We evaluated real-time processes of platelet thrombus formation on a collagen surface in a flow chamber with whole blood from patients with various platelet aggregation disorders, such as Bernard-Soulier syndrome (BSS), Glanzmann’s thrombasthenia (GTA), type 3 von Willebrand disease (vWD), and congenital afibrinogenemia (Af), who lack platelet glycoprotein (GP) Ib-IX complex, GP IIb-IIIa, von Willebrand factor (vWF), and fibrinogen, respectively. Blood from GTA patients showed impaired thrombus growth but significant initial platelet-surface interaction under all shear conditions tested (50 to 1,500 s−1). By contrast, blood from patients with BSS or type 3 vWD showed no platelet-surface interaction under high shear (≥1,210 s−1) but normal thrombus formation under low shear (≤340 s−1). When shear rate was increased stepwise to 1,500 s−1 during perfusion, the thrombus growth observed in type 3 vWD or BSS under low shear was arrested, whereas that in control blood was sharply accelerated as a function of shear rate. Overall thrombus formation in Af appeared indistinguishable from that of a control under shear rates between 50 and 1,500 s−1. However, Af thrombi formed under such conditions collapsed immediately when shear rate was further increased to 4,500 s−1, whereas thrombi of type 3 vWD or BSS formed under low shear were stable even when shear rate was elevated to 9,000 s−1 during perfusion. These findings suggest that distinct molecular mechanisms underlie the pathologic bleeding in these diseases and point to the distinct roles of two major adhesive proteins, vWF and fibrinogen. In mural thrombus formation under flow conditions, vWF, perhaps mainly through its interaction with GP Ib-IX, acts as an “initiator and promoter,” whereas fibrinogen, via its binding to GP IIb-IIIa, acts as a “stabilizer” against heightened shear forces that could lead to peeling off of platelets from the surface.
PLATELET PLUG FORMATION in vivo at sites of vessel wall damage is critical in ensuring blood flow to vital organs, but may also contribute to the generation of pathologic intravascular thrombosis.1-4 Many platelet membrane receptors and plasma adhesive proteins are thought to be involved in this crucial event, although the precise mechanisms involved are not fully understood, particularly under flow conditions. Indeed, most previous studies of platelet function were performed in a static or closed stirring experimental system. More recent platelet functional studies that take blood flow into consideration indicate the significant effect of shear rate on platelet aggregation mechanisms.5-7 Indeed, studies that use a cone-and-plate–type viscometer have shown that platelet aggregation induced by experimental high shear stress (≥80 dyne/cm2) in a soluble phase is a consequence of the interaction between von Willebrand factor (vWF) and platelet glycoprotein (GP) Ib-IX and GP IIb-IIIa, whereas platelet aggregation under low shear stress (6 to 12 dyne/cm2) is mediated by the interaction between fibrinogen and GP IIb-IIIa.8,9 However, the mechanisms of platelet aggregate accumulation on a thrombogenic surface (mural thrombus formation) under flow conditions, ie, mechanisms relevant for in vivo hemostatic plug formation at sites of vascular injury, may be more complex or different from those of soluble-phase shear-induced platelet aggregation. Recent studies using experimental flow systems to analyze mural thrombus formation on several types of collagen, a major component of vascular subendothelium, have demonstrated that at least four platelet receptors, GP Ib-IX, Ia-IIa, IIb-IIIa, and VI play a role in this event.10-15 Although recent studies suggest the critical role of the interaction between plasma vWF immobilized to collagen and platelet GP Ib in initial platelet adhesion to the surface,15 16 the precise mechanisms of overall mural thrombus formation on a collagen surface, especially shear-specific functions of platelet receptors and adhesive proteins, remain to be addressed.
In the present study, we observed the real-time process of mural thrombogenesis on a type I collagen-coated surface under flow conditions with various shear rates in the blood of patients with major congenital platelet aggregation disorders. Our experimental approach indicates distinct pathogenic mechanisms relevant for the life-long bleeding symptoms in these diseases and illustrates in detail the shear-specific and time-course–dependent functions of major platelet receptors and adhesive proteins in platelet thrombus formation under dynamic blood flow conditions.
MATERIALS AND METHODS
Patient profiles.
Two unrelated patients with Bernard-Soulier syndrome (BSS) fulfilled the criteria of this disease, namely, giant platelets and mild thrombocytopenia (120 × 103/μL and 160 × 103/μL, respectively), with a typical lack of ristocetin- (1.2 mg/mL) induced platelet aggregation. Flow cytometric analysis confirmed no measurable α-chain of GP Ib, with trace amounts of GP IX on the surface of platelets from these patients. Blood of a patient with May-Hegglin anormaly, whose platelets were similar to those of the BSS patients in population size and counts, served as a control for BSS.
Three independent Glanzmann’s thrombasthenia (GTA) patients were all categorized as type I GTA with a defect of clot retraction, complete lack of platelet aggregation by adenosine diphosphate or collagen, and normal platelet counts (200 to 350 × 103/μL), on the surface of which only 1% to 2.2% of GP IIb-IIIa complex were expressed, as judged by flow cytometry. Normal plasma levels of vWF (75% to 130%) and fibrinogen (250 to 350 mg/dL) were confirmed in patients with BSS and GTA by enzyme-linked immunosorbent assay for vWF and electroimmunoassay for fibrinogen, respectively.17-19
One patient with type 3 von Willebrand disease (vWD) had no detectable vWF antigen in plasma or platelets, as confirmed by multimer analysis with sodium dodecyl sulfate (SDS) (1.5%) agarose gel electrophoresis.20 21 Thus, patient exhibited complete lack of ristocetin-induced platelet aggregation, with normal ranges of platelet counts and plasma fibrinogen.
Plasma fibrinogen levels of two unrelated afibrinogenemia (Af) patients were below the detection limit by electroimmunoassay and no significant fibrinogen in their platelets was confirmed by SDS-polyacrylamide gel electrophoresis, followed by immunoblotting. These Af patients showed complete lack of platelet aggregation by adenosine diphosphate or collagen, with normal platelet counts and plasma vWF antigen levels. In all patients with congenital platelet aggregation disorders, bleeding time was markedly prolonged (>20 minutes). Thrombus formation in 10 nonsmoking healthy volunteers, who were not taking any medications for the previous 2 weeks and whose platelet counts were all between 200 to 350 × 103/μL, was analyzed as a normal control. Blood donors, including patients in this study, showed no significant anemia, with hematocrit values always greater than 35%.
Blood collection and platelet labeling.
Blood was collected by using the specific thrombin inhibitor argatroban (MD-805, final concentration 0.125 mg/mL; Mitsubishi Chemical, Corp, Tokyo, Japan) as an anticoagulant to maintain physiologic concentrations of divalent cations in blood. This argatroban concentration was used to ensure elimination of a possible thrombin generation during the entire processes of platelet thrombogenesis. Indeed, preliminary experiments confirmed no observable fibrin clot formation for at least 3 hours after blood collection at this argatroban concentration. Anticoagulated whole blood was kept at 37°C and used in perfusion studies within 1 hour after blood collection. Before perfusion, the fluorescent dye mepacrine (quinacrine dihydrochloride; final concentration 0.01 mmol/L, Sigma-Aldrich Co, Tokyo, Japan), was added to the blood to label platelets, allowing visualization of platelet-surface interaction with epifluorescence videomicroscopy. At the concentration used, mepacrine, which immediately accumulates in the dense granules of platelets, does not interfere with normal platelet function.13,16 22
Preparation of collagen solution and collagen-coated coverslips.
Suspensions of type I acid-insoluble collagen fibrils (2.1 mg/mL) were prepared from bovine Achilles tendon (Sigma-Aldrich Co, Tokyo, Japan) in 0.5 mol/L acetic acid (pH 2.8) as described.13,22 23Glass coverslips (24 × 50 mm; Matsunami Glass Co Ltd, Japan) were coated with 200 μL of the collagen solution, placed in a humid environment for 60 minutes, rinsed with 10 mL of 50 mmol/L phosphate buffered saline (pH 7.35) to remove nonadherent collagen, and placed in a flow chamber (see below).
Flow chamber and epifluorescence videomicroscopy.
A flow chamber that varies shear rate in a linear manner was assembled and mounted on a microscope (BX60; Olympus Co, Tokyo, Japan) equipped for epifluorescent illumination (BX-FLA; Olympus Co) and charge-coupled device (CCD) camera system (U-VPT-N; Olympus Co) as described.22-24 A whole blood sample was aspirated through the flow chamber and across the collagen-coated coverslip at a constant flow rate via a syringe pump (Model 935; Harvard Apparatus, South Natick, MA). Unless otherwise indicated, the entire thrombus generation process, from initial platelet-surface interaction to platelet aggregate accumulation on the surface, was observed in real time at positions of the flow chamber corresponding to 50 s−1 and 1,500 s−1 and recorded with a video cassette recorder (Hi8 VIEWCAM; Sharp Co, Ltd, Osaka, Japan). These two shear rates were selected to represent a typical low and high shear rate, respectively, based on recent studies indicating that platelet functions and the mechanisms of mural thrombus formation under these two shear rates differ from each other.16,22 23
Evaluation of platelet thrombi generated on the surface.
Time-course images of thrombus formation in a videotape were digitized by a frame grabber (DIG98; DITECT Co, Tokyo, Japan) and subjected to computer-assisted analysis with an image processing application (Win ROOF; Mitani Corp, Fukui, Japan). This program allows evaluation of platelet thrombi generated on a surface at a defined area in each image. First, the average fluorescence intensity corresponding to a single platelet was calculated in each perfusion, with the image representing the earliest period of platelet-surface interaction. At this phase, platelets are not yet visibly cohered on the surface and are clearly distinguishable from one another. This value was used to set the threshold for background subtraction from each image to standardize image intensity. Platelet thrombi generated on the surface in time-course images obtained in the analogous perfusion were then evaluated based on the integration patterns of fluorescence (fluorescence intensity multiplied by the area). Total fluorescence of platelet thrombi in each image, expressed as an arbitrary “pixel unit,” is the sum of fluorescence of each thrombus present in a defined area in each image. Although this value may not directly represent the actual number of adherent platelets, it does reflect at least the extent of platelet thrombus generation on the surface and can be used for relative comparison of thrombus growth among various diseases.
Evaluation of the extent of platelet immobilization to the surface.
The extent of platelet immobilization to the surface was evaluated in frames at a defined observation period by using the method of Savage et al,16 with minor modifications. Consecutive images with 0.033-s intervals for 2 s (total 60 frames) were digitized and binarized after subtracting background as described above. The overlapping area of platelets (the logical AND) of the initial two consecutive frames was derived by superimposing, and the next logical AND of the initial three consecutive frames was also calculated with Win ROOF computer software (Mitani). This process was repeated for a total of 60 frames. The value of logical AND is expected to decrease as a function of time when platelets attached to the surface are reversibly adhered and moving and to remain unchanged when platelets are firmly immobilized. Thus, a “platelet mobility index,” calculated as (1 − each logical AND/total area value of platelets in the first frame) × 100, was used to express the extent of platelet immobilization to the surface during a defined observation period. According to this formula, the platelet mobility index must equal 100 when all platelets in the first frame move by a distance greater than its diameter and 0 when all platelets in the first frame are firmly immobilized to the surface. Although this experimental approach may not follow the precise movement of an individual platelet on the surface, especially when platelet cohesion is extensive at late stages of perfusion, this index does reflect the extent of platelet immobilization on the surface.
RESULTS
Thrombus generation on a collagen-coated surface by normal control blood perfused under low or high shear rate.
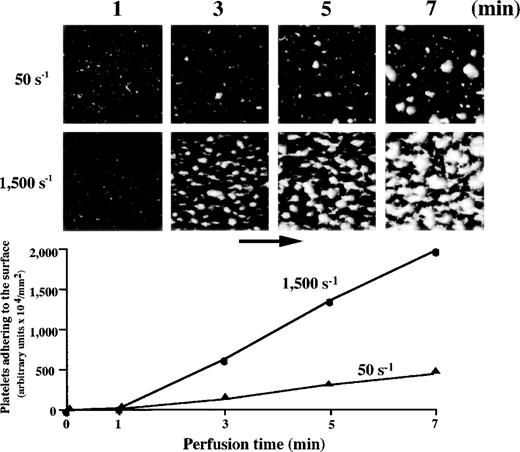
Platelets were gradually immobilized to the surface at either 50 s−1 or 1,500 s−1. The image obtained at 1 minute after the beginning of perfusion, taken as an early phase of platelet-surface interaction, represents “platelet adhesion” in which platelets were adhered to the surface superficially, although small platelet aggregates were already observed in some areas (Fig1). At either 50 s−1 or 1,500 s−1, platelets adhering to the surface gradually assembled to form mural thrombi as a function of time, so that the image obtained at 7 minutes of perfusion, taken as a late phase of platelet-surface interaction, represents “thrombus growth” (Fig1). Mural thrombi formed under high shear, however, tended to cover more surface area than those under low shear. Consistent with the platelet surface coverage visually recognized, the amount of platelets adhering to the surface evaluated by computer-assisted analysis increased in a time-dependent manner (Fig 1).
Thrombus generation on a collagen-coated surface by normal control blood perfused under high or low shear rate. Upper panels: time-course images (taken at 1, 3, 5, and 7 minutes of perfusion) of platelet-surface interaction at 50 s−1 and 1,500 s−1, displayed as accumulated fluorescence, are representative of 10 independent perfusions with blood from 10 individual donors. At either shear rate, the images at 1 minute after the beginning of perfusion indicate superficial platelet adhesion. Platelets adhering to the surface gradually assembled to form mural thrombi as a function of time. Mural thrombi formed under a shear rate of 1,500 s−1 appear to cover much more surface area than those found under 50 s−1. Lower panel: computer-assisted evaluation of amount of platelets adhering to the surface, corresponding to the images displayed above.
Thrombus generation on a collagen-coated surface by normal control blood perfused under high or low shear rate. Upper panels: time-course images (taken at 1, 3, 5, and 7 minutes of perfusion) of platelet-surface interaction at 50 s−1 and 1,500 s−1, displayed as accumulated fluorescence, are representative of 10 independent perfusions with blood from 10 individual donors. At either shear rate, the images at 1 minute after the beginning of perfusion indicate superficial platelet adhesion. Platelets adhering to the surface gradually assembled to form mural thrombi as a function of time. Mural thrombi formed under a shear rate of 1,500 s−1 appear to cover much more surface area than those found under 50 s−1. Lower panel: computer-assisted evaluation of amount of platelets adhering to the surface, corresponding to the images displayed above.
Thrombus generation on a collagen-coated surface by blood from patients with various congenital platelet aggregation disorders perfused under low or high shear rate.
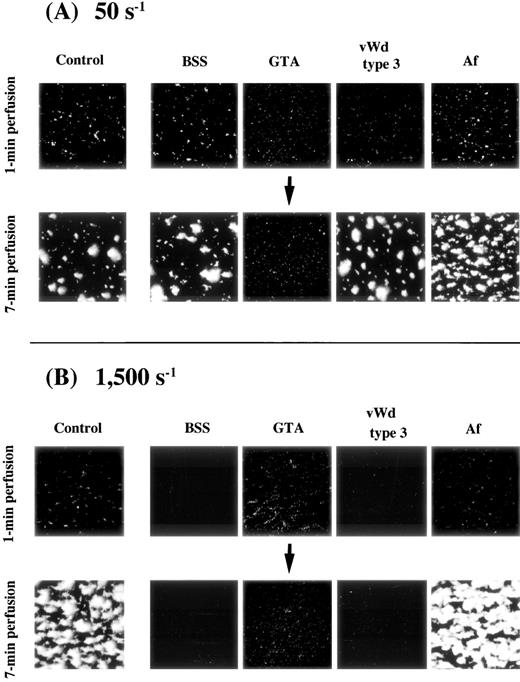
In blood perfusion of BSS, type 3 vWD, and Af under a shear rate of 50 s−1, the extent of initial platelet-surface interaction, as judged by the images and based on the values of platelets adhering to the surface at 1 minute after the beginning of perfusion, as well as thrombus growth, as judged by the results at 7 minutes of perfusion, were comparable with those of a normal control (Fig 2A and Table 1). In perfusion of GTA blood, significant initial platelet interaction comparable with that of the control was observed, but the results at 7 minutes of perfusion remained nearly unchanged from those at 1 minute (Fig 2A and Table 1), indicating the lack of thrombus growth in this disease.
Thrombus generation on a collagen-coated surface by blood from patients with various congenital platelet aggregation disorders perfused under (A) low or (B) high shear rate. BSS, Bernard-Soulier syndrome; GTA, Glanzmann’s thrombasthenia; vWD, von Willebrand disease; Af, congenital afibrinogenemia. Images were taken at 1 minute and 7 minutes after the beginning of perfusion of blood from one patient with each disease (numbered as “−1”; see Table 1); control images are identical to those in Fig 1. (A) Note the nearly unchanged image of thrombus formation in GTA blood at 7 minutes of perfusion; all other images at both 1 minute and 7 minutes of perfusion are comparable with the control. (B) Images correspond to blood of patients in (A). Note the absence of platelet-surface interaction in BSS and type 3 vWD even at 7 minutes of perfusion. Note also that the extent of platelet-surface interaction in GTA blood is comparable with that of the normal control at 1 minute of perfusion, while thrombus growth is absent in the GTA patient even at 7 minutes of perfusion.
Thrombus generation on a collagen-coated surface by blood from patients with various congenital platelet aggregation disorders perfused under (A) low or (B) high shear rate. BSS, Bernard-Soulier syndrome; GTA, Glanzmann’s thrombasthenia; vWD, von Willebrand disease; Af, congenital afibrinogenemia. Images were taken at 1 minute and 7 minutes after the beginning of perfusion of blood from one patient with each disease (numbered as “−1”; see Table 1); control images are identical to those in Fig 1. (A) Note the nearly unchanged image of thrombus formation in GTA blood at 7 minutes of perfusion; all other images at both 1 minute and 7 minutes of perfusion are comparable with the control. (B) Images correspond to blood of patients in (A). Note the absence of platelet-surface interaction in BSS and type 3 vWD even at 7 minutes of perfusion. Note also that the extent of platelet-surface interaction in GTA blood is comparable with that of the normal control at 1 minute of perfusion, while thrombus growth is absent in the GTA patient even at 7 minutes of perfusion.
Evaluation of Platelets Adhering to a Collagen-Coated Surface at 1 Minute and 7 Minutes of Perfusion Under Low or High Shear Rate Conditions in Various Congenital Platelet Aggregation Disorders
| Patients . | Perfusion Time: . | Low Shear (50 s−1) . | High Shear (1,500 s−1) . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | → . | 7 . | 1 . | → . | 7 (minutes) . | ||
| BSS-1 | 36.2* | 437 | 1 | 2.1 | |||
| BSS-2 | 49.7 | 417.3 | 0.8 | 2 | |||
| GTA-1 | 15.3 | 12.9 | 16.5 | 24.9 | |||
| GTA-2 | 29.6 | 33.7 | 33.2 | 35.9 | |||
| GTA-3 | 12.3 | 13.5 | 11.8 | 12 | |||
| vWD type 3 | 29.8 | 465 | 1.6 | 1.5 | |||
| Af-1 | 24.2 | 316.1 | 39.3 | 3006.2 | |||
| Af-2 | 43 | 285.2 | 37.4 | 2522 | |||
| Control (n = 10) | 22.2 ± 14.3 | 368.6 ± 108.5 | 30.9 ± 20.4 | 2278.4 ± 544.5 | |||
| Patients . | Perfusion Time: . | Low Shear (50 s−1) . | High Shear (1,500 s−1) . | ||||
|---|---|---|---|---|---|---|---|
| 1 . | → . | 7 . | 1 . | → . | 7 (minutes) . | ||
| BSS-1 | 36.2* | 437 | 1 | 2.1 | |||
| BSS-2 | 49.7 | 417.3 | 0.8 | 2 | |||
| GTA-1 | 15.3 | 12.9 | 16.5 | 24.9 | |||
| GTA-2 | 29.6 | 33.7 | 33.2 | 35.9 | |||
| GTA-3 | 12.3 | 13.5 | 11.8 | 12 | |||
| vWD type 3 | 29.8 | 465 | 1.6 | 1.5 | |||
| Af-1 | 24.2 | 316.1 | 39.3 | 3006.2 | |||
| Af-2 | 43 | 285.2 | 37.4 | 2522 | |||
| Control (n = 10) | 22.2 ± 14.3 | 368.6 ± 108.5 | 30.9 ± 20.4 | 2278.4 ± 544.5 | |||
The amount of platelets adhering to the surface (arbitrary units × 104/mm2) derived from the image obtained by each single perfusion. Control values represent mean ± SD of 10 independent perfusions.
In blood perfusion of BSS and type 3 vWD under a shear rate of 1,500 s−1, no platelet-surface interaction was observed even at 7 minutes of perfusion (Fig 2B and Table 1). In the case of BSS, this result is assumed to reflect the lack of GP Ib and not the presence of giant platelets or mild thrombocytopenia, because thrombus growth in blood of a May-Hegglin patient, whose platelets were similar to those of BSS patients in population size and counts, was comparable with normal under such flow conditions (data not shown). In contrast, thrombus generation in Af blood appeared indistinguishable from that of the control (Fig 2B and Table 1). In the case of GTA blood, the initial platelet-surface interaction appeared comparable with that of the control, although thrombus growth was undetectable at 7 minutes of perfusion (Fig 2B and Table 1).
Shear-dependency of thrombus generation on a collagen-coated surface in BSS, GTA, and type 3 vWD.
To further confirm the discrepant thrombus generation at high versus low shear rate conditions in BSS and type 3 vWD, the amount of platelets adhering to the surface at 7 minutes of perfusion was analyzed at various shear rates. In the control, the amount of platelet adhering to the surface basically increased as a function of shear rate, whereas maximal values at 340 s−1 in BSS and type 3 vWD decreased as a function of shear rate, and no platelet adhesion was detected at shear rates greater than 1,210 s−1 (Fig3). No thrombus growth was detected under any shear rates examined for GTA blood (Fig 3).
Amount of platelets adhering to a collagen-coated surface in BSS, GTA, and type 3 vWD at various shear rates. Data represent the amount of adherent platelets on the surface, expressed as an arbitrary units, at 7 minutes of perfusion of blood from each patient (numbered as “−1”; see Table 1) with BSS (○), type 3 vWD (▿), and GTA (▵) at the indicated shear rates. In BSS and type 3 vWD, the maxmal value at 340 s−1 decreased as a function of shear rate, with no detectable platelet adhesion at shear rates greater than 1,210 s−1, whereas adhesion in control blood (•) increased as a function of shear rate. Thrombus growth in GTA blood was absent at all shear rates.
Amount of platelets adhering to a collagen-coated surface in BSS, GTA, and type 3 vWD at various shear rates. Data represent the amount of adherent platelets on the surface, expressed as an arbitrary units, at 7 minutes of perfusion of blood from each patient (numbered as “−1”; see Table 1) with BSS (○), type 3 vWD (▿), and GTA (▵) at the indicated shear rates. In BSS and type 3 vWD, the maxmal value at 340 s−1 decreased as a function of shear rate, with no detectable platelet adhesion at shear rates greater than 1,210 s−1, whereas adhesion in control blood (•) increased as a function of shear rate. Thrombus growth in GTA blood was absent at all shear rates.
Evaluation of the extent of platelet immobilization to a collagen-coated surface in GTA.
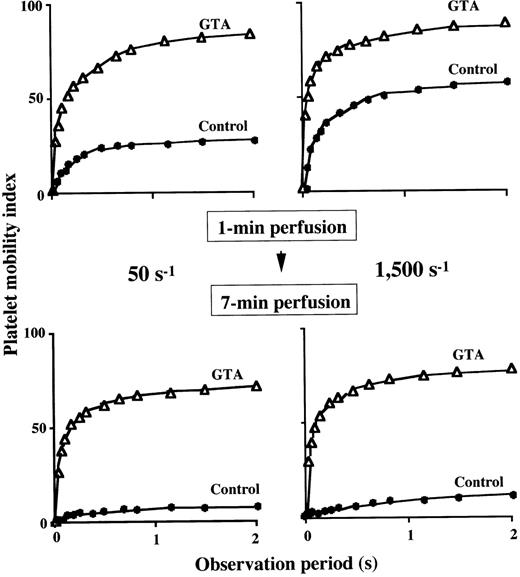
To characterize in detail the impaired thrombus growth in GTA blood under all the shear rates examined, we evaluated the extent of platelet immobilization to a collagen surface. Under a shear rate of 1,500 s−1, the platelet mobility index with a plateau value of about 60 at 1 minute of perfusion in the control decreased to 12 at 7 minutes of perfusion (Fig 4), suggesting that firm platelet adhesion progressed as a function of perfusion time. In contrast, the plateau value of the index in GTA blood at 1 minute of perfusion (86) decreased only slightly at 7 minutes of perfusion (77) (Fig 4), indicating the reversible interaction of GTA platelets with the surface even at a late stage of perfusion. A similar tendency was also observed under a shear rate of 50 s−1 (Fig 4), indicating that firm adhesion of GTA platelets was incomplete even under flow conditions insufficient to peel off platelets from the surface.
Extent of platelet immobilization to a collagen-coated surface in GTA. The platelet mobility index (Materials and Methods) during 2-second observation period was calculated at 1 minute (upper panels) and 7 minutes (lower panels) of perfusion of blood from a patient (numbered as “−1”) with GTA (▵) under a shear rate of 50 s−1 (left panels) and 1,500 s−1 (right panels). At either shear rate, the relatively high platelet mobility index of a normal control (•) at 1 minute of perfusion decreased significantly at 7 minutes of perfusion, indicating that firm platelet adhesion progressed as a function of time. Note that the platelet mobility index for GTA, which was higher than that of normal at 1 minute of perfusion, decreased only slightly at 7 minutes of perfusion, indicating only limited firm adhesion of platelets in GTA during perfusion.
Extent of platelet immobilization to a collagen-coated surface in GTA. The platelet mobility index (Materials and Methods) during 2-second observation period was calculated at 1 minute (upper panels) and 7 minutes (lower panels) of perfusion of blood from a patient (numbered as “−1”) with GTA (▵) under a shear rate of 50 s−1 (left panels) and 1,500 s−1 (right panels). At either shear rate, the relatively high platelet mobility index of a normal control (•) at 1 minute of perfusion decreased significantly at 7 minutes of perfusion, indicating that firm platelet adhesion progressed as a function of time. Note that the platelet mobility index for GTA, which was higher than that of normal at 1 minute of perfusion, decreased only slightly at 7 minutes of perfusion, indicating only limited firm adhesion of platelets in GTA during perfusion.
Strength of thrombi against increasing shear rates in BSS or type 3 vWD.
Although platelet thrombi were found to form to an extent comparable with normal in BSS and vWD type 3 under low shear rate conditions (Figs2A and 3 and Table 1), the quality of these thrombi generated in the absence of vWF or GP Ib, especially at high shear rates, is uncertain. Therefore, we observed the changes in thrombi of BSS and type 3 vWD during stepwise increases in shear rate during perfusion. Based on the amount of platelets adhering to the surface, the time-dependent thrombus growth during the 7-minute perfusion of BSS or type 3 vWD blood with a shear rate of 340 s−1 was arrested when the applied shear rate was raised to 1,500 s−1, although thrombus growth in control blood was greatly accelerated in a shear-dependent manner (Fig 5). Moreover, real-time observations of thrombus growth processes (not shown here) and the platelet mobility index (Table 2) confirmed that thrombi of BSS or type 3 vWD generated under low shear, like those of control, were stable and firmly fixed during stepwise increases of shear rates to 9,000 s−1. These results indicate that vWF and GP Ib, in addition to their critical contribution to initial platelet adhesion, play a determining role in thrombus growth under high shear rate conditions. Further, the resistance of thrombi formed in the abence of vWF or GP Ib against very high shear rates, a force that could peel off platelets from the surface, suggests that neither vWF nor GP Ib plays a substantial role in thrombus stability.
Changes in platelet thrombi generated under low shear rate conditions by stepwise increase of shear rate during perfusion in BSS or type 3 vWD. Blood from control (•), BSS (patient No. 1) (○), or type 3 vWD (▿) was perfused over a collagen surface under 340 s−1 for 7 minutes. The shear rate applied was then increased stepwise to 1,500 s−1 for 2 minutes and subsequently to 4,500 s−1 for 1 minute and to 9,000 s−1 for 1 minute. Based on the amount of platelets adhering to the surface, the time-dependent thrombus growth in BSS or type 3 vWD was arrested when the applied shear rate was raised to 1,500 s−1, although thrombus growth in control blood was greatly accelerated in a shear-dependent manner.
Changes in platelet thrombi generated under low shear rate conditions by stepwise increase of shear rate during perfusion in BSS or type 3 vWD. Blood from control (•), BSS (patient No. 1) (○), or type 3 vWD (▿) was perfused over a collagen surface under 340 s−1 for 7 minutes. The shear rate applied was then increased stepwise to 1,500 s−1 for 2 minutes and subsequently to 4,500 s−1 for 1 minute and to 9,000 s−1 for 1 minute. Based on the amount of platelets adhering to the surface, the time-dependent thrombus growth in BSS or type 3 vWD was arrested when the applied shear rate was raised to 1,500 s−1, although thrombus growth in control blood was greatly accelerated in a shear-dependent manner.
Changes in the Platelet Mobility Index Around the Transitional Time Point From 1,500 s−1 to 4,500 s−1 During Blood Perfusion of a BSS, vWD Type 3, and Af Patient*
| . | Shear Rate: . | 1,500 s−1 . | → . | 4,500 s−1 . |
|---|---|---|---|---|
| BSS-1 | 11.2 ± 2.2 | → | 9.8 ± 1.9 | |
| vWD type 3 | 10.6 ± 1.6 | → | 9.6 ± 1.4 | |
| Af-1 | 9 ± 2.5 | → | ||
| Control | 12 ± 1.6 | → | 9 ± 2.4 |
| . | Shear Rate: . | 1,500 s−1 . | → . | 4,500 s−1 . |
|---|---|---|---|---|
| BSS-1 | 11.2 ± 2.2 | → | 9.8 ± 1.9 | |
| vWD type 3 | 10.6 ± 1.6 | → | 9.6 ± 1.4 | |
| Af-1 | 9 ± 2.5 | → | ||
| Control | 12 ± 1.6 | → | 9 ± 2.4 |
Data are from the experiments described in the legends to Fig 5(BSS and type 3 vWD) and Fig 6 (Af) and obtained at 10 seconds before (1,500 s−1) and after (4,500 s−1) the shear rate transition. Each platelet mobility index (during 2 seconds; Materials and Methods) represents the mean ± SD of five index values derived from five nonoverlapping areas (110 × 73 μm) in a single frame. Note that the platelet mobility index of Af (underlined) greatly increased after the shear rate elevation, suggesting the breakdown of platelet immobilization to the surface once established.
Strength of thrombi against very high shear rates in congenital Af.
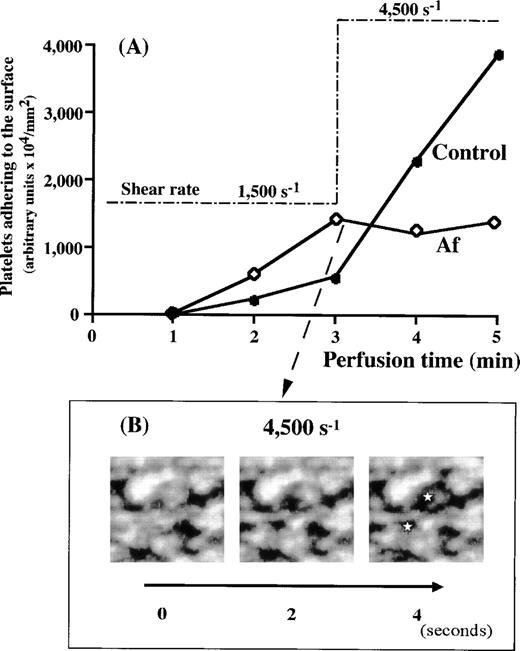
The overall process of mural thrombus formation in Af appeared to be comparable with normal under either low or high shear rate conditions (Fig 2 and Table 1). However, all prior experiments suggested that increasing shear rates induce a decrease in thrombus formation in most of the bleeding diseases while apparently upregulating thrombus growth in the normal control (Fig 3). Therefore, we examined thrombus formation by Af blood under higher shear rate conditions. Real-time observations showed progressive loosening of thrombi formed under a 1,500 s−1 shear rate, with gradual collapse after a stepwise increase of the shear rate to 4,500 s−1 (Fig6B). Based on the values of platelets adhering to the surface, thrombus growth observed under a shear rate of 1,500 s−1 in Af blood perfusion was arrested when shear rate was shifted to 4,500 s−1, whereas thrombus formation in control blood was sharply accelerated under 4,500 s−1conditions (Fig 6A). This result (Fig 6A) is similar to those in the case of BSS and vWD type 3 (Fig 5) and does not directly reflect the gradual collapse of Af thrombi, because flowing collapsed pieces of thrombi continuously came into the observed field from the outside of frame. Besides, the initiation of platelet adhesion and aggregation (although not much growing), concomitantly with the collapse, occurred newly and repeatedly as a function of vWF, not resulting in a drastic decrease in the amount of platelets in a examined frame. However, the platelet mobility index of Af blood around the transitional time point from 1,500 s−1 to 4,500 s−1 greatly increased from 9 to 42.3 (Table 2), apparently because of the alteration of shear rate, demonstrating the breakdown of platelet immobilization to the surface once established under lower shear rates. The control index, as well as BSS and vWD index showed no such increase (Table 2). Moreover, consecutive images obtained under a shear rate of 4,500 s−1 and at 2-second intervals provided a direct evidence of the gradual collapse of the thrombi formed in Af that have never been observed in the case of vWD (Fig 6B). These results indicate the critical involvement of fibrinogen in maintaining thrombus strength against heightened shearing forces.
Evaluation of thrombi formed in Af blood at 1,500 s−1 against a very high shear rate. (A) Control (•) or Af (patient No. 1) (◊) blood was perfused over a collagen-coated surface under 1,500 s−1 for 3 minutes. Based on the amount of platelets adhering to the surface, thrombi of Af formed more rapidly than those of the control. When the shear rate applied was increased stepwise to 4,500 s−1 at 3 minutes after the beginning of perfusion, the time-dependent thrombus growth in Af blood was arrested, while thrombus growth in control blood was accelerated. (B) Consecutive images of thrombi in Af blood collapsing under a shear rate of 4,500 s−1. The images at 2-second intervals were captured immediately after the shear rate transition to 4,500 s−1. Note that thrombi in Af blood collapse as a function of time, especially around the areas indicated (⋆).
Evaluation of thrombi formed in Af blood at 1,500 s−1 against a very high shear rate. (A) Control (•) or Af (patient No. 1) (◊) blood was perfused over a collagen-coated surface under 1,500 s−1 for 3 minutes. Based on the amount of platelets adhering to the surface, thrombi of Af formed more rapidly than those of the control. When the shear rate applied was increased stepwise to 4,500 s−1 at 3 minutes after the beginning of perfusion, the time-dependent thrombus growth in Af blood was arrested, while thrombus growth in control blood was accelerated. (B) Consecutive images of thrombi in Af blood collapsing under a shear rate of 4,500 s−1. The images at 2-second intervals were captured immediately after the shear rate transition to 4,500 s−1. Note that thrombi in Af blood collapse as a function of time, especially around the areas indicated (⋆).
DISCUSSION
Recent flow studies using a vWF-coated glass surface showed that the interaction of surface-immobilized vWF with GP Ib on flowing platelets mediates tethering and translocation of platelets along the surface.16 This initial transient attachment of platelets is assumed to be an activating signal for platelets, leading to firm platelet adhesion through binding of activated GP IIb-IIIa to surface-immobilized vWF.16,25 Although an analogous event on a collagen-coated surface, to which plasma vWF bound first in the early stage of blood perfusion, was recently confirmed,14 15 the overall mechanisms of platelet thrombogenesis on a collagen surface could be more complex than those that underlie the event on a purified vWF surface because of involvement of a variety of adhesive proteins and platelet membrane receptors on this thrombogenic surface.
To gain insight into platelet thrombogenesis on a collagen surface under physiologic flow conditions, we observed real-time processes of platelet thrombus formation on this surface in several congenital platelet aggregation disorders. Although several previous studies have analyzed the thrombogenicity of bleeding diseases under flow conditions,26-29 ours is the first to simultaneously monitor the entire process of thrombus formation in real time under identical flow experimental conditions, in platelet aggregation disorders that respectively lack a single component of two major platelet receptors (GP Ib-IX and IIb-IIIa), or two major soluble adhesive proteins (vWF and fibrinogen).
With the exception of GTA, the platelet aggregation disorders examined under low shear rate conditions showed thrombus generation processes comparable with those of a normal control, indicating compensation for the lack of a single component by other adhesive proteins or platelet receptors under conditions in which platelets flow at a relatively low speed. This conclusion contrasts with previous studies of soluble-phase shear-induced platelet aggregation, in which platelet GP IIb-IIIa strictly required fibrinogen, not vWF, as an adhesive ligand for platelet cohesion under low shear stress conditions.8,9However, among the factors examined in our study, only GP IIb-IIIa appeared to be indispensable in establishing firm platelet adhesion even under low shear rate conditions. Although a recent flow study indicated the critical involvement of GP Ia-IIa in firm platelet adhesion to a collagen-surface,30 the contribution of GP Ia-IIa might be insufficient in the complete absence of GP IIb-IIIa.
Consistent with previous reports,28,29 the interaction of surface-fixed vWF with GP Ib is absolutely required for initiation of the platelet-surface interaction, because neither BSS nor type 3 vWD showed evidence of platelet-surface interaction under high shear rate conditions. When platelets flow much faster than under low shear rates, the extremely high association rate of bond formation between vWF and GP Ib is critical in mediating the initial communication of flowing platelets with the surface, even on a collagen-coated surface where the known platelet collagen receptors GP Ia-IIa, GP IV, and GP VI may play a role. Further, our results confirmed that the interaction of vWF with GP Ib is also crucial for thrombus growth under high shear rate conditions. Although it remains unclear exactly how the vWF-GP Ib interaction functions in thrombus growth, the requirement of this interaction in platelet communication with the surface under high shear rates suggests a scenario whereby vWF flowing at high speed can be captured only through its transient interaction with GP Ib on platelets adhering to and immobilized on the surface, followed by the irreversible binding of vWF transiently trapped by GP Ib to neighboring activated GP IIb-IIIa. Captured vWF on platelets adhering to the surface might then mediate the second layer platelet adhesion via the vWF-GP Ib interaction and via the subsequent vWF-GP IIb-IIIa interaction, in a mode similar to the initial platelet adhesion on the surface. Thus, basic mechanisms of mural platelet aggregate accumulation on the surface might represent repeated cycles of these events under high shear rate conditions. Based on this interpretation, the interaction of vWF with GP Ib, regardless of whether vWF is in solution or immobilized, is an absolute prerequisite for the overall thrombus generation process. Indeed, a recent inhibition study, using function-blocking antibodies and confocal laser microscopy in combination with a flow chamber system analogous to ours, showed that the interaction of vWF with GP Ib was essential for mural thrombus growth on a type I collagen-coated surface.31
Fibrinogen is probably not involved in fundamental mechanisms of mural thrombogenesis under high shear flow conditions. Indeed, mural thrombi of Af blood formed in a manner indistinguishable from that of the normal control at the high shear rate (1,500 s−1) in this study. However, the observation that platelet thrombi, once formed under such flow conditions, began to collapse when the shear rate was greatly elevated suggests that fibrinogen is needed to maintain the strength of thrombi under heightened hemodynamic shearing forces that could peel off platelets from the surface. Thus, unlike the initial phase of platelet-surface interaction, blood flow situations above the surface may become heterogeneous during mural thrombogenesis, especially at sites around thrombi, which, by their bulk and ensuing collisions with blood components, create local low shear rate situations that favor binding of fibrinogen to GP IIb-IIIa. Flowing plasma fibrinogen is, therefore, thought to be gradually integrated as a function of time into thrombi, which are composed of vWF and platelets in the early phase of the process, through binding to GP IIb-IIIa even under high shear rates. Interestingly, thrombi formed in the absence of vWF or GP Ib, unlike those formed without fibrinogen, showed sufficient strength against very high shear rates, suggesting no substantial role for vWF as an adhesive ligand in thrombus stability. Together, results clearly show the distinct roles of two major adhesive proteins, vWF and fibrinogen, in mural thrombus formation under flow conditions with high shear rates; vWF, perhaps mainly through its interaction with GP Ib-IX, acts as an “initiator and promoter,” whereas fibrinogen, via its binding to GP IIb-IIIa, acts as a “stabilizer” against heightened shearing forces that could lead to peeling off of platelets from the surface.
In conclusion, the present study shows that distinct pathogenic mechanisms underlie the life long bleeding symptoms associated with prolonged bleeding time in these diseases. Impaired mural thrombus generation was observed to some extent in all diseases under high shear rate conditions and those under low shear were mostly normal except for GTA, implying the physiologic relevance of platelet functions under high shear blood flow situations. The shear specific and time-course–dependent functions of GP Ib, GP IIb-IIIa, vWF, and fibrinogen in our study might shed light on the complex mechanisms involved in mural thrombogenesis under physiologic flow conditions and might provide the groundwork for therapeutic strategies against pathologic intravascular thrombosis formed under high shear stress, such as coronary occlusive diseases.
ACKNOWLEDGMENT
We thank Marina Hoffman for editorial assistance.
Part of this work was presented at the American Society of Hematology Meeting in San Diego, CA, December 5-9, 1997 (abstract No. 92), and in Miami Beach, FL, December 4-8, 1998 (abstract No. 1422).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Mitsuhiko Sugimoto MD, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: sugi-ped@nmu-gw.cc.naramed-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal