared with wild-type mice. These derangements occurred despite the fact that leukocyte emigration into the peritoneal space was preserved in these mice. Leukocyte activation may be one of these alternative processes, as interaction of unstimulated neutrophils with E-selectin upregulates the adhesive activity of Mac-1 (αMβ2),30,31 and neutrophils from transgenic mice constitutively expressing E-selectin show increased oxidative activity.32 Therefore, in this patient, the diminished surface expression of endothelial E-selectin may result in the loss of important signals required for the activation of neutrophils and their subsequent ability to kill bacteria.
The presence of markedly elevated levels of sE-selectin33represents yet a third potential mechanism that may contribute to this patient’s increased susceptibility to infection. Cultured endothelial cells have been shown to release E-selectin following activation presumably as a result of proteolytic cleavage of surface E-selectin.34-36 Elevated levels of sE-selectin have been detected in patients with diabetes, inflammatory skin disorders, vasculitis, and scleroderma, although these levels have correlated only weakly or not all with disease activity.37,38 High levels of sE-selectin have also been observed in sepsis with higher and/or persistent elevations correlating with greater mortality and severity of disease.33,35 In our patient, sE-selectin was determined on 2 occasions, 5 months apart, when she was well without clinical evidence of infection and the levels were noted to be 2 to 3 times that of age-matched controls.39,40 Although its precise in vivo function is unknown, sE-selectin in the blood is biologically active in that it is able to bind to and mediate adhesion and activation of neutrophils.30-32 Therefore the high circulating levels of sE-selectin observed in this patient may result in inappropriate intravascular leukocyte activation or may compromise adhesive interactions with activated endothelium.
In conclusion, we have identified a patient with recurrent severe bacterial infections and clinically impaired pus formation associated with markedly reduced surface expression of E-selectin on inflamed endothelium, although E-selectin message was present in this tissue and significantly elevated levels of sE-selectin were detected in the patient’s serum. As gene sequencing failed to show any significant mutations, these data suggest that in this patient, E-selectin is rapidly shed from the endothelium of her vasculature resulting in markedly decreased endothelial surface expression of E-selectin and increased circulating sE-selectin. We speculate that this pattern of expression may be part of a wider process in which there is increased proteolytic activity on the endothelium. Regardless of the mechanism, these data provide additional human evidence for the importance of endothelial selectins in leukocyte recruitment and the ability to fight infections.
ACKNOWLEDGMENT
The authors are extremely grateful to Steven M. Albelda for his advice and support of the work in this article. We also thank Mildred Daise, Eugenia Argyris, Kate Veksler, Patricia Sassoli, Ann Leone, and Miae Oh for their expert technical assistance. We are grateful to Enyi Okereke, MD, for providing us with tissue from chronic diabetic lower extremity ulcers.
Supported by grants from the Robert Wood Johnson Foundation Minority Faculty Development Program (H.M.D.), National Institutes of Health Grant No. K14 HL-03382 (H.M.D.), The Wallace Chair (K.E.S.), and the Lupus Foundation (K.E.S.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Horace M. DeLisser, MD, 894 Maloney Bldg, University of Pennsylvania Medical Center, 3600 Spruce St, Philadelphia, PA 19104-4283; e-mail: delisser@mail.med.upenn.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal