Granulocyte-colony stimulating factor (G-CSF) has been found to act on the neutrophilic lineage. We recently showed that human G-CSF (hG-CSF) has effects similar to early-acting cytokines such as interleukin-3 (IL-3) in the development of multipotential hematopoietic progenitors in transgenic (Tg) mice expressing receptors (R) for hG-CSF. In the present study, we examined the effects of hG-CSF on more mature hematopoietic cells committed to megakaryocytic lineage in these Tg mice. The administration of hG-CSF to the Tg mice increased the numbers of both platelets in peripheral blood and megakaryocytes in the spleen, indicating that hG-CSF stimulates megakaryopoiesis in the Tg mice in vivo. The stimulatory effect of hG-CSF was also supported by the results of studies in vitro. hG-CSF supported megakaryocyte colony formation in a dose-dependent fashion in clonal cultures of bone marrow cells derived from the Tg mice. Direct effects of hG-CSF on megakaryocytic progenitors in the Tg mice were confirmed by culture of single-cell sorted from bone marrow cells. hG-CSF showed a stronger effect on maturation of megakaryocytes in the Tg mice than that of IL-3 alone, but weaker than that of TPO alone. In addition, hG-CSF induced phosphorylation of STAT3 but not Jak2 or STAT5, while TPO induced phosphorylation of both. In contrast to TPO, hG-CSF did not enhance ADP-induced aggregation. Thus, hG-CSF has a wide variety of functions in megakaryopoiesis of hG-CSFR-Tg mice, as compared with other megakaryopoietic cytokines, but the activity of hG-CSF in megakaryocytes and platelets does not stand up to a comparison with that of TPO. Specific signals may be required for the full maturation and activation of platelets.
THE PROLIFERATION, differentiation and maturation of hematopoietic cells are regulated by a number of cytokines.1 These molecules exert biological functions through specific receptors expressed on the surface of target cells.2 Binding of the cytokines to the receptors induces phosphorylation of a variety of cellular proteins, including a group of signal transducers and activators of transcription (STAT) proteins, involved in intracellular signal transduction.3 Although it was earlier postulated that each cytokine has specific functions, recent studies have shown that the specific activity of each cytokine depends on the cellular context in which the receptor is expressed. Dubart et al4 reported that erythropoietin (EPO) supported the formation of multilineage colonies comprising not only erythrocytes but also granulocytes, macrophages and megakaryocytes from multipotential progenitor cells transduced with the EPO receptor cDNA. We have also shown that human granulocyte-macrophage colony-stimulating factor (hGM-CSF) stimulated development of multiple lineages of cells, such as erythrocytic and megakaryocytic cells in addition to myelocytic cells in transgenic (Tg) mice expressing hGM-CSF receptors (R).5 Thus, cytokines specific for erythropoiesis or myelopoiesis can support megakaryopoiesis to some extent if their receptors are expressed on megakaryocytic cells. Whether intracellular signals emanating from ectopic receptors can activate and otherwise mimic the biological effects on megakaryopoiesis in a fashion identical to that of natural receptors for megakaryopoietic cytokines has remained unclear.
Megakaryopoiesis is a multistage developmental process governed by a series of megakaryopoietic cytokines.6,7 Some, such as interleukin-3 (IL-3), called megakaryopoietic colony-stimulating factors, support proliferation of megakaryocytic progenitors and differentiation into immature megakaryocytes. In contrast, some such as IL-6, IL-11, leukemia inhibitory factor, and oncostatin M, which are known as Mk-potentiators, affect the maturation of megakaryocytes. Thrombopoietin (TPO) has been found to be a major physiological regulator of megakaryopoiesis. TPO stimulates both growth of megakaryocytic progenitors and megakaryocyte maturation.8TPO also enhances human platelet aggregation, and induces tyrosine phosphorylation of Jak2, STAT3, and STAT5.9
Recently, we generated Tg mice expressing receptors for human granulocyte colony-stimulating factor (hG-CSFR), a myelopoietic growth factor.10 A 3-kb fragment of G-CSFR cDNA was inserted into the EcoRI site of a pLG1 expression vector that is under control of mouse major histocompatibility L-locus gene promoter, resulting in ubiquitous expression of the transgene. hG-CSF induces colony formation that includes multiple lineages when incubated with bone marrow (BM) from those mice.10 In the present study, we scrutinized effects of hG-CSF on megakaryopoiesis and thrombopoiesis in the Tg mice in vivo and in vitro and compared these effects with cytokines known to stimulate megakaryopoiesis and thrombopoiesis. In addition, we examined activities of hG-CSF and TPO on aggregation and STAT phosphorylation in platelets of the Tg mice. The biological, signaling, and functional effects of hG-CSF on megakaryopoiesis of the Tg mouse differ from those of any other megakaryopoietic cytokines, including TPO.
MATERIALS AND METHODS
Mice.
Tg mice constitutively expressing high-affinity hG-CSFR were generated in our laboratory, as described.10 The Tg mice and their normal littermates were 8 weeks old, of both sexes, and their backgrounds were C3H/HeN and (C57BL/6 × C3H/HeN) F1. These mice were maintained in an environmentally controlled clean room with 12-hour light-dark cycles under specific pathogen-free conditions in micro-isolator cages. The mice were not used in more than two protocols, and were not repeatedly bled. Injection schedules of hG-CSF were according to the modified method described elsewhere.11 Briefly, the Tg mice and normal littermates were injected intraperitoneally with 0.2 mL of phosphate-buffered saline (PBS) containing 1% of C3H/HeN mice serum (serum/PBS) or 500 μg/kg of hG-CSF twice a day at 12-hour intervals for 7 consecutive days at 9:00 am and 9:00 pm, and peripheral blood (PB), BM and spleen cells were harvested 12 hours after the last injection.
Cytokines and antibodies.
Recombinant hG-CSF and hTPO were kindly provided by Kirin Brewery (Tokyo, Japan). Recombinant mouse IL-3 (mIL-3) and recombinant hIL-6 were provided by Amgen (Thousand Oaks, CA), and Tosoh Co (Kanagawa, Japan), respectively. Fluorescein isothiocyanate (FITC)-conjugated hamster 1C2 antibody that immunoreacts with mouse platelets and megakaryocytes is a gift from Dr Junichiro Fujimoto12(National Children’s Medical Research Center, Tokyo, Japan). Allophycocyanin (APC)-conjugated anti-c-Kit antibody (ACK-2) was a generous gift from Dr Shin-Ichi Nishikawa (Kyoto University, Kyoto, Japan). Biotin-conjugated monoclonal antibodies (MoAbs) specific for CD45R (B220, RA3-6B2), Gr-1 (Ly-6G, RB6-8C5), CD4 (L3T4, RM4-5), CD8a (Ly-2, 53-6.7), TR119 (TER119), Mac-1 (CD11b, M1/70), phycoerythrin (PE)-conjugated anti-mouse Sca-1 (Ly-6A/E, E13-161.7), APC-conjugated rat IgG2b, rat anti-mouse CD32/CD16 (FcγII/III receptor, 2.4G2), and mouse anti-STAT5A and B polyclonal antibodies (pAb) were purchased from Pharmingen (San Diego, CA). An anti-Jak2 pAb and an antiphosphotyrosine MoAb (4G10) were from Upstate Biotechnology (Lake Placid, NY). PE-conjugated streptavidin, rat IgG2a, texas red (TR)-conjugated streptavidin, and R-phycoerythrin-cyanine 5 (RPE-Cy5)-conjugated streptavidin were purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA), Cedarlane Laboratories, Ltd (Ontario, Canada), Life Technologies, Inc (Gaithersburg, MD), and DAKO (Glostrup, Denmark), respectively. Anti-STAT3 pAb was from Santa Cruz (Santa Cruz, CA).
Cell and platelet preparations.
Mice were anesthetized with ether, and killed by rapid cervical dislocation. BM cells were flushed from femurs and tibiae into a minimum essential medium (α-MEM; Flow Laboratories, Rockville, MD) with 2% fetal bovine serum (FBS; Hyclone, Logan, UT) using 21-gauge needles. Then cells were passed through a 70-μm nylon cell strainer (no. 2350; Becton Dickinson Labware, Franklin Lakes, NJ). BM mononuclear cells (MNC) were prepared using a density gradient centrifugation method. Cells were diluted with PBS, layered over Lympholyte-M (Cosmo Bio, Tokyo, Japan), and centrifuged for 30 minutes at 1,500 rpm at room temperature. Interface cells were collected and washed twice with PBS. For platelets preparation, mice were anesthetized with ether, and blood was drawn from superior vena cava into 40 mmol/L D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK; Sigma, St Louis, MO), and gently mixed. Platelet-rich plasma (PRP) was prepared by centrifuging the whole blood at 200g for 20 minutes and aspirating PRP. PRP was incubated with aspirin (2 mmol/L) for 30 minutes at room temperature. To prepare washed platelets, prostaglandin E1 (1 mmol/L) was added from a stock solution in absolute ethanol (1 mmol/L). The PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL of a modified HEPES-Tyrode buffer (129 mmol/L NaCl, 8.9 mmol/L NaHCO3, 0.8 mmol/L KH2PO4, 0.8 mmol/L MgCl2, 5.6 mmol/L dextrose, and 10 mmol/L HEPES, pH 7.4) also containing apyrase (2 U/mL) and washed twice with PBS. Platelets were resuspended in the same buffer at a concentration of 3 to 9 × 108 cells/mL with apyrase (2 U/mL) containing 1 mmol/L CaCl2 at 37°C.
Clonal cell culture.
Clonal cell culture was performed in triplicate, as described previously.13-15 Briefly, 1 mL of culture mixture containing 2.5 × 104 BM cells, α-MEM, 1.2% methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% FBS, 1% deionized fraction V bovine serum albumin (BSA; Sigma), 10−4 mol/L mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), and varying concentrations of hG-CSF, 20 ng/mL of hTPO, 10 ng/mL of mIL-3, or 100 ng/mL of hIL-6 was plated in each 35-mm suspension culture dish (no. 171099; Nunc, Inc, Naperville, IL) followed by incubation at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Colony types were determined on days 7 and 14 of incubation by in situ observation according to documented criteria14,16,17 using an inverted microscope. To assess the accuracy of in situ identification of the colonies, individual colonies were lifted using an Eppendorf micropipette under direct microscopic visualization, spread on glass slides using a cytocentrifuge (Cytospin 2; Shandon Inc, Pittsburgh, PA), then stained with May-Grünwald-Giemsa (Muto Chemica, Tokyo, Japan) or acetylcholine esterase (AChE) for megakaryocytes.18 Except for megakaryocyte colonies, cell aggregates consisting of more than 50 cells were scored as colonies. Megakaryocyte colonies were scored as such when they had four or more megakaryocytes.19 20Abbreviations for the colony types are as follows: Mk, megakaryocyte colonies; M-Mix, mixed hematopoietic colonies containing megakaryocytes; GM, granulocyte and/or macrophage colonies; others, other colonies including mast and blast cell colonies.
Clone-sorting and single cell culture.
Clone-sorting of lineage (Lin)−c-Kit+Sca-1+ and Lin−c-Kit+Sca-1− cells from BM cells of (C57BL/6 × C3H/HeN) F1 Tg mice was performed using a modification of a previously described technique.21Briefly, BMMNC were enriched by negative selection with streptavidin-conjugated beads (PerSeptive Biosystems, Framingham, MA), using a cocktail of biotin-conjugated MoAb specific for CD45R/B220, Gr-1, CD4, CD8, TR119, and Mac-1. After incubation with rat anti-mouse CD32/CD16 to avoid nonspecific antibody binding, the lineage marker–negative cells were stained with PE-conjugated Sca-1 and APC-conjugated anti–c-Kit antibodies. The cells were then washed twice with PBS and incubated with TR-conjugated streptavidin. The negative controls were stained with PE-conjugated rat IgG2a, APC-conjugated rat IgG2b, or only TR-conjugated streptavidin. Individual Lin−c-Kit+Sca-1+ and Lin−c-Kit+Sca-1− cells were sorted into each well of 96-well flat-bottomed plates (no. 163320, Nunc) with a FACSVantage equipped with an automatic cell deposition unit (ACDU; Becton Dickinson). The clone-sorted cells were then cultured with 200 μL culture medium containing 30% FBS, 1% deionized fraction V BSA, 10−4 mol/L mercaptoethanol, and with the addition of 20 ng/mL of hG-CSF, 100 ng/mL of hIL-6, 20 ng/mL of hTPO, or 10 ng/mL of mIL-3. On days 5 and 8 of culture, colony formations were observed and classified using of an inverted microscope.
Determination of size of megakaryocytes in megakaryocyte colonies.
BM cells (2.5 × 104) were cultured in methycellulose medium containing 20 ng/mL of hG-CSF, 20 ng/mL of hTPO, or 10 ng/mL of mIL-3. After 5 days culture, Mk colonies were lifted from methylcellulose medium, pooled, and washed with α-MEM. The cells were cytospun and stained with AChE. The diameter of each megakaryocyte identified by AChE-staining was measured using a microscope equipped with an ocular micrometer. The mean of two perpendicular diameters was calculated.17
Peripheral blood cell counts.
Mice were anesthetized with ether, and PB was taken from superior vena cava using a 1-mL syringe with a 21-gauge needle. After the collection, PB was quickly mixed with 2 mg EDTA to avoid aggregation. The numbers of red blood cells (RBCs), white blood cells (WBCs), platelets, and the hemoglobin concentration (Hb) were counted using a hemacytometer (Sysmex K1000; Sysmex, Kobe, Japan). Smear preparations were made on glass slides and stained with May-Grünwald-Giemsa solution.
Histological examination of spleens.
Spleens of the Tg mice and normal littermates treated with hG-CSF or serum/PBS were taken for histological examination. After fixation with 10% formalin solution, the sections were stained with hematoxylin-eosin.
Examination of hG-CSFR expression on platelets.
Flow cytometry analysis was performed by a modified method previously described.10 Briefly, PRP was incubated with PE-conjugated hG-CSF (R & D Systems, Inc, Minneapolis, MN) or FITC-conjugated 1C2 MoAb for 60 minutes at room temperature, diluted with staining buffer (PBS containing 2% FBS and 0.1% sodium azide), then analyzed using a FACScan (Becton Dickinson). PE-conjugated streptavidin or FITC-conjugated goat anti-hamster IgG (Cedarlane, Ontario, Canada) was used as a control, respectively.
Measurement of platelet aggregation.
Immunoprecipitation.
Platelet stimulation was terminated by adding an equal amount of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 [vol/wt], pH 7.4). After 20 minutes of incubation on ice, the lysates were centrifuged at 10,000g for 20 minutes at 4°C. The supernatant (from 3 to 9 × 108 platelets) was removed and incubated with preimmune serum and Protein A-Sepharose (Transduction Laboratories, Lexington, KY) (40 μL of 50% slurry) for 1 hour. After preclearing, the desired pAb were added followed by incubation for 2 to 3 hours on ice. Protein A-Sepharose (40 μL of 50% slurry) was then added and the preparation was incubated for several hours. The immune complexes were washed three times with 1 mL of cold washing buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 1% Triton X-100 [vol/wt], pH 7.4) and then resuspended in Laemmli’s sample buffer (10% glycerol, 1% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, 50 mmol/L Tris-HCl, pH 6.8). After boiling at 95°C for 5 minutes, one-dimensional SDS-electrophoresis was performed on 10% or 7.5% to 15% polyacrylamide gels. Separated proteins were electrophoretically transferred from the gels onto polyvinylidene difluoride (PVDF) membranes in buffer containing Tris (25 mmol/L), glycine (192 mmol/L) and 20% methanol at 0.2 A for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (TBS: Tris-buffered-saline, 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.6 with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 2 μL/mL (for STAT5 A and B) or 2 μg/mL (for STAT3) in TBST. The primary antibody was removed and the blots were washed four times in TBST and incubated with horseradish peroxidase-conjugated second antibody diluted 1:5,000 in TBST. Blots were then washed four times with TBST. Antibody reactions were detected with chemiluminescence, according to the manufacturer’s instructions.
Statistical analysis.
The data are expressed as the mean ± SD of triplicate plates in colony formation. Student’s t-test was used to determine the statistical significance.
RESULTS
hG-CSF administration to hG-CSFR-Tg mice.
The peripheral blood WBC, RBC, Hb and platelet counts of the Tg mice were similar to those of normal littermates. In vivo effects of hG-CSF on megakaryopoiesis in the Tg mice and normal littermates were examined after administration of hG-CSF (1,000 μg/kg/d) for 7 consecutive days. After the administration of hG-CSF or serum/PBS, both the Tg mice and normal littermates looked healthy. The number of PB RBCs and the Hb level showed no remarkable changes on day 8, and the number of WBCs increased to similar levels in both the Tg mice and normal littermates (Table 1). The most marked difference was in the number of platelets; while a 1.4-fold increase of platelets was seen in the Tg mice treated with hG-CSF, a decrease in platelet count was seen in normal littermates.
Analysis of Peripheral Blood of hG-CSFR Tg-Mice and Littermates Injected With hG-CSF
| hG-CSF (μg/kg/d) . | Tg Mice . | Littermates . | ||
|---|---|---|---|---|
| 0 . | 1,000 . | 0 . | 1,000 . | |
| WBC (×103/μL) | 3.3 ± 0.5 | 41.9 ± 7.7 | 3.2 ± 0.6 | 48.7 ± 8.4 |
| RBC (×106/μL) | 8.2 ± 0.3 | 8.3 ± 0.8 | 8.2 ± 0.4 | 8.0 ± 0.5 |
| Hb (g/dL) | 14.9 ± 0.6 | 14.1 ± 0.2 | 15.2 ± 0.3 | 14.6 ± 0.5 |
| Platelets (×106/μL) | 1.0 ± 0.1 | 1.4 ± 0.2 | 1.0 ± 0.1 | 0.6 ± 0.1 |
| hG-CSF (μg/kg/d) . | Tg Mice . | Littermates . | ||
|---|---|---|---|---|
| 0 . | 1,000 . | 0 . | 1,000 . | |
| WBC (×103/μL) | 3.3 ± 0.5 | 41.9 ± 7.7 | 3.2 ± 0.6 | 48.7 ± 8.4 |
| RBC (×106/μL) | 8.2 ± 0.3 | 8.3 ± 0.8 | 8.2 ± 0.4 | 8.0 ± 0.5 |
| Hb (g/dL) | 14.9 ± 0.6 | 14.1 ± 0.2 | 15.2 ± 0.3 | 14.6 ± 0.5 |
| Platelets (×106/μL) | 1.0 ± 0.1 | 1.4 ± 0.2 | 1.0 ± 0.1 | 0.6 ± 0.1 |
Results are expressed as mean ± SD for 10 mice per group.
Abbreviations: WBC, white blood cell count; RBC, red blood cell count; Hb, hemoglobin.
After the injection of hG-CSF, a significant splenomegaly was observed in all the mice. The spleen weight increased 5-fold in the Tg mice and normal littermates injected with hG-CSF (431.2 ± 76.8 and 421.4 ± 75.6 mg, respectively) compared to mice injected with serum/PBS (75.9 ± 11.4 and 79.9 ± 11.3 mg, respectively). The numbers of spleen cells increased in a parallel with spleen weights in the Tg mice and normal littermates (3.6 ± 0.8 and 3.5 ± 0.9 × 108, respectively, v serum/PBS 1.0 ± 0.2 and 1.1 ± 0.1 × 108, respectively). Histological analysis showed that the spleens of the Tg mice contained an increased number of megakaryocytes compared with normal littermates (Fig 1). The megakaryocytes in spleens of the Tg mice included various stages of maturation ranging from small immature to huge mature megakaryocytes. These results indicate that hG-CSF simulates megakaryopoiesis and thrombopoiesis in the Tg mice in vivo.
Photographs of spleens of a littermate (A) and a Tg mouse (B) injected with hG-CSF. Note increased numbers of megakaryocytes in the spleen of the Tg mouse (original magnification ×100).
Photographs of spleens of a littermate (A) and a Tg mouse (B) injected with hG-CSF. Note increased numbers of megakaryocytes in the spleen of the Tg mouse (original magnification ×100).
Effects of hG-CSF on megakaryocyte colony formation in the Tg mice.
To clarify effects of hG-CSF on megakaryopoiesis in the Tg mice, we used methylcellulose clonogenic assay of BM cells derived from Tg mice and normal littermates. hG-CSF significantly stimulated Mk and M-Mix colonies from BM cells of the Tg mice but not from littermates (Table2). In the Tg mice, hG-CSF supported a larger number of Mk and M-Mix colonies than mIL-3, hTPO, or hIL-6. mIL-3, hTPO, or hIL-6 showed the same effects on Mk and M-Mix colonies in both the Tg mice and their normal littermates. While a maximal effect on Mk colony formation was seen with 20 ng/mL of hG-CSF in the Tg mice (data not shown), no Mk colonies were observed at concentrations of up to 100 ng/mL in the case of littermates. To assess the accuracy of in situ identification of Mk colonies (Fig2A), cytospin preparations of individual colonies were stained with AChE. All Mk colonies consisted of AChE-positive megakaryocytes (Fig 2B).
Effects of hG-CSF on Colony Formation of BM Cells
| Mice . | Cytokines . | No. of Colonies (2.5 × 104 BM cells) . | |||
|---|---|---|---|---|---|
| Mk . | M-Mix . | GM . | Others . | ||
| Littermate | No factor | 0 | 0 | 0 | 0 |
| hG-CSF | 0 | 0 | 28 ± 5 | 0 | |
| mIL-3 | 2 ± 1 | 2 ± 1 | 106 ± 7 | 10 ± 2 | |
| TPO | 2 ± 1 | 1 ± 1 | 3 ± 2 | 0 | |
| hIL-6 | 1 ± 1 | 0 | 18 ± 4 | 0 | |
| Tg Mouse | No factor | 0 | 0 | 0 | 0 |
| hG-CSF | 10 ± 2 | 3 ± 1 | 61 ± 6 | 8 ± 2 | |
| mIL-3 | 2 ± 1 | 2 ± 1 | 111 ± 1 | 9 ± 1 | |
| TPO | 2 ± 1 | 1 ± 1 | 4 ± 1 | 0 | |
| hIL-6 | 1 ± 1 | 0 | 21 ± 4 | 0 | |
| Mice . | Cytokines . | No. of Colonies (2.5 × 104 BM cells) . | |||
|---|---|---|---|---|---|
| Mk . | M-Mix . | GM . | Others . | ||
| Littermate | No factor | 0 | 0 | 0 | 0 |
| hG-CSF | 0 | 0 | 28 ± 5 | 0 | |
| mIL-3 | 2 ± 1 | 2 ± 1 | 106 ± 7 | 10 ± 2 | |
| TPO | 2 ± 1 | 1 ± 1 | 3 ± 2 | 0 | |
| hIL-6 | 1 ± 1 | 0 | 18 ± 4 | 0 | |
| Tg Mouse | No factor | 0 | 0 | 0 | 0 |
| hG-CSF | 10 ± 2 | 3 ± 1 | 61 ± 6 | 8 ± 2 | |
| mIL-3 | 2 ± 1 | 2 ± 1 | 111 ± 1 | 9 ± 1 | |
| TPO | 2 ± 1 | 1 ± 1 | 4 ± 1 | 0 | |
| hIL-6 | 1 ± 1 | 0 | 21 ± 4 | 0 | |
BM cells (2.5 × 104) were incubated in the presence of hG-CSF (20 ng/mL), mIL-3 (10 ng/mL), TPO (20 ng/mL), or hIL-6 (100 ng/mL). Values are mean ± SD of triplicate plates.
Abbreviations: Mk, megakaryocyte colonies; M-Mix, mixed hematopoietic colonies containing megakaryocytes; GM, granulocyte-macrophage colonies; Others, including blast-cell colonies and mast-cell colonies.
(A) In situ appearance of a representative Mk colony induced by 20 ng/mL of hG-CSF from BM cells of hG-CSFR-Tg mice (original magnification ×200). (B) AChE-positive megakaryocytes in the Mk colony (original magnification ×400).
(A) In situ appearance of a representative Mk colony induced by 20 ng/mL of hG-CSF from BM cells of hG-CSFR-Tg mice (original magnification ×200). (B) AChE-positive megakaryocytes in the Mk colony (original magnification ×400).
Colony formation from clone-sorted Lin−c-Kit+Sca-1+/−cells.
In the Tg mice, accessory cells expressing hG-CSFR may also be responsive to hG-CSF and produce various cytokines which in turn induce growth of megakaryopoietic progenitors. To exclude this possibility, we sorted Lin−c-Kit+Sca-1+ and Lin−c-Kit+Sca-1− cells, which have been shown to reflect murine primitive hematopoietic progenitors and more mature populations, respectively,24 from BM cells of the Tg mice and normal littermates. Because it was reported that only some of primitive hematopoietic cells expressed Sca-1 antigen in C3H/HeN,25 we used BM cells of (C57BL/6 × C3H/HeN) F1 backgrounds as the source of sorted cells. The single-sorted cells were cultured with hG-CSF, or three megakaryopoietic cytokines, hIL-6, mIL-3, and hTPO, and the colony formation was compared (Table 3). hIL-6, mIL-3, and hTPO had the same effects on Mk colony formation on sorted cells derived from the Tg mice and normal littermates. In both, hIL-6 supported no or only a few Mk colonies, mIL-3 supported Mk colonies from both Lin−c-Kit+Sca-1+ and Lin−c-Kit+Sca-1− cells, and hTPO supported Mk colonies from only Lin−c-Kit+Sca-1− cells. Conversely, hG-CSF supported Mk colonies from both fractions (Sca-1+/Sca-1−) of Tg mice, whereas Mk colonies were never observed in response to G-CSF in normal littermates. The number of Mk colonies induced by hG-CSF was larger than that by mIL-3 in the Tg mice. These results confirmed the direct effect of hG-CSF on megakaryocytic progenitors of Tg mice and further indicate that hG-CSF has differential effects on Mk colony formation compared with those other megakaryopoietic cytokines.
Colony Formation From Clone-Sorted Lin−c-Kit+Sca-1+/−Cells
| Mice . | Cytokines . | Sorted Cells . | No. of Sorted Cells . | No. of Colonies . | |||
|---|---|---|---|---|---|---|---|
| Mk . | M-Mix . | GM . | Others . | ||||
| Littermates | hG-CSF | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 16 | 0 |
| Sca-1− | 384 | 0 | 0 | 78 | 0 | ||
| mIL-3 | Lin−c-Kit+Sca-1+ | 384 | 4 | 26 | 140 | 56 | |
| Sca-1− | 384 | 14 | 12 | 168 | 10 | ||
| TPO | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 5 | 0 | |
| Sca-1− | 384 | 20 | 0 | 0 | 0 | ||
| hIL-6 | Lin−c-Kit+Sca-1+ | 384 | 1 | 0 | 38 | 0 | |
| Sca-1− | 384 | 0 | 0 | 38 | 0 | ||
| Tg mice | hG-CSF | Lin−c-Kit+Sca-1+ | 384 | 20 | 18 | 117 | 54 |
| Sca-1− | 384 | 14 | 5 | 127 | 12 | ||
| mIL-3 | Lin−c-Kit+Sca-1+ | 384 | 6 | 24 | 148 | 59 | |
| Sca-1− | 384 | 13 | 13 | 153 | 10 | ||
| TPO | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 4 | 0 | |
| Sca-1− | 384 | 22 | 0 | 0 | 0 | ||
| hIL-6 | Lin−c-Kit+Sca-1+ | 384 | 1 | 0 | 36 | 0 | |
| Sca-1− | 384 | 0 | 0 | 38 | 0 | ||
| Mice . | Cytokines . | Sorted Cells . | No. of Sorted Cells . | No. of Colonies . | |||
|---|---|---|---|---|---|---|---|
| Mk . | M-Mix . | GM . | Others . | ||||
| Littermates | hG-CSF | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 16 | 0 |
| Sca-1− | 384 | 0 | 0 | 78 | 0 | ||
| mIL-3 | Lin−c-Kit+Sca-1+ | 384 | 4 | 26 | 140 | 56 | |
| Sca-1− | 384 | 14 | 12 | 168 | 10 | ||
| TPO | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 5 | 0 | |
| Sca-1− | 384 | 20 | 0 | 0 | 0 | ||
| hIL-6 | Lin−c-Kit+Sca-1+ | 384 | 1 | 0 | 38 | 0 | |
| Sca-1− | 384 | 0 | 0 | 38 | 0 | ||
| Tg mice | hG-CSF | Lin−c-Kit+Sca-1+ | 384 | 20 | 18 | 117 | 54 |
| Sca-1− | 384 | 14 | 5 | 127 | 12 | ||
| mIL-3 | Lin−c-Kit+Sca-1+ | 384 | 6 | 24 | 148 | 59 | |
| Sca-1− | 384 | 13 | 13 | 153 | 10 | ||
| TPO | Lin−c-Kit+Sca-1+ | 384 | 0 | 0 | 4 | 0 | |
| Sca-1− | 384 | 22 | 0 | 0 | 0 | ||
| hIL-6 | Lin−c-Kit+Sca-1+ | 384 | 1 | 0 | 36 | 0 | |
| Sca-1− | 384 | 0 | 0 | 38 | 0 | ||
Lin−c-Kit+Sca-1+/− cells were sorted from BM of hG-CSFR-Tg mice and littermates, and cultured for 8 days in the presence of hG-CSF (20 ng/mL), mIL-3 (10 ng/mL), TPO (20 ng/mL) or hIL-6 (100 ng/mL).
Abbreviations: Mk, megakaryocyte colonies; M-Mix, mixed hematopoietic colonies containing megakaryocytes; GM, granulocyte-macrophage colonies; Others, including blast cell colonies and mast cell colonies.
Effect of hG-CSF on megakaryocyte maturation.
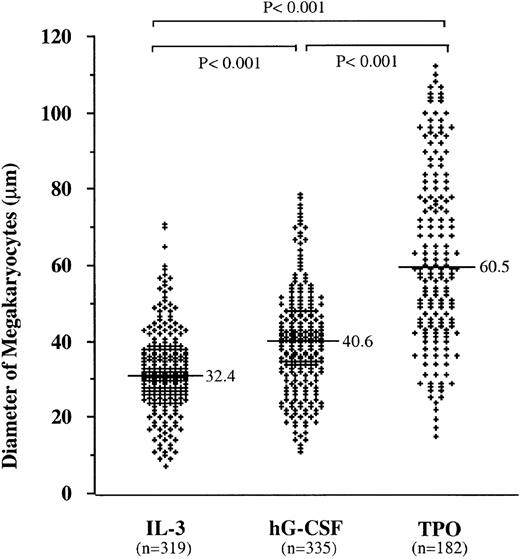
We next examined the effect of hG-CSF on megakaryocyte maturation. Because of the simultaneous presence of a large number of myelocytic cells stimulated by hG-CSF in the culture system, it was not feasible to analyze the ploidy of megakaryocytes by flow cytometry. Therefore, the size of megakaryocytes was used to evaluate megakaryocyte maturation, since the size achieved by each megakaryocyte is proportional to its ploidy.26 On day 5 of methylcellulose culture of BM cells from the Tg mice or normal littermates, Mk colonies were pooled from 120 plates containing hG-CSF, 120 plates containing mIL-3, and 150 plates containing hTPO, then processed for measurement of the size of constituent megakaryocytes. As shown in Fig3, hG-CSF induced larger megakaryocytes compared to mIL-3, although the size was smaller than that stimulated with hTPO (P < .001). Therefore, it appears that hG-CSF stimulates not only the proliferation of megakaryocytic progenitors but also the maturation of megakaryocytes in the Tg mice.
Effects of mIL-3, hG-CSF, and hTPO on megakaryocyte size in Mk colonies. Three independent experiments were analyzed to generate this figure. The horizontal bars in each group represent the mean perpendicular diameters of megakaryocytes in Mk colonies. The numbers of measured megakaryocytes are presented in parentheses. The megakaryocytes from Mk colonies supported by hG-CSF were significantly larger than those in case of mIL-3, but smaller than those in case of hTPO (P < .001, for both).
Effects of mIL-3, hG-CSF, and hTPO on megakaryocyte size in Mk colonies. Three independent experiments were analyzed to generate this figure. The horizontal bars in each group represent the mean perpendicular diameters of megakaryocytes in Mk colonies. The numbers of measured megakaryocytes are presented in parentheses. The megakaryocytes from Mk colonies supported by hG-CSF were significantly larger than those in case of mIL-3, but smaller than those in case of hTPO (P < .001, for both).
Effect of hG-CSF on platelet aggregation.
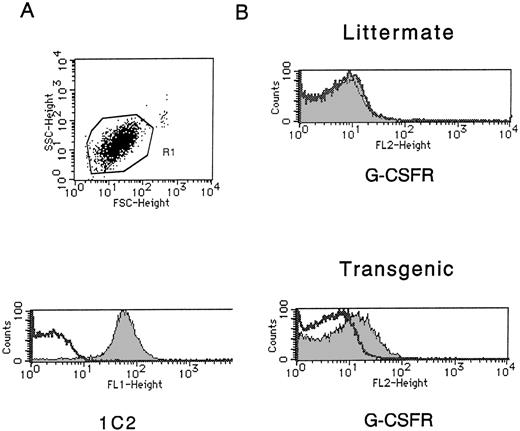
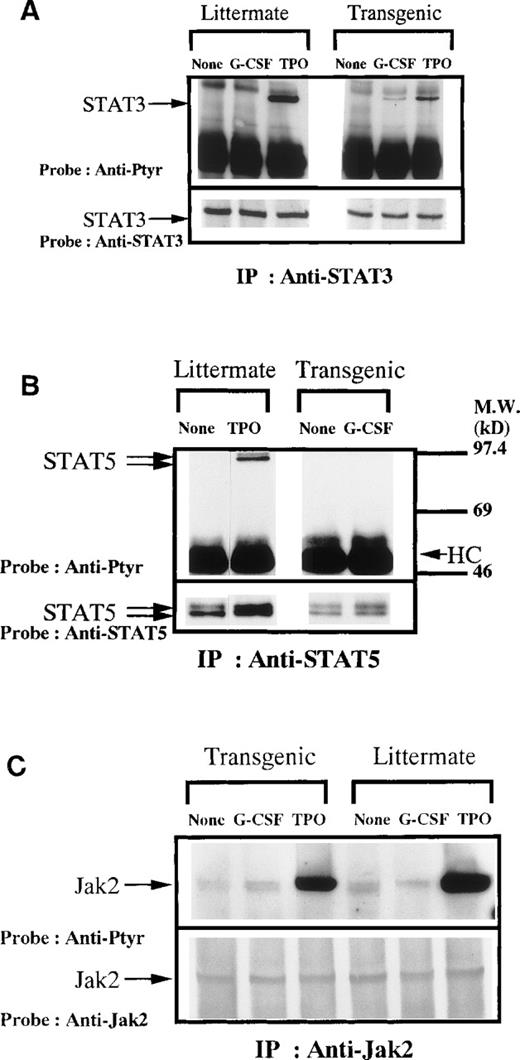
We examined the expression of hG-CSFR on platelets using PE-conjugated hG-CSF. Platelets in PRP from both the Tg mice and normal littermates were confirmed by staining with 1C2, an antibody reacting with mouse platelets (Fig 4A). As shown in Fig 4B, hG-CSF bound to the platelets of the Tg mice, but not normal littermates, indicating the expression of hG-CSFR on platelets of the Tg mice. We next compared the effect of hG-CSF with that of hTPO on platelet aggregation induced by ADP in the Tg mice, since we earlier reported that hTPO enhances human platelet functions.23 As shown in Fig 5, the addition of hG-CSF to PPACK PRP did not enhance the aggregation of murine platelets induced by ADP, while hTPO enhanced this aggregation, as was observed in human platelets. Phosphorylation of STAT3 and STAT5 (Fig6) showed that hTPO stimulated tyrosine phosphorylation of STAT3, STAT5, and Jak2 in platelets from both the Tg mice and normal littermates, which are consistent with our previous data on human platelets. In the Tg mice, however, STAT3 but not STAT5 was modestly phosphorylated in platelets stimulated by hG-CSF, while hG-CSF did not induce tyrosine phosphorylation of either STAT3 or STAT5 in littermates. Thus, platelets of the Tg mice express functional hG-CSFR but hG-CSFR-mediated signals and their biological effects differ from those of the c-Mpl, the TPO receptor, in the Tg mouse platelets.
The expression of hG-CSFR on platelets of the Tg mice (the unshaded areas represent isotype control, the shaded areas represent specific staining). (A) Platelets gated in R1 of the Tg mice were stained with 1C2, an anti-mouse platelet antibody. The same finding was obtained with platelets of normal littermates. (B) PE-conjugated hG-CSF bound to platelets of the Tg mice, but not littermates.
The expression of hG-CSFR on platelets of the Tg mice (the unshaded areas represent isotype control, the shaded areas represent specific staining). (A) Platelets gated in R1 of the Tg mice were stained with 1C2, an anti-mouse platelet antibody. The same finding was obtained with platelets of normal littermates. (B) PE-conjugated hG-CSF bound to platelets of the Tg mice, but not littermates.
hTPO and hG-CSF were diluted in autologous platelet-poor plasma (PPP, final concentration 10 μg/mL). PRP from Tg mice was diluted with PPP to adjust the platelet concentration at 2.0 × 108/mL. hTPO or hG-CSF was added 5 minutes before adding ADP (1 mmol/L).
hTPO and hG-CSF were diluted in autologous platelet-poor plasma (PPP, final concentration 10 μg/mL). PRP from Tg mice was diluted with PPP to adjust the platelet concentration at 2.0 × 108/mL. hTPO or hG-CSF was added 5 minutes before adding ADP (1 mmol/L).
(A) Tyrosine phosphorylation of STAT3. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before or 5 minutes after stimulation with hTPO (100 ng/mL) or hG-CSF for 5 minutes. STAT3 was immunoprecipitated from 6.0 × 108 platelets from littermate mice or 2.0 × 108 platelets from Tg mice. Immune complexes were resuspended in SDS-sample buffer and divided into two. Proteins were separated by 15 to 7.5% SDS-PAGE and transferred onto PVDF membranes. One immunoblot was probed with anti-phosphotyrosine antibodies and bands were visualized by chemiluminescence (top). The other blot was probed for STAT3 (bottom). The arrows indicate the bands of interest. (B) Tyrosine phosphorylation of STAT5. The same as in (A) except the combination of STAT5A and B antisera were used instead of STAT3 antisera. (C) Tyrosine phosphorylation of Jak2. The same as in (B) except the combination of an anti-Jak2 polyclonal antibody was used instead of STAT5 antisera.
(A) Tyrosine phosphorylation of STAT3. Platelets were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before or 5 minutes after stimulation with hTPO (100 ng/mL) or hG-CSF for 5 minutes. STAT3 was immunoprecipitated from 6.0 × 108 platelets from littermate mice or 2.0 × 108 platelets from Tg mice. Immune complexes were resuspended in SDS-sample buffer and divided into two. Proteins were separated by 15 to 7.5% SDS-PAGE and transferred onto PVDF membranes. One immunoblot was probed with anti-phosphotyrosine antibodies and bands were visualized by chemiluminescence (top). The other blot was probed for STAT3 (bottom). The arrows indicate the bands of interest. (B) Tyrosine phosphorylation of STAT5. The same as in (A) except the combination of STAT5A and B antisera were used instead of STAT3 antisera. (C) Tyrosine phosphorylation of Jak2. The same as in (B) except the combination of an anti-Jak2 polyclonal antibody was used instead of STAT5 antisera.
DISCUSSION
Accumulating data were shown that G-CSF specifically regulates the proliferation and differentiation of myelocytic progenitor cells.27 However, the ectopic expression of the G-CSFR in hematopoietic progenitor cells has also been shown to allow for G-CSF–dependent multilineage differentiation.28 We also demonstrated that hG-CSF supports the development of various types of hematopoietic progenitors, including Mast, Mix, and Mk other than GM progenitors in hG-CSFR-Tg mice.10 These reports indicate that hG-CSF can stimulate megakaryopoiesis if hG-CSFR is expressed on cells of megakaryocytic lineage.
In the present study, we extended these observations to determine more precisely the effects of hG-CSF on megakaryopoiesis. Evidence for the stimulatory effect of hG-CSF on megakaryopoiesis in the Tg mice was supported by findings in vivo and in vitro. The administration of hG-CSF resulted in an increase in platelets in PB and megakaryocytes in spleen of the Tg mice, indicating that hG-CSF stimulates megakaryopoiesis and thrombopoiesis in vivo. Because mouse and human G-CSF have been shown to crossreact with the receptors of both species,10,29 30 the normal platelet count in uninjected Tg mice indicates that the concentrations of endogenous mouse G-CSF are not sufficient to affect megakaryocytopoiesis in the Tg mice in vivo.
In vitro studies showed the effect of hG-CSF on megakaryopoiesis in even more detail. The effect of hG-CSF on megakaryocytic progenitors of the Tg mice was confirmed in a dose-response study of hG-CSF and single-cell cultures of sorted hematopoietic progenitors. The results clearly show that the proliferation of megakaryocytic progenitors is directly triggered by hG-CSF, without the influence of accessory cells. hG-CSF also stimulated the enlargement of megakaryocytes, reflecting megakaryocyte maturation, in the Tg mice. Thus, hG-CSF showed a wide variety of functions in megakaryopoiesis of the Tg mice.
In the single-cell culture of sorted hematopoietic progenitors, hIL-6, mIL-3, and hTPO had different effects on Mk colony formation. hIL-6, an Mk potentiator, had no activity related to Mk colony formation, and mIL-3, an Mk-CSF, supported Mk colony formation in case of both Lin−c-Kit+Sca-1+ and Lin−c-Kit+Sca-1− cells. hTPO supported Mk colony formation for only Lin−c-Kit+Sca-1− cells, suggesting that colony-forming activity of hTPO predominantly acts on relatively mature megakaryocytic progenitors. Effects of hG-CSF on Mk colony formation differed from effects of these three megakaryopoietic cytokines in the Tg mice. hG-CSF induced Mk colony formation in the cases of both Lin−c-Kit+Sca-1+and Lin−c-Kit+Sca-1− cells of the Tg mice, but the number of Mk colonies derived from Lin−c-Kit+Sca-1+ cells exceeds that seen with mIL-3. Thus, hG-CSF, as a single factor, is the most potent stimulator of Mk colony formation, although the combination of mIL-3 and hTPO supported almost the same number of Mk colonies from Lin−c-Kit+Sca-1+ cells (data not shown). Differences in activities between hG-CSF and other megakaryopoietic cytokines were also observed in case of megakaryocyte maturation. hG-CSF showed greater activity than mIL-3 in enlarging megakaryocytes, albeit the activity being weaker than that of hTPO. Because Lin−c-Kit+Sca-1+ cells are only about 10% of Lin−c-Kit+Sca-1− cells in BM (data not shown), it can be estimated that the majority of megakaryocytes cultured from BM cells by hG-CSF are derived from Lin−c-Kit+Sca-1− cells. Therefore, the difference in size of megakaryocytes supported by hG-CSF and hTPO is unlikely to be caused by the immaturity of the target progenitor population by hG-CSF.
The distinct activities of hG-CSF on megakaryocytic development may be explained by differences in the distribution between hG-CSFR and each cytokine receptor. The present study suggests that IL-6R is not expressed on murine megakaryocytic progenitors, and IL-3R is predominantly expressed on early stage of cells in murine megakaryopoiesis, mainly megakaryocytic progenitors. Although TPO acts on all stages of megakaryocytic cells, our observation shows that c-Mpl may be predominantly expressed at the late stage of cells. There is also a possibility that these receptors are present but not functional at certain stages. On the other hand, it is conceivable that hG-CSFR is expressed throughout the development of megakaryocytic cells in the Tg mice, so that hG-CSF has a wide variety of functions in the Tg mouse megakaryopoiesis.
Alternatively, hG-CSFR on megakaryocytic cells may activate different signals from those by natural receptors for megakaryopoietic cytokines, resulting in different effects on the Tg mouse megakaryopoiesis. Platelets are an excellent model system for investigating signaling induced by TPO. Platelets from patients with thrombocytopenia with absent radii have been shown to have reduced responses to exogenous TPO.31 In platelets of these patients, exogenous TPO did not induce protein tyrosine phosphorylation or prime platelet aggregation. Accordingly, we examined TPO– and G-CSF–induced proximal signaling in murine platelets. Our studies show for the first time presence of Jak2, STAT3 and STAT5 in murine platelets and the inducible tyrosine phosphorylation following stimulation by TPO. We earlier found that these signaling molecules were tyrosine phosphorylated in hTPO-stimulated platelets.9,32 Coexpression of hG-CSFR and c-Mpl on platelets of the Tg mice was shown by the phosphorylation of STAT3 in platelets stimulated by hG-CSF and hTPO, although the degrees of tyrosine phosphorylation was much less in G-CSF–stimulated platelets than in TPO-stimulated platelets. However, neither Jak2 nor STAT5 was phosphorylated in Tg mouse platelets stimulated by hG-CSF. Interestingly, Parganas et al33 have shown G-CSF signaling was still functional in Jak2-deficient mice; the hG-CSFR may be able to activate different kinases.
The difference in activities between hG-CSF and hTPO was also observed in platelet aggregation studies. hTPO but not hG-CSF enhanced the platelet aggregation induced by ADP. Because STAT3 was tyrosine phosphorylated by hG-CSF stimulation, there is a possibility that the intensity of signal activated by hG-CSF could explain the lack of response of Tg mouse platelets to hG-CSF. Drachman et al34found that TPO induced tyrosine phosphorylation of Jak2, STAT3, and STAT5 in murine mature megakaryocytes. In contrast, G-CSF may not be able to induce tyrosine phosphorylation of signaling molecules like Jak2 and STAT5 in mature megakaryocytes from the Tg mice. G-CSF, as a result, may have only weak biological effects on the late stage of megakaryocyte maturation. Shimoda et al35 reported that human platelets possessed functional G-CSFR, and G-CSF augmented ADP-induced aggregation. However, other workers could not confirm the costimulatory effects of G-CSF on human platelets,36 and the presence or absence of functional G-CSFRs on human platelets has remained controversial. If human platelets do express G-CSFR, mechanisms of aggregation between human and murine platelets may differ.
We previously reported that hG-CSF, as well as early acting cytokines such as IL-3, stimulated the proliferation of multipotential progenitor cells of the Tg mice, but had no effect on their commitment to specific hematopoietic lineages.10 Similar results have been reported from studies using murine hematopoietic cells expressing transgenes of receptors for mouse macrophage colony-stimulating factor, EPO receptor, c-Mpl, hGM-CSFR, or mIL-5R α chain-Tg mice.4,5 37-39 We propose that cytokines function as proliferation-promoting factors in multipotential progenitors and that cellular differentiation is determined by an intrinsic program. Although intracellular signal-transducing pathways of these transreceptors in murine multipotential progenitors are unknown, there may be common signals for mitosis. In the present study, hG-CSF had a wide variety of effects on Tg mouse megakaryopoiesis, but the activities cannot be compared with those of TPO with regard to megakaryocytes and platelets of the Tg mice. We suggest that specific intracellular signals may be required for full maturation of megakaryocytic progenitors committed to megakaryocytes and for activation of functions in platelets.
ACKNOWLEDGMENT
We thank Dr. David A. Williams for critical reading of the manuscript, M. Ohara for comments on the manuscript, and I. Hirose and K. Sudo for excellent technical assistance.
Supported in part by the Japan Society for the promotion of Science.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Tatsutoshi Nakahata, MD, DM Sci, Department of Clinical Oncology, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan; e-mail:nakahata@ims.u-tokyo.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal