Abstract
The activation of phospholipase A2 (PLA2) with release of eicosanoids and prostanoids in mature myeloid cells and the augmentation (priming) of this activity by cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) are central to the inflammatory process. Yet, there are few data concerning PLA2 activity and its regulation by growth factors in primary hematopoietic cells. We therefore analyzed the PLA2activity of mobilized human CD34 antigen-positive (CD34+) stem cells by quantitation of the extracellular release of3H-arachidonate. The PLA2 activity of CD34+ cells stimulated with calcium ionophore (A23187) was of similar magnitude to that of mature neutrophils and monocytes. Preincubation of CD34+ cells with stem cell factor (SCF) before A23187-stimulation resulted in primed PLA2 activity, whereas interleukin-3 (IL-3), GM-CSF, and tumor necrosis factor had no significant effect. When CD34+ cells were induced to differentiate, PLA2 activity remained responsive to SCF for several days, but after 8 days, at which stage morphological and functional evidence of maturation was occurring, priming of PLA2 by SCF could no longer be elicited, whereas responses to GM-CSF and IL-3 had developed. The further metabolism of arachidonic acid to eicosanoids by CD34+ cells was not detected by either thin-layer chromatography, enzyme immunoassay, or differential spectroscopy. SCF stimulated the rapid but transient activation of ERK2 (p42 MAP kinase) in CD34+ cells, and we used the MAP kinase kinase inhibitor, PD 098059, which at 30 μmol/L blocks ERK2 activation in CD34+ cells, to investigate whether SCF-mediated priming of arachidonate release was mediated by this kinase. PD 098059 only partially inhibited A23187-stimulated PLA2 activity primed by SCF, suggesting the involvement of ERK2 and possibly a further signal transduction pathway. Methyl arachidonyl fluorophosphonate (5 μmol/L), a dual inhibitor of i and cPLA2 isoforms, completely inhibited arachidonate release without affecting ERK2 activation, demonstrating the lack of cellular toxicity. These data provide the first evidence that primitive myeloid cells have the capacity to release arachidonate, which is regulated by an early acting hematopoietic growth factor important for the growth and survival of these cells.
PRIMITIVE MYELOID CELLS have a blastic appearance and a characteristic immunophenotype that includes the expression of the CD34 antigen.1,2 These cells are able to undergo a series of amplification divisions within the bone marrow that are associated with phenotypic differentiation and the acquisition of mature phagocytic cell functions such as respiratory burst activity.3,4 Many of the growth factors that induce proliferation of immature cells also modulate the function of the mature end cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF), for instance, not only stimulates proliferation of neutrophil and monocyte progenitor cells, but also augments (primes) many of the agonist-induced functions of the mature end-cells, such as phagocytosis and killing of micro-organisms.5
Phospholipase A2 (PLA2) has a key role in mature phagocytes in releasing arachidonic acid from cell membranes to serve as substrate for the production of the eicosanoids and prostanoids.6,7 These molecules are important in host-defense and inflammation, because they regulate the migration and activation of phagocytic cells. However, arachidonate itself is important as a signal transduction molecule in raising intracellular calcium levels,8,9 activating enzymes such as protein kinase C,10 MAP kinase,11 and neutral sphingomyelinase12 and modulating GTP protein function13 and gene expression,14 and might also serve these functions in immature cells. The PLA2enzymes form a superfamily comprising at least 9 groups,15which can be divided into 3 mechanistic classes, the low-molecular weight secretory group of PLA2 enzymes that require calcium for catalytic activity (sPLA2)16; the 80-kD cytosolic PLA2 that does not require calcium for catalysis but for translocation to the location of its substrate in cellular membranes (cPLA2)17,18; and the calcium-independent enzymes (iPLA2).19 The expression of the different PLA2 isoforms varies according to cell type, and some cells express multiple isoforms, such as macrophages that express group II sPLA2, group IV cPLA2, and group VI iPLA2.20
Stimulated PLA2 activity in phagocytes can be increased by short-term incubation with inflammatory mediators such as tumor necrosis factor α (TNFα)21 and GM-CSF22-24during the process known as cell priming that is not dependent on new protein synthesis. At present, it is unclear which PLA2isoforms are activated during cytokine-mediated priming of arachidonate release, although it was recently reported that both group IV cPLA2 and group II sPLA2 are activated during the TNFα-mediated priming of neutrophils.21 The mechanism of cytokine-mediated regulation of the different PLA2enzymes by cytokines has not been fully elucidated. Activation of cPLA2 is associated with translocation of cPLA2to cell membranes in response to an increase in intracellular calcium and is mediated by its CalB/C2 domain25,26; however, many growth factors do not stimulate a calcium flux. Serine phosphorylation is reported to be important for activating cPLA2activity,27 and several serine kinases that are activated by growth factors may be involved, including protein kinase C,28 mitogen-activated protein kinase (p42/Erk-2 MAP kinase),29,30 and stress-activated protein kinase (p38).31 Ser-505 in the cPLA2 sequence is a major phosphorylation site29 and lies within an MAPK consensus sequence. However, inhibition of p38 kinase was shown to only partially inhibit the phosphorylation of both Ser-505 and Ser-727; therefore, another kinase may be involved downstream of p38.31
Many of the studies of primitive myeloid cells and the differentiation process have, in the past, been restricted to growth factor-independent leukemic cell lines because of the unavailability of adequate numbers of the rare primary cells. Cell lines such as HL-60 and U-937 express cPLA2 protein,32,33 which is active in in vitro assays, yet intact cells release barely detectable amounts of AA when stimulated via functional receptors or receptor-independent pathways.32,34-36 During in vitro differentiation of HL-60 cells, the capacity to release AA after stimulation increases concomitantly with the capacity to synthesize eicosanoids,37 38 and this is in accord with a pivatol role for AA production in the effector functions of mature phagocytic cells. However, it must be borne in mind that these cell lines are highly abnormal, eg, both HL-60 and U937 cells are completely factor-independent for proliferation.
There is conflicting evidence that leukotrienes can stimulate the proliferation of immature myeloid cells,39-42 and it is unclear whether primary immature cells have functional PLA2and are able to synthesize eicosanoids. A prelimary study reports the presence of cPLA2, cyclooxygenase, and 5-lipoxygenase mRNA in CD34 antigen-positive (CD34+) cells mobilized into the peripheral blood43; however, another study found no cPLA2 mRNA in CD34+ cells derived from cord blood.44 Advances in the ability to mobilize human hematopoietic progenitor/precursor cells from the marrow into the blood45,46 and in the techniques used to collect and isolate reasonably pure primitive cell populations on the basis of CD34 antigen expression47 now make it more readily possible to study such primary cells. Freshly purified peripheral blood CD34+ cells are in a quiescent G0 stage of the cell cycle48,49 but can be induced to cycle in vitro by a range of growth factors.49,50 With continued culture in the presence of appropriate factors, these cells then lose expression of the CD34+ antigen and begin to acquire the appearance and functional properties of mature phagocytes.3 The aim of this study was to determine whether PLA2 activity is present in very primitive myeloid cells, and if so, to determine its cytokine dependence.
MATERIALS AND METHODS
Growth factors, agonists, and inhibitors.
Stock solutions of recombinant human GM-CSF (rhGM-CSF; expressed inEscherichia coli; Hoechst UK/Behringwerke, Marburg, Germany), recombinant human stem cell factor (rhSCF; expressed in E coli), recombinant human interleukin-3 (rhIL-3), and rhIL-6 (all from R&D Systems, Europe, Abingdon, Oxfordshire, UK) were prepared in sterile phosphate-buffered saline (PBS; pH 7.4) containing 1% (vol/vol) fetal calf serum (FCS) and stored at −20°C. MK886 was a gift from Merck-Frosst Canada Inc (Pointe Claire-Dorval, Quebec, Canada). A 100 μmol/L stock solution in dimethyl sulphoxide (DMSO) was prepared immediately before use. 12-O-tetradecanoylphorbol 13-acetate (TPA), calcium ionophore (A23187), and indomethacin were from Sigma Chemical Co (Poole, Dorset, UK). Stock solutions of A23187, prepared in DMSO at 5 mg/mL and stored at −20°C, were diluted to 100 μmol/L in PBS immediately before the experiment. A stock solution of PD 098059 (Alexis Corp [UK] Ltd, Nottingham, UK) at 30 mmol/L DMSO was stored at −20°C and diluted 1,000-fold into final reaction mixtures. Methyl arachidonyl fluorophosphonate (MAFP) was supplied in solution in methyl acetate (Cayman Chemical, Ann Arbor, MI). The solvent was evaporated under nitrogen and MAFP was reconstituted with DMSO at 50 mmol/L and stored at −80°C. It was further diluted with DMSO on the day of the assay.
Purification of CD34+ progenitor cells mobilized into the peripheral blood.
CD34+ peripheral blood progenitor cells (PBPC) were purified from 26 patients (4 with Hodgkin’s disease, 10 with non-Hodgkin’s lymphoma, and 12 with myeloma) and 10 healthy normal donors after obtaining informed consent. Progenitor cells were first mobilized from the bone marrow of patients with either cyclophosphamide (1.5 g/m2) followed by daily granulocyte colony-stimulating factor (G-CSF; 10 mg/kg/d filgrastim or 263 μg/d lenograstim) for 10 days or an ESHAP regime followed by G-CSF (lenograstim) as described before.47 Normal donors received G-CSF alone. White blood cells were isolated from the peripheral blood by apheresis and CD34+ cells were purified either on a CEPRATE SC immunoaffinity column (Cell Pro Inc, Bothwell, WA) using a biotinylated mouse-antihuman CD34 monoclonal antibody and avadin-coated beads47 or with an AmCell Clinimacs device and anti-CD34+ antibody-coated microbeads (Miltenyi Biotech Ltd, Bisley, Surrey, UK). Progenitor cell purity was assessed by flow cytometry using an anti-CD34 antibody HPCA (Becton Dickinson, Franklin Lakes, NY) and by morphological analysis.51 The purity of CD34+ cells after affinity purification on CEPRATE columns was 82% ± 10% (mean ± 1 SD, n = 19) with 2% ± 1% recognizable neutrophil precursors, but no mature neutrophils were seen. The major cell contaminants were lymphocytes (6% ± 2%) and monocytes (10% ± 2%), which were removed, respectively, with anti-CD3 and CD19 magnetic beads according to the manufacturer’s instructions (Dynal, Bromborough, UK) or by adherence to plastic. The purity of CD34+ cells after Clinimacs separation was 98%, with less than 2% contamination with lymphocytes. Contamination with monocytes of the final cell suspension used for experimentation was 5% ± 1% for CEPRATE-separated cells and 0.8% ± 0.1% for Clinimacs cells, and the respective viabilities of these cells were 93% ± 3% and 94% ± 2%. CD34+ cells were used for experimentation when the number obtained exceeded that required for rapid and sustained hematological engraftment.47 Mobilized CD34+ cells were either cultured overnight in Iscove’s Minimal Essential Medium (IMEM; GIBCO-BRL, Paisley, UK) supplemented with 20% (vol/vol) FCS (GIBCO-BRL) in a humidified atmosphere of 5% carbon dioxide in air (day 1 cells) or for up to 8 days in IMEM/20% FCS supplemented with rhSCF, IL-3, and IL-6 (all at 10 ng/mL) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). Before analysis of cellular responses to cytokines, cells were harvested from the culture medium by centrifugation, followed by 3 large volume washes to remove ambient growth factors, and incubated for 18 hours in IMEM/20% FCS to allow re-expression of surface receptors. The morphological maturity of cultured cells was assessed from cytocentrifuge preparations stained with Leishman’s stain. Functional maturity was assessed by the nitroblue tetrazolium (NBT) reduction test of cells stimulated with TPA (1 μg/mL), as previously described.35
Purification of neutrophils and monocytes.
Neutrophils were purified from peripheral blood anticoagulated with 2 mmol/L EDTA (pH 7.4), by dextran sedimentation, by centrifugation through Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), and by hypotonic lysis as described previously,52 using sterile preparations and procedures to minimize contact of cells with endotoxin and reduce inadvertant priming. Platelet-free preparations of monocytes were obtained by centrifugation of whole blood through 12% Ficoll-Paque/Dulbecco’s PBS without calcium and magnesium (PBS−; GIBCO-BRL). The platelet-rich supernatant was removed and the platelet-depleted blood was layered over a single cushion of Ficoll-Paque and centrifuged as described before. T and B lymphocytes were removed from the monocytes using magnetic beads coated with CD19 and CD3 according to the manufacturers’ instructions (Dynal). Purified cells were resuspended in PBS supplemented with 0.9 mmol/L calcium and 0.5 mmol/L magnesium (PBS+) and 5 mmol/L glucose.
Cell lines.
The HL-6053 and TF-1 myeloid cell lines54 were grown in Roswell Park Memorial Institute (RPMI) medium (GIBCO-BRL) supplemented with 10% FCS at concentrations not exceeding 5 × 105/mL. Cultures of TF-1 cells were additionally supplemented with 5 ng/mL GM-CSF every 2 days. Cells were harvested and resupended in PBS+ supplemented with 10 mmol/L glucose. Before analysis of responses to cytokines, the cells were removed from medium containing growth factor and incubated for 18 hours to allow re-expression of GM-CSF receptors.
3H-Arachidonate release from intact cells.
Cells were incubated with [5,6,8,9,11,12,14,15-3H]AA (specific activity, 7.33 TBq/mmol; 202 Ci/mmol; Amersham International, Amersham, Buckinghamshire, UK) at a final concentration of 0.5 μCi/mL for 2 hours with occasional mixing. Purified neutrophils (5 × 106/mL PBS+/5 mmol/L glucose/0.1% vol/vol FCS) were incubated at room temperature. Mobilized CD34+ cells (2.5 × 106/mL 50% IMEM/50% PBS/10 mmol/L glucose/0.1% vol/vol FCS) and TF-1 cells (2.5 × 106/mL 50% RPMI/50% PBS+/10 mmol/L glucose/0.1% vol/vol FCS) were incubated at 37°C for 1 hour followed by 1 hour at room temperature. The radiolabeled cells were centrifuged (300g for 7 minutes) and the supernatants were removed. The cell pellets were washed a further 3 times in PBS+G and finally were resuspended at 5 × 105 cells/mL PBS+G for CD34+ cells and at 2 × 106 cells/mL for all other cell types. Aliquots (0.5 mL) of these cell suspensions were equilibrated to 37°C. Neutrophil samples were incubated at this stage for 5 minutes with 200 nmol/L MK886 to inhibit the conversion of AA to leukotrienes via the action of 5-lipoxygenase.55 MK886 was not added to samples of immature cells, because pilot experiments showed that these cells did not release leukotrienes. All samples were then incubated with the following cytokines and growth factors for 10 minutes: TNFα (500 U/mL), IL-3 or GM-CSF (both at 10 ng/mL), SCF (100 ng/mL), or growth factor diluent (0.01% FCS; final concentrations); or with TPA (500 ng/mL) or DMSO diluent (0.01%). Replicate samples were then stimulated for 20 minutes with or without 1 μmol/L calcium ionophore, A23187, in the presence of fatty acid-free bovine serum albumin (BSA; final concentration, 1 mg/mL), which was added to trap the AA that was released and to prevent re-esterification. The reaction was terminated by placing the samples on ice. The samples were then centrifuged (12,000g for 4 minutes) and 0.4-mL aliquots of the supernatants were assayed for radioactivity by liquid scintillation spectroscopy. Duplicate samples containing 0.4 mL of the initial radiolabeled cell suspension were used to estimate the total amount of incorporated radioactivity, and arachidonate release was expressed as a percentage of this.
Measurement of AA release from CD34+ cells by thin-layer chromatography (TLC).
Freshly purified PBPC were incubated at 2.5 × 105/mL for 24 hours with IL-3, IL-6, and SCF, followed by removal of growth factors and overnight starvation as described above. On day 2, cells were harvested from culture, resuspended at 2.5 × 106/mL of 50% IMEM/50% PBS/10 mmol/L glucose/0.1% vol/vol FCS, and radiolabeled with 3H-AA (1 μCi/mL) as described above, followed by washing to remove unincorporated isotope. PBPC were resuspended in PBS+/10 mmol/L glucose in two 1-mL aliquots of 5.6 × 106 cells and equilibrated to 37°C. MAFP,56 the dual inhibitor of c and iPLA2 isoforms,19 was added at a final concentration of 5 μmol/L to one aliquot and DMSO diluent (0.1% vol/vol) was added to the replicate sample for 15 minutes, followed by 100 ng/mL SCF to both samples for 10 minutes. Both samples were stimulated with 1 μmol/L calcium ionophore in the presence of 2 mg/mL BSA for 30 minutes. The samples were chilled on ice and then centrifuged (12,000g for 4 minutes). The supernatant was taken into glass centrifuge tubes (15 × 100 mm) and 100 μg AA was added to act as carrier. Lipids were extracted by the addition of 3 mL of ice-cold chloroform:methanol: 1/10 formic acid (1:2:0.15). The samples were vortex mixed and left on ice for 15 minutes. One milliliter of ice-cold distilled water and 2 mL of chloroform were added to achieve phase separation. The samples were vortex-mixed, left on ice for 15 minutes, and then centrifuged at 1,800g for 10 minutes at 4°C. Samples were taken from the aqueous and organic phases as well as the protein interface to determine their radioactivity. The organic layer was dried in a vacuum evaporator and then taken up in 10 mL chloroform:methanol, 9:1. Lipids were separated by TLC on silica gel 60 plates (Merck, Darmstadt, Germany) using as the mobile phase, the upper phase from a mixture of ethyl acetate:iso-octane (2,2,4-trimethylpentane):water:acetic acid (4.5:2.5:5:1) as described previously.32 A standard composed of a mixture of 3H-AA and nonlabeled AA (100 μg) was extracted and run on the TLC plate in parallel with the cell samples. The lanes on the TLC plate were marked with a pencil and divided into 16 × 1 cm fractions starting from the origin. The fractions were scraped from the plate, and their radioactivity was determined by β-scintillation spectroscopy. Daily measurements of the radioactivity in the scintillation vials showed that at least 3 days were required before the radioactive lipids had eluted fully from the silica.
Measurement of leukotriene production.
Leukotriene B4 (LTB4), LTC4, LTD4, and LTE4 were detemined by enzymeimmunoassay using Biotrak kits, RPN 223 (LTB4) and RPN 224 (LTC4, LTD4, and LTE4; Amersham International) following the manufacturer’s instructions. LTB4 production by neutrophils stimulated with 1 μmol/L A23187 and peptidoleukotriene production by monocytes also stimulated with A23187 were used as positive controls. Eicosanoid production was also measured by difference spectroscopy of supernatants from 107 cells, using a dual-beam spectrophotometer (Unicam UV2 spectrometer; ATI Unicam, Cambridge, UK) with matched quartz cuvettes. The concentration of LTB4 was calculated from the height of the 270 nm peak (absorption maximum), taking the extinction coefficient of LTB4 as E270 nm = 50 mmol−1 cm−1. Confirmation of the absorbance spectrum of LTB4 was by comparison with authentic LTB4 and its omega oxidation metabolites as well as the peptidoleukotrienes, LTC4, LTD4, and LTE4 (Cascade Biochem Ltd, Reading, Berkshire, UK).
Measurement of MAP kinase (p42/Erk2) activation by gel retardation assay.
Cells (1.3 × 107/mL PBS+G) were stimulated with either cytokine diluent (0.01%), SCF (100 ng/mL), GM-CSF (10 ng/mL), or TPA (500 ng/mL). At timed intervals, 30-μL samples (5 × 105 cells) were taken into 30 μL lysis buffer supplemented with protease inhibitors (20 μg/mL leupeptin, 20 μg/mL pepstatin, 20 μg/mL aprotinin, 1 μmol/L phenylmethylsulfonylfluoride, and 1 mmol/L diisopropylfluorophosphate), phosphatase inhibitors (50 mmol/L sodium orthovanadate, 1 mmol/L β glycerophosphate, 5 mmol/L pyrophosphate, and 50 mmol/L sodium fluoride), and 2 mmol/L n-ethylmaleimide. Sixty microliters of boiling Laemmli sample buffer was added and the samples were heated at 100°C for 3 minutes. Proteins from 1 to 2 × 105cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 15% acrylamide:0.075% bisacrylamide) and immunoblotted first with anti-MAP kinase antibody (0.2 μg/mL, Erk-2 C-14; Santa Cruz Biotechnology, Santa Cruz, CA) and second with a peroxidase-conjugated goat-antirabbit antibody (at 1:2,000 dilution; Dako Ltd, High Wycombe, Buckinghamshire, UK), followed by enhanced chemiluminescence and autoradiography for detection (Amersham International).
Data analysis.
Unless otherwise stated, the data are the mean ± 1 SE of the number of experiments given in the text.
RESULTS
Detection of PLA2 activity in undifferentiated hematopoietic cells.
PLA2 activity was quantitated by measuring the release of radioactivity into the supernatant from cells that had been radiolabeled with 3H-arachidonic acid (AA) and then stimulated with either 500 ng/mL TPA or 1 μmol/L A23187 or by the sequential addition of these 2 agonists. The radioactivity released was then expressed as a percentage of total cell radioactivity to control for any differences in the amount of 3H-AA incorporated by the different cell types. Initial studies performed on cells from the immature erythroleukemia cell line, TF-1, showed levels of arachidonate release in these primitive cells that were comparable to that seen in mature neutrophils, whereas promyelocytic HL-60 cells released very little arachidonate under any condition (Table 1). Further studies were then performed in primary human hematopoietic cells, namely purified CD34+ cells, mobilized into the circulation by a combination of chemotherapy and G-CSF.47 The levels of arachidonate release were again comparable with that seen in mature neutrophils and monocytes (Table 1).
Activation of Arachidonate Release From CD34+ Cells Compared With Other Cell Types
| Cell Type (n) . | Arachidonate Release Stimulated by . | |||
|---|---|---|---|---|
| DMSO . | DMSO + A23187 . | TPA . | TPA + A23187 . | |
| HL-60 (5) | 1.1 ± 0.1* | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.6 ± 0.2 |
| TF-1 (10) | 3.1 ± 0.2 | 5.2 ± 0.5† | 3.6 ± 0.2 | 17.0 ± 1.2† |
| CD34+ (11) | 4.8 ± 0.6 | 6.5 ± 1.0† | 6.4 ± 0.8‡ | 24.5 ± 4.4‡ |
| Neutrophils (7) | 2.7 ± 0.2 | 7.3 ± 1.0‡ | 10.1 ± 1.11-153 | 18.5 ± 1.11-153 |
| Monocytes (10) | 6.9 ± 0.7 | 17.5 ± 2.41-153 | 17.1 ± 1.81-153 | 26.1 ± 2.21-153 |
| Cell Type (n) . | Arachidonate Release Stimulated by . | |||
|---|---|---|---|---|
| DMSO . | DMSO + A23187 . | TPA . | TPA + A23187 . | |
| HL-60 (5) | 1.1 ± 0.1* | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.6 ± 0.2 |
| TF-1 (10) | 3.1 ± 0.2 | 5.2 ± 0.5† | 3.6 ± 0.2 | 17.0 ± 1.2† |
| CD34+ (11) | 4.8 ± 0.6 | 6.5 ± 1.0† | 6.4 ± 0.8‡ | 24.5 ± 4.4‡ |
| Neutrophils (7) | 2.7 ± 0.2 | 7.3 ± 1.0‡ | 10.1 ± 1.11-153 | 18.5 ± 1.11-153 |
| Monocytes (10) | 6.9 ± 0.7 | 17.5 ± 2.41-153 | 17.1 ± 1.81-153 | 26.1 ± 2.21-153 |
CD34+ cells were mobilized from the bone marrow into the peripheral blood and selected on the basis of their CD34 antigen expression. The mean number of monocytes contaminating the CD34+ cell preparations was 2.1% ± 1.0%. TF-1 and CD34+ cells were incubated for 18 hours without growth factors before testing. Neutrophils and monocytes were tested on the day of purification. All cells were radiolabelled with3H-AA and stimulated with either 500 ng/mL TPA or DMSO diluent for 10 minutes, followed by 1 μmol/L A23187 or PBS control for 20 minutes, and AA release was measured as described in Materials and Methods. AA release was expressed as a percentage of the total cell radioactivity, which was 76,341 ± 14,590 cpm/106 HL-60 cells, 92,049 ± 5268 cpm/106 TF-1 cells, 171,863 ± 39,371 cpm/106 CD34 cells, 69,838 ± 12,788 cpm/106 neutrophils, and 61,505 ± 10,380 cpm/106 monocytes. Values are the percentage of cellular radioactivity. P values show the significance of difference from DMSO controls measured by Student’s paired t-test.
The data shown are the mean ± 1 SE of the number of experiments (n).
.05 > P > .01.
.01 > P > .001.
.001 > P.
Regulation of PLA2 activity by growth factors in undifferentiated hematopoietic cells.
We and others have previously shown that PLA2 activity in mature phagocytes stimulated with either receptor-dependent agonists or receptor-independent stimuli such as A23187 is primed by short-term incubation (10 to 20 minutes) with growth factors such as GM-CSF.22-24 We therefore explored the effect on AA release of prior exposure to various growth factors in the TF-1 cell line and primary CD34+ cells, in comparison with purified neutrophils from peripheral blood. The effect of preincubation with optimal concentrations of the growth factors, GM-CSF (10 ng/mL), IL-3 (10 ng/mL), TNFα (500 U/mL), and SCF (100 ng/mL) on ionophore-stimulated AA release was measured. To compare the magnitude of cytokine-mediated priming between cell types, arachidonate release in resting cells (no A23187 stimulation) was subtracted from the A23187-stimulated value and the data for cytokine-primed cells were then expressed as a percentage of the respective FCS diluent control. As shown in Fig 1, each cell type had a different pattern of response to the cytokines. In the primary CD34+ cells (Fig 1A), SCF was the major priming agent of AA release and IL-3 had a weak but statistically significant effect, whereas neither GM-CSF nor TNFα did so. The CD34+ cells used in this study were from patients who had received both chemotherapy and G-CSF during the mobilization procedure as well as normal donors who received G-CSF alone. No statistically significant differences were seen in the CD34+ cell responses to cytokines between patients and normal donors (data not shown). In cultures of CD34+ TF-1 cells (Fig 1B), AA release was equally primed by GM-CSF and IL-3, whereas SCF had a small but significant effect and TNFα had no significant effect. In neutrophils (Fig 1C), GM-CSF had the greatest priming effect and TNFα had a minor but statistically significant effect, whereas neither IL-3 nor SCF had any significant effect, which may reflect the low expression of IL-3 and SCF receptors on these cells. Monocytes purified from peripheral blood were also tested, because these cells were present as very minor contaminants (2% ± 1%, n = 9) in the CD34+ cell preparations used for the experiments in Fig 1. SCF failed to affect AA release in monocytes, with the priming being 99% ± 19% of the FCS control, whereas GM-CSF–mediated priming was 140% ± 22% of control (P = .03, n = 10).
Effect of growth factors on A23187-stimulated AA release in CD34+ cells compared with other cell types. Suspensions of (A) day 1 CD34+ cells, (B) TF-1 cells, and (C) neutrophils were incubated for 10 minutes with either growth factor diluent (0.01% vol/vol FCS), GM-CSF (10 ng/mL), IL-3 (10 ng/mL), TNF (500 U/mL), or SCF (100 ng/mL) and then stimulated with 1 μmol/L A23187. Arachidonate release was measured as described in Materials and Methods and expressed as a percentage of growth factor diluent control. The number of replicate experiments performed are indicated in the figure. The statistical significance of the differences between cytokine- and diluent-mediated priming are shown (*.05 > P > .01; **.01 > P > .001; ***.001 > P; Student’s paired t-test). The absolute values of the background release of AA was 4.6% ± 0.4%, 3.1% ± 0.3%, and 2.3% ± 0.2% of total cell radioactivity for CD34+ cells, TF-1 cells, and neutrophils, respectively. Similarly, A23187-stimulated AA release was 7.2% ± 0.8%, 5.6% ± 0.6%, and 6.9% ± 1.1% in the FCS-primed samples.
Effect of growth factors on A23187-stimulated AA release in CD34+ cells compared with other cell types. Suspensions of (A) day 1 CD34+ cells, (B) TF-1 cells, and (C) neutrophils were incubated for 10 minutes with either growth factor diluent (0.01% vol/vol FCS), GM-CSF (10 ng/mL), IL-3 (10 ng/mL), TNF (500 U/mL), or SCF (100 ng/mL) and then stimulated with 1 μmol/L A23187. Arachidonate release was measured as described in Materials and Methods and expressed as a percentage of growth factor diluent control. The number of replicate experiments performed are indicated in the figure. The statistical significance of the differences between cytokine- and diluent-mediated priming are shown (*.05 > P > .01; **.01 > P > .001; ***.001 > P; Student’s paired t-test). The absolute values of the background release of AA was 4.6% ± 0.4%, 3.1% ± 0.3%, and 2.3% ± 0.2% of total cell radioactivity for CD34+ cells, TF-1 cells, and neutrophils, respectively. Similarly, A23187-stimulated AA release was 7.2% ± 0.8%, 5.6% ± 0.6%, and 6.9% ± 1.1% in the FCS-primed samples.
The effect of in vitro culture with IL-3, IL-6, and SCF on arachidonate release from primary CD34+ cells.
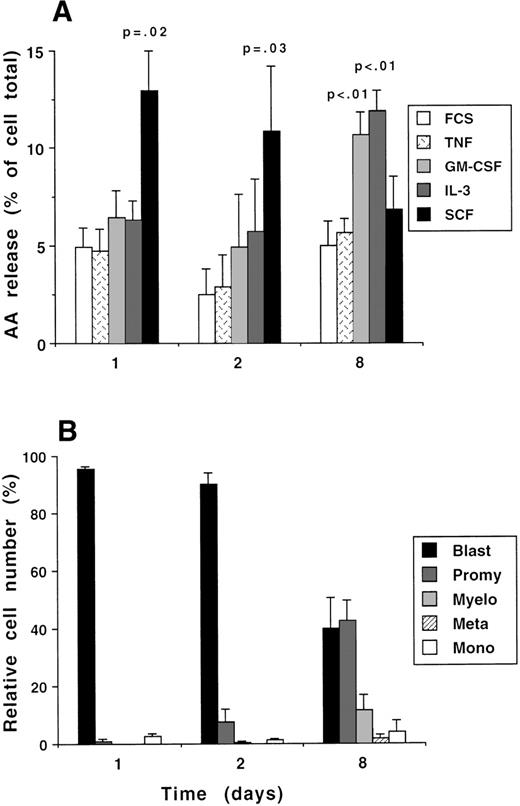
Freshly purified CD34+ cells were grown for up to 8 days in medium supplemented with a cytokine cocktail (IL-3, IL-6, and SCF, all at 10 ng/mL), which induced proliferation and partial differentiation, as was previously reported.50 57 As shown in Fig 2B, morphological maturation was apparent at day 8, and a proportion of cells acquired the functional characteristics of fully mature cells (11% ± 2% NBT-positive cells on day 8 [n = 8]; 2% ± 1% NBT positive on day 1 [n = 12]).
Regulation of PLA2 activity during differentiation of CD34+ cells. (A) Freshly purified CD34+ cells were incubated for up to 8 days with IL-3, IL-6, and SCF (all at 10 ng/mL). After 18 hours of incubation without growth factors, cells were primed for 10 minutes with either TNF (500 U/mL), IL-3 (10 ng/mL), GM-CSF (10 ng/mL), SCF (100 ng/mL), or diluent (0.01% vol/vol FCS), followed by activation with 1 μmol/L A23187 for 20 minutes, and arachidonate release was measured. Basal release in unstimulated samples was subtracted from the A23187-stimulated values and the data are expressed as a percentage of total cell radioactivity. The data shown are the mean ± 1 SE of 3 to 8 experiments. The statistical significance of the differences between cytokine and diluent-treated cells at each time interval are given (Student’s paired t-test). (B) Morphological analysis of cytospin preparations from the cultures as used in (A).
Regulation of PLA2 activity during differentiation of CD34+ cells. (A) Freshly purified CD34+ cells were incubated for up to 8 days with IL-3, IL-6, and SCF (all at 10 ng/mL). After 18 hours of incubation without growth factors, cells were primed for 10 minutes with either TNF (500 U/mL), IL-3 (10 ng/mL), GM-CSF (10 ng/mL), SCF (100 ng/mL), or diluent (0.01% vol/vol FCS), followed by activation with 1 μmol/L A23187 for 20 minutes, and arachidonate release was measured. Basal release in unstimulated samples was subtracted from the A23187-stimulated values and the data are expressed as a percentage of total cell radioactivity. The data shown are the mean ± 1 SE of 3 to 8 experiments. The statistical significance of the differences between cytokine and diluent-treated cells at each time interval are given (Student’s paired t-test). (B) Morphological analysis of cytospin preparations from the cultures as used in (A).
Samples were taken for analysis of AA release on days 1, 2, and 8. Before analysis, cells were washed free of growth factor and incubated for 18 hours in growth factor-free medium to allow the re-expression of receptors that may have been downregulated during culture. Thus, the cells analyzed on day 1 were not exposed to the cytokine cocktail.3H-AA release from radiolabeled cells was measured as described above in cells preincubated for 10 minutes with either TPA or growth factors before stimulation with A23187. Basal AA release from cells stimulated with PBS instead of A23187 was measured in parallel samples and these values were subtracted from the A23187-stimulated values to give the increment in AA release due to A23187.
Maximal stimulation of AA release by TPA + A23187 decreased during the culture period, with levels of AA release being 36% ± 4%, 25% ± 5%, and 23% ± 1% of cell radioactivity on days 1, 2, and 8, respectively (the significance of difference between day 1 and either day 2 or 8 was P = .014 [n = 6]; Wilcoxon’s signed rank test). The differential effects of acute stimulation with growth factors on calcium-stimulated AA release is shown in Fig 2A. The release of AA from A23187-stimulated cells that were preincubated with growth factor diluent (FCS) remained relatively constant over the culture period. The effect of SCF declined concomitantly with the reduction in the proportion of cells with a blast-like morphology in the cultures (Fig 2B), and by day 8 no significant SCF responses were seen. In contrast, the priming effect of GM-CSF and IL-3 on A23187-stimulated PLA2 activity increased during the culture period to levels that were significantly greater than those seen with diluent. The increase in GM-CSF and IL-3 responses coincided with an increase in the proportion of more mature cells (promyelocytes, myelocytes, and metamyelocytes constituted about 60% of cells on day 8). TNFα had little effect on AA release at any stage of culture.
Analysis by TLC of tritiated products released from SCF-primed CD34+ cells.
In 3 experiments, primary CD34+ cells were labeled with3H-AA and incubated with SCF, followed by stimulation with ionophore as described above, and the tritiated lipids released into the supernatant were analyzed by TLC (see Materials and Methods). It was not possible to analyze AA release using mass measurements because of the limited number of primary cells available. The distribution of radiolabel in the different phases of the lipid extraction was 91% ± 1% (mean ± 1 SD) in the organic phase, 9% ± 1% in the aqueous phase, and 1% ± 1% in the BSA protein layer. The recovery of radioactivity from the cell supernatants in the organic phase was 97% ± 5%, and this confirmed the efficiency of the extraction procedure. A sample of the 3H-AA that was used for cell labeling was also extracted and subject to TLC in parallel with the cell supernatants. Figure 3 shows that the profile of the radioactive lipids released from cells primed with SCF and stimulated with A23187 was similar to the 3H-AA standard. These data demonstrated that AA was the predominant lipid released from SCF-stimulated CD34+ cells and validated the radiometric AA release assay.
Analysis of arachidonate release by TLC. Day-1 CD34+ cells labeled with 3H-arachidonic acid were incubated with either 5 μmol/L MAFP or DMSO diluent before priming for 10 minutes with 100 ng/mL SCF, followed by activation with 1 μmol/L A23187 for 20 minutes. The lipids were extracted, spotted onto the origin (0) of the TLC plate, and separated as described in Materials and Methods. The distribution of radioactivity in 1-cm fractions scraped from the plate is shown. The data shown are from a single experiment representative of 3 that were performed.
Analysis of arachidonate release by TLC. Day-1 CD34+ cells labeled with 3H-arachidonic acid were incubated with either 5 μmol/L MAFP or DMSO diluent before priming for 10 minutes with 100 ng/mL SCF, followed by activation with 1 μmol/L A23187 for 20 minutes. The lipids were extracted, spotted onto the origin (0) of the TLC plate, and separated as described in Materials and Methods. The distribution of radioactivity in 1-cm fractions scraped from the plate is shown. The data shown are from a single experiment representative of 3 that were performed.
Studies with the PLA2 inhibitor, MAFP.
To confirm that the arachidonate released from primitive myeloid cells was attributable to PLA2 activity, studies were performed with MAFP, which acts as a transition state analogue at the active site of PLA2 and is a dual inhibitor of both cPLA2and iPLA2.19 Primary CD34+ cells were preincubated with 5 μmol/L MAFP or DMSO diluent for 10 minutes, and AA release in response to priming with TPA, SCF, and GM-CSF, followed by stimulation with A23187, was measured with the radiometric assay. The data from 3 experiments are shown in Fig 4. In control samples incubated with DMSO diluent, TPA and SCF increased the cells’ responses to A23187 above that seen with growth factor diluent controls, whereas GM-CSF had no enhancing effect, which confirms the selective effect of SCF in priming AA release shown in Figs 1 and 2. Preincubation with 5 μmol/L MAFP inhibited AA release from the CD34+ cells, irrespective of the priming agent used. The arachidonate released from the primary CD34+ cells that were incubated with and without 5 μmol/L MAFP, followed by priming with SCF and stimulation with ionophore, was analyzed by TLC as described above. The data shown in Fig 3 confirm that MAFP inhibited AA release to near basal levels. MAFP at 5 μmol/L also inhibited A23187-stimulated AA release from CD34+ cells that had been cultured for 8 days with IL-3, IL-6, and SCF (94% inhibition compared with DMSO control; single experiment) as well as AA release from peripheral blood neutrophils (98% and 92% inhibition compared with DMSO control; data from 2 separate experiments). There is always concern that the effects of an inhibitor represent nonspecific toxicity to the cell, but it should be noted that 5 μmol/L MAFP had no inhibitory effect on TPA- or SCF-mediated MAP kinase (ERK2) activity in CD34+ cells (see below, Fig 6A). The data shown in Figs 3 and 4 provide evidence for PLA2 activity being the major enzyme responsible for cytokine-primed AA release.
The effect of MAFP on arachidonic acid release. Day-2 cultured CD34+ cells were incubated with either 5 μmol/L MAFP or DMSO diluent for 15 minutes, followed by priming with either 0.01% FCS diluent, 100 ng/mL SCF, or 500 ng/mL TPA, and activated with either 1 μmol/L A23187 or PBS. The data shown are the mean ± 1 SE of 3 experiments. The significance of the difference between MAFP- and DMSO-treated samples is shown (Student’s pairedt-test).
The effect of MAFP on arachidonic acid release. Day-2 cultured CD34+ cells were incubated with either 5 μmol/L MAFP or DMSO diluent for 15 minutes, followed by priming with either 0.01% FCS diluent, 100 ng/mL SCF, or 500 ng/mL TPA, and activated with either 1 μmol/L A23187 or PBS. The data shown are the mean ± 1 SE of 3 experiments. The significance of the difference between MAFP- and DMSO-treated samples is shown (Student’s pairedt-test).
Investigation of eicosanoid release by primary CD34+cells and their in vitro progeny.
Experiments were performed to determine whether the arachidonate produced by the CD34+ cells was metabolised by 5-lipoxygenase or cyclo-oxygenase. In initial experiments, cells radiolabeled with 3H-AA were preincubated with either DMSO diluent or 200 nmol/L MK886 (a specific inhibitor of 5-lipoxygenase)55 or 20 μmol/L indomethacin to inhibit cyclo-oxygenase, before stimulation with A23187. The radioactivity released into the extracellular medium was counted. No delipidated albumin was added to the reaction mixtures (such as was used in the assays of arachidonate release), because this would trap arachidonate and prevent its metabolism to eicosanoids. In these experiments, the radioactivity released from TF-1 cells or freshly purified CD34+ cells after stimulation with A23187 remained at basal levels, and no effect of MK886 or indomethacin was seen. In contrast, there was significant radioactive release from neutrophils stimulated with A23187 (11.7% ± 0.9% of total cell radioactivity, n = 17), which was inhibited to basal levels by preincubation with MK886 (1.1% ± 0.2%). In 2 experiments with monocytes, A23187 stimulated the release of 10.3% and 10.4% of cell radioactivity and this was inhibited to 5.1% and 5.4%, respectively, by indomethacin. These data suggested that there was little lipoxygenase or cyclo-oxygenase activity present in the primitive cells.
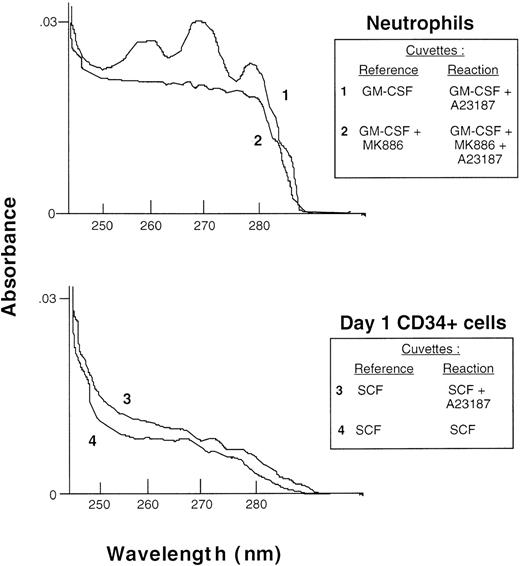
The data from the radiometric assays was confirmed by enzymeimmunoassays of nonradiolabeled cells. In 3 experiments, the amount of LTB4 released from day 1 CD34+ cells was 8 ± 2, 6 ± 1, and 6 ± 2 pg/5 × 105cells when these were stimulated with diluent, 100 ng/mL SCF, or SCF + 1 μmol/L A23187, respectively. Similar data were obtained with TF-1 cells. In comparison, neutrophils stimulated with A23187 produced approximately 2,000 times more LTB4 (12.3 ng/5 × 105 cells). Production of the peptidoleukotrienes LTC4, LTD4, and LTE4 in CD34+ cells stimulated as described before with either SCF or SCF + A23187 was less than 1 pg/5 × 105 cells, whereas monocytes stimulated with A23187 produced more than 1.2 ng/5 × 105 cells. As a further confirmation of these data, eicosanoid production by neutrophils primed with GM-CSF and by day 1 CD34+ cells primed with SCF was analyzed by difference spectroscopy. In these experiments, the absorption spectrum of supernatants from cells stimulated with A23187 was measured after subtraction of the spectrum of supernatants from unstimulated cells. Figure 5 (trace 1) shows the characteristic absoption spectrum of LTB4 produced by neutrophils after stimulation with A23187, with peaks at 260, 270, and 280 nm. In 4 experiments, the mean production of LTB4 and its 20 omega hydroxy- and carboxy-metabolites by neutrophils was determined from its extinction coefficient at 270 nm and was 24.6 ± 2.6 ng/106 cells, which is in close agreement with the concentration of LTB4 measured by the enzyme immunoassays. The absoption spectrum of LTB4 was not visible in trace 2 when neutrophils had been preincubated with 5-lipoxygenase inhibitor, MK886. In contrast, no leukotriene peaks were seen in SCF-primed CD34+ cells stimulated with A23187 (trace 3) or unstimulated control cells (trace 4).
Analysis of eicosanoid production by difference spectroscopy. Neutrophils (top panel) and day 1 CD34+cells (bottom panel), both at 1 × 107 cells/mL, were incubated, respectively, with 10 ng/mL GM-CSF or 100 ng/mL SCF, followed by 1 μmol/L A23187 or PBS diluent as indicated in the inset, and the absorption spectrum of the supernatants was measured in a dual-beam spectrometer with the cuvettes arranged as indicated.
Analysis of eicosanoid production by difference spectroscopy. Neutrophils (top panel) and day 1 CD34+cells (bottom panel), both at 1 × 107 cells/mL, were incubated, respectively, with 10 ng/mL GM-CSF or 100 ng/mL SCF, followed by 1 μmol/L A23187 or PBS diluent as indicated in the inset, and the absorption spectrum of the supernatants was measured in a dual-beam spectrometer with the cuvettes arranged as indicated.
Further studies were then performed on primary CD34+ cells that had been cultured for 8 days and were at an intermediate stage of differentiation, at which time they had lost CD34 antigen expression and were beginning to acquire functional respiratory burst activity. However, no leukotriene production was detected in response to stimulation with A23187 (data not shown).
Activation of p42/Erk2 MAP kinase in CD34+ cells.
A possible mechanism by which growth factors can prime cPLA2 activity is by phosphorylation and activation of MAP kinase (ERK2) with subsequent phosphorylation of the cPLA2isoform on serine 505, resulting in increased enzyme activity.28,29 This has been previously suggested as a mechanism of GM-CSF–mediated priming of PLA2 activity in neutrophils.23 We therefore sought to determine whether SCF, GM-CSF, and TPA could activate MAP kinase in primary CD34+ cells. Cells were stimulated with either 500 ng/mL TPA, 10 ng/mL GM-CSF, or 100 ng/mL SCF, and activation (phosphorylation) of p42/Erk2 MAP kinase was measured by gel retardation assay and Western blotting. The results from 3 experiments show that both TPA and SCF activated MAP kinase, but with different kinetics (Fig 6). MAP kinase activation by SCF and TPA was rapid, being apparent within 1 minute and maximal within 2 to 3 minutes of stimulation (Fig 6A). However, responses to SCF were transient and were virtually undetectable at 11 minutes poststimulation (Fig 6B), whereas the responses to TPA persisted at these time intervals. In contrast, no response to GM-CSF was apparent in the day 1 CD34+ cells (Fig 6B), in confirmation of our previous study.57 These data are consistent with the ability of TPA and SCF and the inability of GM-CSF to prime PLA2 activity in these primitive cells. However, GM-CSF activated ERK2 in day-8 cultured cells (Fig7A), as was previously reported.57
Activation of p42/Erk2 MAP kinase in primary CD34+ cells measured by gel retardation assay and Western blotting (see Materials and Methods). (A) Cells were stimulated with either cytokine diluent (0.001% FCS), SCF (100ng/mL), or TPA (500 ng/mL) for the times indicated. Replicate samples were preincubated with either DMSO diluent or the PLA2 inhibitor MAFP (5 μmol/L) for 15 minutes before cytokine stimulation. (B) Cells were incubated with cytokines as in (A) as well as GM-CSF (10 ng/mL) for the times indicated, and Erk2 activation was measured as in (A).
Activation of p42/Erk2 MAP kinase in primary CD34+ cells measured by gel retardation assay and Western blotting (see Materials and Methods). (A) Cells were stimulated with either cytokine diluent (0.001% FCS), SCF (100ng/mL), or TPA (500 ng/mL) for the times indicated. Replicate samples were preincubated with either DMSO diluent or the PLA2 inhibitor MAFP (5 μmol/L) for 15 minutes before cytokine stimulation. (B) Cells were incubated with cytokines as in (A) as well as GM-CSF (10 ng/mL) for the times indicated, and Erk2 activation was measured as in (A).
Effect of the MEK1 inhibitor, PD 098059, on CD34+ cells. (A) Cells cultured for 8 days in IL-3, IL-6, and SCF (day-8 cells) were incubated for 18 hours in the absence of growth factors to allow re-expression of growth factor receptors. Cells were then preincubated with 30 μmol/L PD 098059 or DMSO diluent for 30 minutes before stimulation with GM-CSF for 7 minutes, and p42/ERK2 activation was measured by gel retardation assay. Data from 2 separate experiments are shown. (B) Effect of PD 098059 on PLA2activity of primary CD34+ cells (day-1 cells, n = 5) or day-8 cultured cells (n = 3). Cells were incubated for 30 minutes with 30 μmol/L PD 098059 or DMSO diluent, and arachidonate release was measured after 10 minutes of priming with 100 ng/mL SCF, 10 ng/mL GM-CSF, or 500 ng/mL TPA as indicated, followed by activation with 1 μmol/L A23187 for 20 minutes. Basal release of arachidonate in unstimulated samples was subtracted from the A23187-stimulated values, and the data are expressed as the percentage of incorporated cellular radioactivity. The statistical significance of the difference between DMSO and PD-treated cells is shown: *.05 > P > .01; **.01 > P > .001 (Student’s paired t-test).
Effect of the MEK1 inhibitor, PD 098059, on CD34+ cells. (A) Cells cultured for 8 days in IL-3, IL-6, and SCF (day-8 cells) were incubated for 18 hours in the absence of growth factors to allow re-expression of growth factor receptors. Cells were then preincubated with 30 μmol/L PD 098059 or DMSO diluent for 30 minutes before stimulation with GM-CSF for 7 minutes, and p42/ERK2 activation was measured by gel retardation assay. Data from 2 separate experiments are shown. (B) Effect of PD 098059 on PLA2activity of primary CD34+ cells (day-1 cells, n = 5) or day-8 cultured cells (n = 3). Cells were incubated for 30 minutes with 30 μmol/L PD 098059 or DMSO diluent, and arachidonate release was measured after 10 minutes of priming with 100 ng/mL SCF, 10 ng/mL GM-CSF, or 500 ng/mL TPA as indicated, followed by activation with 1 μmol/L A23187 for 20 minutes. Basal release of arachidonate in unstimulated samples was subtracted from the A23187-stimulated values, and the data are expressed as the percentage of incorporated cellular radioactivity. The statistical significance of the difference between DMSO and PD-treated cells is shown: *.05 > P > .01; **.01 > P > .001 (Student’s paired t-test).
Effect of the MEK1 kinase inhibitor, PD 098059, on PLA2activity.
To test whether activation of MAP kinase was required for PLA2 activity in CD34+ cells, we sought to block MAP kinase activity and look at the effect on AA release. PD 098059 is a selective inhibitor of MAP kinase kinase (MEK1) and is therefore an upstream inhibitor of MAP kinase (p42 ERK2).58Figure 7A confirms that 30 μmol/L PD 098059 inhibited the activation of ERK2. CD34+ cells were then incubated for 15 minutes with either 30 μmol/L PD 098059 or DMSO diluent control, and arachidonate release after priming with growth factors and activation with ionophore was measured (see Materials and Methods). Figure 7B shows that PD 098059 partially inhibited AA release in day 1 cells primed with either growth factor diluent (to 84% ± 36% of no PD control; n = 5), SCF (to 46% ± 5% of control; .01 >P > .001), or TPA (to 31% ± 10% of control; .01 >P > .001). Similarly, in 3 experiments, PD 098059 partially inhibited AA release in day-8 cells primed by diluent (to 66% ± 8% of no PD control), GM-CSF (to 45% ± 8% of control; .01 >P > .001), and TPA (to 57% ± 8% of control).
DISCUSSION
This study has demonstrated that primitive CD34+hematopoietic cells express PLA2 activity, which was measured in intact cells by the extracellular release of arachidonate using a radiometric assay and confirmed by TLC. The magnitude of PLA2 activity in CD34+ cells stimulated by calcium ionophore was similar to that of peripheral blood neutrophils and monocytes. This degree of PLA2 activity in hematopoietic progenitor cells was relatively unexpected, because immature cells from the promyelocytic HL-60 line only reach their full capacity to release AA when they are stimulated to differentiate.34 37 It should be borne in mind that the CD34+ cells had been mobilized into the peripheral blood by in vivo G-CSF, and this may have altered the PLA2 activity of the cells in some way. However, in confirmation of the data with primary CD34+ cells, we found that primitive CD34+ TF-1 cells also express levels of PLA2activity that are comparable with those of mature cells.
Regulation of AA release by myeloid cell-specific growth factors such as GM-CSF has been demonstrated in mature human phagocytic cells.5,22-24 In the current study, we found that A23187-stimulated AA release from freshly purified CD34+cells was selectively enhanced by preincubation with SCF and to a minor degree by IL-3, but not significantly by GM-CSF or TNFα. This differential activation by SCF was confirmed in experiments in which SCF was able to activate p42 MAP kinase, whereas no responses to GM-CSF were seen. The activation of CD34+ cells by SCF at this early stage of differentiation is consistent with SCF being an early acting factor for hematopoiesis.60 Preliminary experiments with other early acting factors such as Flt-3 ligand and thrombopoietin failed to detect enhancement of PLA2 activity, suggesting that SCF has a specific role in this respect PLA2 (data not shown). At the present time, the difference in the ability of Flt-3 and SCF to mobilize AA is not understood. Both bind to receptors that are members of the tyrosine kinase-containing family of receptors, but they may have more subtle differences in signal transduction pathways; this is an area for future work.
It was previously shown that differentiation of myeloid CD34+ cells can be achieved by in vitro culture with IL-3, IL-6, and SCF, resulting in both morphological and partial functional maturation,57 and we therefore investigated whether changes in PLA2 activity also occurred under these conditions. There was a minor decrease over time in the responses to TPA + A23187 (which elicit maximal PLA2 responses in intact cells),59 which contrasts with the significant increase in PLA2 activity during HL-60 cell differentiation that was previously reported.34,37 When CD34+ cells were induced to differentiate, the pattern of responsiveness of PLA2 to growth factors also changed, in that A23187-stimulated PLA2 activity ceased to be enhanced by SCF, but was able to be upregulated by both IL-3 and GM-CSF, whereas the lack of response to TNFα remained unchanged. The differentiation-dependent changes in factor-enhanced PLA2activity may reflect either a change in receptor expression or changes in signaling pathways. The responsiveness to SCF during the first 2 days of culture is in accord with the report that 80% of progenitor cells freshly purified from peripheral blood express c-kit, the receptor for SCF,61 and the reduction in PLA2responses to SCF that we saw after 8 days in culture is in accord with the reported gradual loss of SCF receptors from progenitor cells induced to differentiate down the granulocytic pathway.61-63 Morphological analysis of the cultures confirmed that the loss of SCF responsiveness was associated with the reduction in the proportion of blast cells and development of promyelocytes, myelocytes, and metamyelocytes. In contrast, we recently reported that freshly purified CD34+ cells express very few high-affinity GM-CSF receptors, but these increased in number after culture in SCF, IL-3, and IL-6.57 However, the limiting factor for the acquisition of PLA2 responses to GM-CSF by cultured cells may not reside at the level of receptor expression, because our previous study also showed that there was a differentiation-linked increase in GM-CSF–mediated signaling down the JAK2, ERK2, and STAT5 transduction pathways that reached maximal levels well before there was any increase in the number of high-affinity GM-CSF receptors.57
At this stage, we do not know which isoform of PLA2mediates the capacity for AA release in CD34+ cells. AA release was enhanced by an increase in intracellular calcium stimulated by the calcium ionophore, A23187, and this was further increased by preincubation of the cells with the phorbol ester, TPA. These are characteristics shared by the 80- to 110-kD cytosolic isoform of PLA2,59 whose presence in mobilized peripheral blood CD34+ cells was recently detected at the mRNA level by RT-PCR.43 However, this group recently reported that the iPLA2 isoform was also expressed in stem cells.64 We also showed that the PLA2 activity could be enhanced by growth factors, which is again characteristic of cPLA2. SCF was previously shown to stimulate cPLA2 phosphorylation and arachidonate release within 10 minutes in mouse bone marrow-derived mast cells65 as well as increasing the expression of cPLA2 at the protein and mRNA level over a longer time course.66 Our experiments with MAFP, which is reported to be a dual inhibitor of both cPLA2 and iPLA2,19 show inhibition of both unprimed and growth factor-primed AA release and suggest that either of these PLA2 isoforms are candidates. When primary CD34+ cells were incubated with the MEK1 inhibitor, PD 098059,58 PLA2 activity, whether in SCF-primed or unprimed cells, was partially inhibited (to 30% to 50% of control responses), suggesting the involvement of MEK1 and thus most probably ERK2 in PLA2 activation. This evidence increases the likelihood that the isoform in CD34+ cells is cPLA2, because this isoform can be phosphorylated and activated by ERK2.28,29 However, inhibition of PLA2 by the MEK1 inhibitor was only partial, and it is possible that there is another pathway of activation. Indeed, recent evidence from platelet studies suggests that p38 and other kinases have a role in the activation of cPLA2 in certain cell types.30 Although not previously reported, it is possible that ionophore stimulation of PLA2 may also be partially mediated by ERK2.
The kinetics of activation of p42 MAP kinase by SCF were extremely rapid and transient as measured by a gel retardation assay (optimal at 2 minutes and decreasing by 5 to 11 minutes). The first demonstration of the activation of MAP kinase by SCF in human myeloid MO7 cells showed reponses as sustained as 15 minutes poststimulation.67 However, our data are in accord with a previous study using human fetal liver cells that showed similarly rapid and transient activation of JAK-2 kinase by SCF.68Rapid and transient ERK2 activation appears to be a feature of growth factor signaling in immature myeloid cells, because previous work with both HL-60 cells69,70 and CD34+cells57 show a conversion from transient to prolonged ERK2 activation after differentiation induction.
Although there is preliminary evidence that CD34+ cells express mRNA for both prostaglandin H synthase I and II, as well as 5-lipoxygenase,43 we have shown that stimulation of PLA2 activity was not associated with the release of leukotrienes. This is in contrast with mature phagocytes that metabolize endogenously generated AA to eicosanoid molecules that are important for regulating host defense. The apparent lack of 5-lipoxygenase activity in these progenitor cells is consistent with the observation that the acquisition of 5-lipoxygenase activity depends on the synthesis of the 5-lipoxygenase activating protein later during myeloid maturation.38 There are conflicting reports that myeloid cell proliferation is regulated by eicosanoids.39-42 Because we observed the proliferation and differentiation of myeloid stem cells during in vitro culture in the absence of eicosanoid production, our data suggest that proliferation of CD34+ cells may not depend on endogenous eicosanoid production.
This study presents evidence for active PLA2 in very primitive myeloid cells. Its regulation by SCF, which promotes the maintenance and proliferation of these early cells, supports the notion that PLA2 activity has a role in the physiology of human stem cells. This report has also shown that AA release is under the regulation of later growth factors in a time-dependent pattern of regulation during CD34+ cell differentiation and suggests that AA may be needed at several specific stages of myeloid cell differentiation. Further work is underway to investigate its role in regulating both proliferation and differentiation of human hematopoietic cells.
ACKNOWLEDGMENT
The authors are grateful to Stuart J Ings for preparation of CD34+ cells.
Supported by the Kay Kendall Leukaemia Fund (P.J.R.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pamela J. Roberts, PhD, Department of Haematology, University College London Medical School, 98 Chenies Mews, London WC1E 6HX, UK; e-mail: pamela.roberts@ucl.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal