Abstract
Expansion of early lymphoid progenitors requires interleukin-7 (IL-7), which functions through γc-mediated receptor activation of Jak3. Jak3 deficiency is a cause of severe combined immunodeficiency (SCID) in humans and mice. IL-3 activates many of the same signaling pathways as IL-7, such as Stat5, but achieves this effect through the activation of Jak2 rather than Jak3. We hypothesized that expansion of an IL-7–responsive precursor population through a Jak3-independent pathway using IL-3 may stimulate early lymphoid progenitors and restore lymphopoiesis in Jak3−/− mice. Newborn Jak3−/− mice that were injected with IL-3 demonstrated thymic enlargement, a 2- to 20-fold increase in thymocyte numbers, and up to a 10-fold expansion in the number of CD4+, CD8+, and B220+/IgM+ splenic lymphocytes, consistent with an effect upon an early lymphoid progenitor population. In contrast to control mice, IL-3–treated Jak3−/− mice challenged with the allogeneic major histocompatibility complex (MHC) class I-bearing tumor P815 developed a specific CD8-dependent cytotoxic T lymphocyte (CTL) response. IL-3–treated mice also mounted influenza-specific CTL responses and survival was prolonged. The beneficial effects of IL-3 are proposed to be produced by stimulation of a lymphoid precursor population of IL-7R+/IL-3R+ cells that we identified in wild-type bone marrow. In vitro, we show that an early IL-7R+ lymphoid progenitor population expresses IL-3R and proliferates in response to IL-3 and that IL-3 activates Stat5 comparably to IL-7. Clinically, IL-3 may therefore be useful treatment for X-linked and Jak3-deficient SCID patients who lack bone marrow donors.
LYMPHOID DEVELOPMENT is regulated by cytokines, including interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15. The receptors for these cytokines share the common γ chain (γc), mutations of which cause the X-linked form of severe combined immunodeficiency (SCID). This mutation accounts for approximately half of all cases of SCID.1,2 The γc chain binds the cytoplasmic tyrosine kinase Jak3 and mediates the activation of Jak3 after ligand binding.3-7Jak3 mutations occur in an autosomal recessive subset of SCID that comprises approximately 10% of all cases of SCID.2,8,9 Defects in signaling via the γcand Jak3 are virtually indistinguishable, as are the phenotypes of Jak3-deficient and X-linked SCID. The phenotype of Jak3-deficient mice is similar to that of the human condition, except that a profound B lymphopenia exists in the mutant mice.10-12
Among the cytokines that use the γc-Jak3 pathway, IL-7 plays an essential role in the early development of the lymphoid lineages. Mutant mice that lack IL-7 or that lack the IL-7–specific receptor α-chain (IL-7Rα) also suffer from SCID.13,14Nonetheless, in all of these murine models of SCID, some lymphoid cells are generated, and in humans there may be a significant expansion of lymphoid populations particularly affecting B cells.9 15These observations have lead us to investigate whether other cytokines might be able to mediate early lymphoid expansion, even in the absence of a functional response to the cytokines that use γc and Jak3, and thereby compensate for the severe immunodeficiency associated with mutations in these pathways.
One of the cytokines that had previously been suggested to affect early progenitors for both the myeloid and lymphoid lineages is IL-3.16 Indeed, IL-3 was first isolated because of its inferred role in thymocyte maturation.17 Recent studies of cytokine signaling have demonstrated a remarkable similarity in the signaling pathways that are activated. In particular, IL-3 and IL-7 activate virtually identical pathways, although the IL-7 receptor system requires Jak3, whereas the IL-3 receptor system requires the highly related Jak2.7 18 Hence, if the receptor for IL-3 was expressed in early lymphoid progenitors, it should allow some degree of expansion of this population by a Jak2-dependent pathway and compensate for later failure to expand cells via a Jak3-dependent pathway. The studies described here demonstrate that IL-3 can significantly expand early lymphoid progenitors and enhance immune function in vivo.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained in the Animal Resources Center of St Jude Children’s Research Hospital (Memphis, TN). Jak3−/− mice were generated by targeted gene disruption10 and were housed under specific pathogen-free (SPF) conditions. Age-matched wild-type mice were either Jak3+/+ mice from the knockout colony or B6J129SVF2 mice that have a similar genetic background. SCID mice were BALB/cByJSCID and were also held under SPF conditions.
Antibodies and reagents.
Phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) specific for CD45R/B220 (clone RA3-6B2), CD43 (clone S7), and CD4 (clone RM4-4) and fluorescein isothiocyanate (FITC)-conjugated MoAbs specific for c-kit (clone 2B8), CD19 (clone 1D3), CD8 (clone 53-6.7), CD24 (clone M1/69), BP-1 (clone 6C3), and IgM (clone R6-60.2) were purchased from PharMingen (San Diego, CA). Unlabeled rat MoAbs specific for IL-7Rα chain (clone A7R34) and IL-3Rα chain (clone 5B11) were kindly provided by Drs S. Nishikawa19 (Kyoto University, Kyoto, Japan) and by D. Gorman20(DNAX Research Institute, Palo Alto, CA), respectively. Anti–IL-7Rα and IL-3Rα MoAbs were directly conjugated to R-PE and Alexa 488 dyes, respectively, according to the manufacturer’s recommendations (Molecular Probes, Eugene, OR). All cytokines were purchased from R&D Systems (Minneapolis, MN). [3H]-thymidine was obtained from Amersham Life Sciences (Cleveland, OH).
Preparation of bone marrow cell suspensions.
Femurs and tibiae were flushed with Hanks’ balanced salt solution (HBSS) containing 10% fetal calf serum (FCS). Cell debris was depleted by passage through nylon mesh screens.
Flow cytometric analysis.
To examine the expression of surface antigens, the cells were washed and then resuspended in phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin and 0.02% sodium azide (PBSA). Saturating concentrations of antibodies were added and incubated for 10 minutes at room temperature. Cells were washed in PBSA after each incubation step. Red blood cells from primary bone marrow cell suspensions were lysed by treatment with FACS lysis solution (Becton Dickinson, San Jose, CA). Finally, cells were fixed in 0.5% paraformaldehyde in PBS for analysis on a FACScan flow cytometer (Becton Dickinson). Fluorescence data were analyzed by the CellQuest program and presented either in the form of histograms or 2-parameter log contour plots. Dead cells and debris were excluded by characteristic forward and side scatter profiles.
Enrichment of IL-7R positive cells in vitro.
Cells from normal bone marrow were treated with ammonium chloride-potassium bicarbonate solution (150 mmol/L NH4Cl and 10 mmol/L KHCO3) to lyse red blood cells. Cells (1 × 106/mL) were cultured in RPMI-1640 medium containing 5% FCS, 2 mmol/L L-glutamine, 100 U/mL each of penicillin and streptomycin, and 50 μmol/L 2-mercaptoethanol (R5 medium) on irradiated NIH-3T3 cells that retrovirally expressed the mIL-7 cDNA (T220-29 cells; kindly provided by Dr Owen N. Witte [University of California, Los Angeles, CA] and Dr Martine F. Roussel [St Jude Children’s Research Hospital, Memphis, TN]). After 4 days in culture, greater than 95% of nonadherent cells were B220+.
Proliferation assays.
Wild-type (B6J129SVF2) bone marrow cells were grown on T220-29 cells for 8 to 10 days. The cells were washed and resuspended at a final concentration of 5 × 105 cells/mL in R5 medium alone or in medium that contained growth factors: recombinant murine IL-7 (rmIL-7; 5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). Cells (1 × 105) were added to each well of a 96-well flat-bottomed plate and incubated for 36 hours at 37°C in 5% CO2 in air. The cells were then pulse-labeled with 3 μCi/well of [3H]-thymidine for 14 hours and the incorporation of [3H]-thymidine was measured. Individual thymi were removed to tubes containing HBSS, dissociated into a single-cell suspension, washed in HBSS, and resuspended to 107 cells/mL in TCM (S-minimal essential medium [S-MEM] plus 10% FCS, 1% L-glutamine, 1% antibiotic/antimycotic, 1% nonessential amino acids, 0.5% essential amino acids, and 50 μmol/L 2-mercaptoethanol). Thymocytes were stimulated for 72 hours with either anti-CD3ε (clone 145-2C11; 10 μg/mL), anti-CD3ε plus phorbol 12-myristate 13-acetate (PMA; 1 ng/mL), Concanavalin A (CA; 20 μg/mL), CA plus IL-2 (10 U/mL), or CA plus IL-7 (5 ng/mL) and were pulsed with 1 μCi [3H]-thymidine for 18 hours before harvest. Assays were performed in triplicate and the incorporation of [3H]-thymidine was measured. Stimulation indices were determined as the mean experimental values over the mean control values for each experiment.
STAT5 gel mobility shift assay.
Cells were stimulated with IL-3 (10 ng/mL) or IL-7 (50 ng/mL) for 30 minutes and used for the assay as described previously.10The synthetic oligonucleotide probe used as the STAT5A binding site was as follows: 5’ GATCCGAATTCCAGGAATTCA 3’; 3’ GCTTAAGGTCCTTAAGTCTAG 5’. The probe was labeled with a32P-dCTP by blunt-end filling with the Klenow fragment.
Cytokine injections.
Recombinant murine IL-3 was reconstituted in RPMI-1640 medium (1 mg/mL) and administered daily via subcutaneous injection into newborn Jak3−/− mice from days 1 to 14 (10 μg/kg/d) after birth. Recombinant murine SCF was prepared (5 mg/mL) and, similarly, injected subcutaneously for the first 14 postnatal days (50 μg/kg/d). The same volume of medium was injected into −/− littermates as a negative control.
Allogeneic tumor challenge and cytotoxic T lymphocyte (CTL) assay.
P815 mastocytoma cells grown in RPMI-1640 medium containing 10% FCS were washed in HBSS and resuspended in PBS to 2 × 106cells/mL. Four mice from each group each received 1 mL of cell suspension: 0.5 mL intraperitoneally and 0.5 mL subcutaneously. Two weeks later, 2 mice from each group were killed, and splenocytes were isolated, washed in HBSS, and resuspended in S-MEM medium with 10% FCS. Splenocytes (H-2b) were diluted in medium and cocultured with either 51Cr-labeled P815 (H-2d) target cells or 51Cr-labeled SV-B6KH(H-2b) target cells in a standard 6-hour in vitro assay.21 The percentage of specific lysis based on 51Cr release was determined by the following ratio: (target cell release with effector splenocytes from P815 injected mice − spontaneous release)/(maximum release − spontaneous release).
In vivo CD8 depletion.
Mice were depleted in vivo of CD8+ T cells22 by successive intraperitoneal administration of 0.5 mL of diluted mouse ascitic fluid that contained the 2.43.1 (anti-CD8) MoAb.
Influenza A virus infection experiments.
Five-week-old mice were anesthetized by intraperitoneal administration of Avertin (2,2,2-tribromoethanol) and then infected intranasally with 30 μL of PBS containing 240 hemagglutinating units of the influenza A HKx31 virus.23
Statistical analysis.
Thymocyte numbers were compared using a 2-tailed Student’st-test.
RESULTS
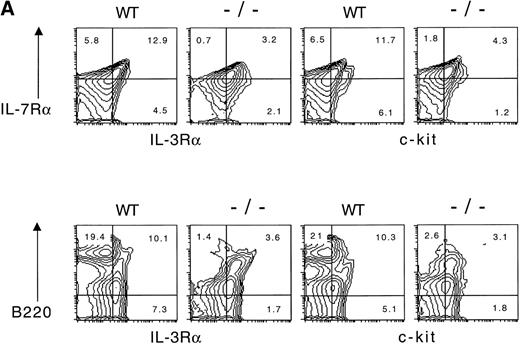
We initially examined the bone marrow from wild-type and Jak3−/− mice for the presence of cells that expressed the IL-7 receptor α (IL-7Rα) chain and the IL-3 receptor α (IL-3Rα) chain by flow cytometry (Fig1). As shown, approximately 19% of wild-type bone marrow cells express the IL-7Rα chain, and this population is clearly reduced in bone marrow from Jak3−/− mice. Among the wild-type IL-7Rα+ cells, approximately 70% also express the IL-3Rα. Similarly, a subpopulation of B220+ cells in wild-type mice also expressed IL-3Rα and were reduced in frequency in the bone marrow of Jak3−/− mice. Because stem cell factor (SCF) has been implicated in early hematopoietic progenitor expansion, we examined the pattern of expression of the SCF receptor, c-kit. As indicated, a population of cells expressing either IL-7Rα or B220 together with c-kit was evident in wild-type mice but reduced in Jak3−/− mice. The results are consistent with the hypothesis that a population of IL-7Rα+/IL-3Rα+ cells exists in the bone marrow, which is reduced in the Jak3−/− mice. This population may be a precursor for an IL-7Rα+/IL-3Rα− lymphoid-committed cell.
(A) IL-7R and IL-3R are coexpressed by a minor fraction of wild-type and Jak3-deficient bone marrow cells. Bone marrow cells were obtained from wild-type (WT) or Jak3−/−(−/−) and were stained, fixed, and analyzed by flow cytometry. One hundred and fifty thousand cells were analyzed in a lymphoid gate defined by scatter criteria. (B) Staining with isotype control antibodies for anti–IL-7R and anti–IL-3R antibodies is shown for wild-type and Jak3−/− lymphoid-gated cells from bone marrow. The numbers in each quadrant indicate the percentage of positive cells in total bone marrow.
(A) IL-7R and IL-3R are coexpressed by a minor fraction of wild-type and Jak3-deficient bone marrow cells. Bone marrow cells were obtained from wild-type (WT) or Jak3−/−(−/−) and were stained, fixed, and analyzed by flow cytometry. One hundred and fifty thousand cells were analyzed in a lymphoid gate defined by scatter criteria. (B) Staining with isotype control antibodies for anti–IL-7R and anti–IL-3R antibodies is shown for wild-type and Jak3−/− lymphoid-gated cells from bone marrow. The numbers in each quadrant indicate the percentage of positive cells in total bone marrow.
We next examined whether IL-7Rα+ cells contained a population of cells capable of responding to IL-3. For these experiments, wild-type IL-7Rα+ bone marrow cells were enriched by culturing on stromal cells secreting IL-7. In these cultures, the majority of the IL-7Rα+ cells were B cells (B220+, CD19+) and expressed c-kit and had a pro-B–cell or early pre-B–cell phenotype (B220+, CD43+ [Fig 2A] and CD24+, BP-1+/−, IgM−[data not shown]).24 In contrast, no cells were obtained from the cultures when bone marrow cells from Jak3−/− mice were used. Nearly all cells in the cultures also expressed a low level of IL-3Rα. Moreover, there was not a significant fraction of IL-3Rα+ cells that were either IL-7Rα− or B220−, demonstrating the enrichment for lymphoid progenitors relative to myeloid lineage cells (Fig 2A). The responses of these cells to various cytokines are shown in Fig 2B. As indicated, the cells responded to both IL-7 and IL-3, and the response to IL-3 was approximately 80% that of IL-7. As another functional measure of the response of the cells, we examined the ability of IL-7 or IL-3 to induce the activation of Stat5 DNA binding activity. As shown in Fig 2C, both cytokines induced comparable levels of Stat5 DNA binding activity.
IL-7–responsive bone marrow cells are pro-B cells and early pre-B cells that express IL-7R, IL-3R, and c-kit. (A) Flow cytometric analysis of IL-7R, IL-3R, B220, and c-kit expression of IL-7–responsive normal bone marrow cells. The cells are IL-7R+, predominantly CD19+ and c-kit+, and B220+, CD43+. They also express IL-3R at low density, and after gating, these cells were found to coexpress IL-7R and B220 (lower histograms). Bone marrow cells from a 7-week-old B6J129SVF2 mouse were cultured for 10 days on NIH-3T3 cells that secrete mIL-7 (T220-29 cells) and then stained, fixed, and analyzed by flow cytometry. Fifty thousand cells were analyzed without gating for size. The percentage of positive cells is shown in the quadrant. Histograms: dotted line, isotype-matched control antibody; heavy line, antibody. (B) IL-3 and SCF induce the proliferation of IL-7–responsive cells. IL-7–responsive cells (wild-type bone marrow cells cultured for 9 days on T220-29 cells) were washed and cultured without feeder cells in the presence of rmIL-7 (5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). The cells were pulse-labeled with [3H]-thymidine and the incorporation of [3H]-thymidine was measured as counts per minute (cpm). Error bars show the standard deviation of 12 replicates. (C) IL-3 and IL-7 both activate STAT5 in IL-7R+ B-lymphoid progenitors from normal bone marrow. A gel mobility shift assay was performed after 10 hours of IL-7 starvation by using +/+ bone marrow cells that had been cultured for 14 days on T220-29 cells. The addition of anti-Stat5A antiserum50 reduced STAT5-DNA complex formation, whereas control serum did not. The arrowhead indicates the position of the Stat5-DNA complex.
IL-7–responsive bone marrow cells are pro-B cells and early pre-B cells that express IL-7R, IL-3R, and c-kit. (A) Flow cytometric analysis of IL-7R, IL-3R, B220, and c-kit expression of IL-7–responsive normal bone marrow cells. The cells are IL-7R+, predominantly CD19+ and c-kit+, and B220+, CD43+. They also express IL-3R at low density, and after gating, these cells were found to coexpress IL-7R and B220 (lower histograms). Bone marrow cells from a 7-week-old B6J129SVF2 mouse were cultured for 10 days on NIH-3T3 cells that secrete mIL-7 (T220-29 cells) and then stained, fixed, and analyzed by flow cytometry. Fifty thousand cells were analyzed without gating for size. The percentage of positive cells is shown in the quadrant. Histograms: dotted line, isotype-matched control antibody; heavy line, antibody. (B) IL-3 and SCF induce the proliferation of IL-7–responsive cells. IL-7–responsive cells (wild-type bone marrow cells cultured for 9 days on T220-29 cells) were washed and cultured without feeder cells in the presence of rmIL-7 (5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). The cells were pulse-labeled with [3H]-thymidine and the incorporation of [3H]-thymidine was measured as counts per minute (cpm). Error bars show the standard deviation of 12 replicates. (C) IL-3 and IL-7 both activate STAT5 in IL-7R+ B-lymphoid progenitors from normal bone marrow. A gel mobility shift assay was performed after 10 hours of IL-7 starvation by using +/+ bone marrow cells that had been cultured for 14 days on T220-29 cells. The addition of anti-Stat5A antiserum50 reduced STAT5-DNA complex formation, whereas control serum did not. The arrowhead indicates the position of the Stat5-DNA complex.
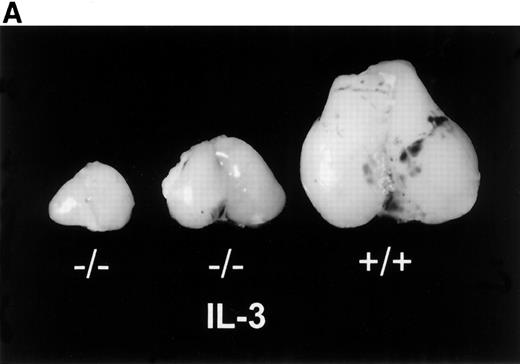
The results given above demonstrated the existence of an IL-3–responsive population of IL-7Rα+ lymphoid progenitors. However, the essential question was whether an IL-3–responsive lymphoid progenitor population could be demonstrated in vivo. We reasoned that such a population might be difficult to detect during normal lymphoid development, during which IL-7 is the predominant functional cytokine. Because the IL-3 receptor does not require Jak3, unlike the IL-7 receptor, we examined the ability of IL-3 to expand early lymphoid progenitors in the absence of IL-7 signaling by using Jak3−/− mice. In these experiments, newborn Jak3−/− mice were injected subcutaneously with recombinant IL-3 (10 μg/kg/d) for 14 days and analyzed at various times thereafter. For comparison, Jak3−/− mice were also injected with SCF (50 μg/kg/d). At 2 to 3 weeks of age, there was a clear response in the IL-3–treated mice, as was evident from thymic size (Fig 3A). The thymocyte numbers in IL-3–treated mice were 2- to 20-fold higher than those of littermates injected with control media (Fig 3B) or with SCF (data not shown). The mean number of thymocytes (±SD) from wild-type mice was 1.09 (±0.25) × 108. The mean thymocyte number in IL-3–treated Jak3-deficient mice (1.44 [±0.47] × 107) was significantly different from that for control-treated Jak3-deficient mice (2.95 [±2.87] × 106; P < .001). This effect was sustained such that IL-3–treated Jak3−/− mice had 4 to 10 times the number of thymocytes compared with control mice at 4 to 6 weeks of age (data not shown). Thymocytes from control or IL-3–treated Jak3−/− mice, as well as thymocytes from wild-type mice, had a comparable pattern of expression of CD4 and CD8 single- and double-positive cells. These results indicate that the primary effect of IL-3 was to expand the normal, IL-7–responsive, thymocyte populations.
Effects of IL-3 treatment on lymphoid populations. IL-3–treated Jak3−/− mice have thymic enlargement and increased numbers of thymocytes and peripheral T and B cells. (A) Thymi from 15-day-old mice are shown. (B) Thymocyte number from mice at 2 to 3 weeks of age. Control-treated Jak3−/− mice (•; n = 17), IL-3–treated Jak3−/− littermates (○; n = 16), and Jak3+/+ mice (□; n = 13). (C) Flow cytometric analysis of splenocytes from 3-week-old mice. The percentage of positive cells is shown for each quadrant. This experiment is representative of 4 independent experiments.
Effects of IL-3 treatment on lymphoid populations. IL-3–treated Jak3−/− mice have thymic enlargement and increased numbers of thymocytes and peripheral T and B cells. (A) Thymi from 15-day-old mice are shown. (B) Thymocyte number from mice at 2 to 3 weeks of age. Control-treated Jak3−/− mice (•; n = 17), IL-3–treated Jak3−/− littermates (○; n = 16), and Jak3+/+ mice (□; n = 13). (C) Flow cytometric analysis of splenocytes from 3-week-old mice. The percentage of positive cells is shown for each quadrant. This experiment is representative of 4 independent experiments.
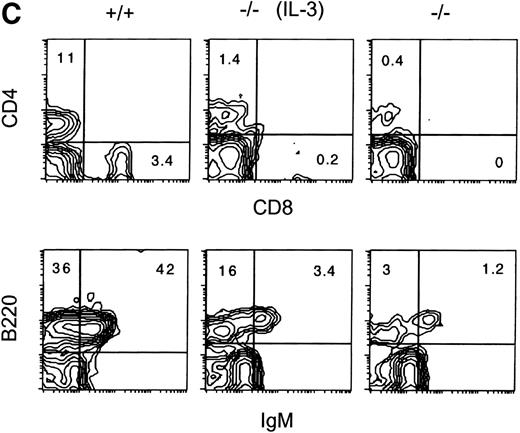
The effects of IL-3 treatment of Jak3−/− mice on peripheral T and B cells were also examined (Fig 3C). The numbers of splenic CD4+ or CD8+ T cells were increased 2- to 10-fold and 2- to 6-fold, respectively, relative to control-treated littermates. The percentage and absolute numbers of B220+, IgM+ splenic cells also increased in the spleens of IL-3–treated Jak3−/− mice relative to control-treated mice. This value ranged from 3- to 10-fold, depending on the individual animals (Table 1). In contrast to IL-3, there were no significant differences in the percentage or absolute numbers of splenic T or B cells in SCF-treated mice relative to the controls. As anticipated, IL-3 also increased the percentage of cells expressing the myeloid lineage markers Gr-1 and Mac-1 by 3- to 4-fold and the total number of splenocytes was increased by 2- to 3-fold (data not shown).
IL-3 Treatment of Jak3-Deficient Mice Increased the Proportion of Peripheral CD4+ and CD8+ T Cells and B220+IgM+ B Cells
| Experimental Group . | Average Percentage of Positive Splenocytes . | ||
|---|---|---|---|
| CD4+ . | CD8+ . | B220+IgM+ . | |
| Jak3+/+ | 7.9 (±3.1) | 3.0 (±0.4) | 40.1 (±6.2) |
| Jak3−/− | 0.4 (±0.1) | 0.3 (±0.2) | 1.0 (±0.3) |
| Jak3−/− (IL-3 treatment) | 1.6 (±0.2) | 1.0 (±0.8) | 4.9 (±1.5) |
| Experimental Group . | Average Percentage of Positive Splenocytes . | ||
|---|---|---|---|
| CD4+ . | CD8+ . | B220+IgM+ . | |
| Jak3+/+ | 7.9 (±3.1) | 3.0 (±0.4) | 40.1 (±6.2) |
| Jak3−/− | 0.4 (±0.1) | 0.3 (±0.2) | 1.0 (±0.3) |
| Jak3−/− (IL-3 treatment) | 1.6 (±0.2) | 1.0 (±0.8) | 4.9 (±1.5) |
In 4 independent experiments, splenocytes from individual mice (2 to 4 mice in each group) were pooled and analyzed. The average percentage of splenocytes positive (±SD) for each set of phenotypic markers is shown.
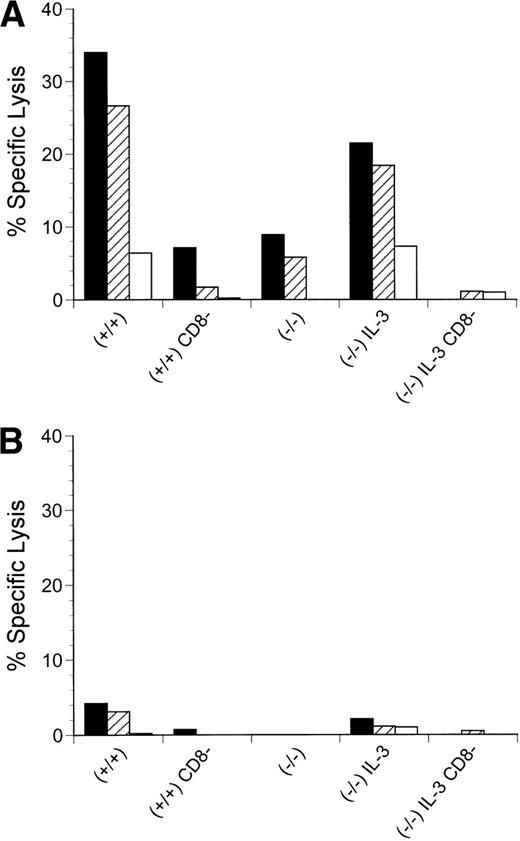
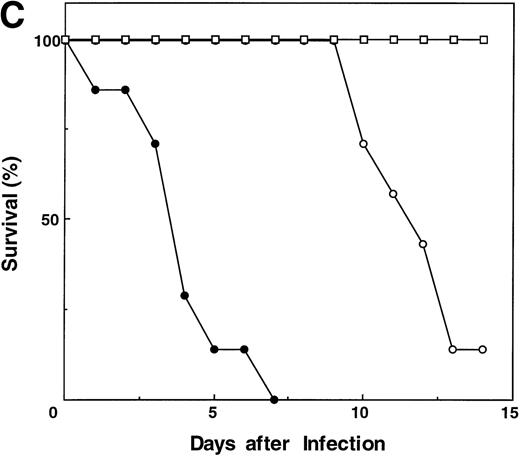
Although IL-3 can expand the number of lymphoid populations by expanding the early progenitor population, it would be anticipated that the lack of Jak3 would preclude functional reconstitution, because the cells would not be able to respond to any of the other cytokines that require γc and Jak3. This point is evident in the lack of a detectable response of thymocytes from IL-3–treated Jak3−/− mice to anti-CD3 in combination with PMA (Fig 4). In addition, peripheral T cells were not responsive (data not shown). However, some level of function exists in the IL-3–amplified, peripheral T cells, even in the absence of Jak3. When we examined the T-cell response to intraperitoneal injections of the allogeneic major histocompatibility complex (MHC) class I-bearing tumor, P815 (Fig 5A and B), a P815-specific CTL response was mounted by IL-3–treated, Jak3−/− mice but not by either control-treated Jak3−/− mice or SCID mice (data not shown). Both in vitro (data not shown) and in vivo depletion of CD8+ cells abolished this response. However, survival of the IL-3–treated Jak3−/− mice was not significantly prolonged relative to the control-treated Jak3−/− mice. Similarly, IL-3–treated Jak3−/− mice, but not controls, displayed influenza-specific cytolytic activity after infection with an attenuated strain of influenza virus. This was associated with a prolonged survival to influenza, although ultimately all of the mice succumbed to the infection, whereas control Jak3+/+ mice survived such infections (Fig 5C).
Proliferative responses of thymocytes from Jak3+/+ mice (+/+), control-treated Jak3−/− mice (−/−), and IL-3–treated Jak3−/− littermates (−/− IL-3). Jak3−/− thymocytes do not respond to T-cell mitogenic and Jak3-dependent cytokine stimuli. Thymocytes were stimulated with mitogen/cytokine combinations as described in Materials and Methods, and their proliferation was measured by incorporation of [3H]-thymidine. Values are average stimulation indices ± SD (n = 2 in each group).
Proliferative responses of thymocytes from Jak3+/+ mice (+/+), control-treated Jak3−/− mice (−/−), and IL-3–treated Jak3−/− littermates (−/− IL-3). Jak3−/− thymocytes do not respond to T-cell mitogenic and Jak3-dependent cytokine stimuli. Thymocytes were stimulated with mitogen/cytokine combinations as described in Materials and Methods, and their proliferation was measured by incorporation of [3H]-thymidine. Values are average stimulation indices ± SD (n = 2 in each group).
Effects of IL-3 treatment on lymphocyte function. CD8-mediated cytolytic activity of IL-3–treated Jak3−/− splenocytes after challenge with allogeneic P815 tumor cells. P815 cells were injected into wild-type (+/+) mice, Jak3-deficient (−/−) mice, or Jak3−/− mice receiving IL-3 (IL-3) in the presence or absence (CD8−) of CD8+ T cells. The CTL response from pooled splenocytes was measured 14 days after the administration of P815 cells (n = 2 in each group). Triplicate wells for each sample were analyzed and the mean value is shown. The effector to target ratios of 30:1 (▪) are presented using either (A) allogeneic P815 or (B) syngeneic SVB6KHA target cells. This experiment is representative of 3 independent experiments. (C) Survival of mice after influenza A HKx31 virus infection (n = 7 in each group). Control-treated Jak3−/− mice (−/−; •), IL-3–treated Jak3−/− littermates (−/− IL-3; ○), and Jak3+/+ mice (+/+; □).
Effects of IL-3 treatment on lymphocyte function. CD8-mediated cytolytic activity of IL-3–treated Jak3−/− splenocytes after challenge with allogeneic P815 tumor cells. P815 cells were injected into wild-type (+/+) mice, Jak3-deficient (−/−) mice, or Jak3−/− mice receiving IL-3 (IL-3) in the presence or absence (CD8−) of CD8+ T cells. The CTL response from pooled splenocytes was measured 14 days after the administration of P815 cells (n = 2 in each group). Triplicate wells for each sample were analyzed and the mean value is shown. The effector to target ratios of 30:1 (▪) are presented using either (A) allogeneic P815 or (B) syngeneic SVB6KHA target cells. This experiment is representative of 3 independent experiments. (C) Survival of mice after influenza A HKx31 virus infection (n = 7 in each group). Control-treated Jak3−/− mice (−/−; •), IL-3–treated Jak3−/− littermates (−/− IL-3; ○), and Jak3+/+ mice (+/+; □).
DISCUSSION
The results show that the functional redundancy of the cytokines involved in lymphoid development may provide an opportunity to compensate for genetic disorders involving their signaling pathways. Jak3−/− mice represent a suitable model to test this hypothesis, because most of the cytokines affecting lymphoid development do not function, thus permitting otherwise redundant cytokines to be identified. The model has the additional advantage of a low background of cytokine effects, because functional T cells, which could produce cytokines such as IL-3, are not generated. Furthermore, Jak3−/− mice may be used to investigate the potentially useful therapeutic effects of later-acting cytokines such as IL-12, which, although required for T helper 1 maturation, do not use Jak3-dependent pathways.25
In vivo, we identified among lymphoid cells in the bone marrow of wild-type and Jak3−/− mice an IL-7Rα+, IL-3Rα+ subpopulation that was predominantly B220lo. Although this subpopulation was reduced approximately 4-fold in Jak3−/− mice, it was relatively well preserved in contrast to the markedly reduced subpopulation of mature B220+, IgM+ B cells. The relationship of the IL-7Rα+/ IL-3Rα+cell population to the Lin− IL-7R+Thy-1− Sca-1lo c-Kitlo common lymphoid progenitor (CLP) described by Kondo et al26 is not defined, but investigation of IL-3Rα expression on the CLP may contribute to further understanding of lymphoid lineage development.
Treatment of Jak3−/− mice with IL-3 increased thymic size, thymocyte number, and the numbers of peripheral B and T cells and enhanced cytolytic T-cell function. These effects of IL-3 upon lymphopoiesis in vivo are consistent with an effect on an early lymphoid progenitor population. We hypothesized that IL-3 acts upon a progenitor cell population that also expresses IL-7 but which cannot respond to IL-7 in Jak3-deficient mice. To test this hypothesis, we enriched wild-type bone marrow for IL-7–responsive cells and found that they also expressed IL-3 receptor. Although not proven, we expected that this relatively pure population of IL-7–responsive cells would represent a physiological target for IL-3 action equivalent to the presumed target population in Jak3-deficient mice. Thus, our in vitro data indirectly support the notion that IL-7Rα+, IL-3Rα+ bone marrow cells were the target of IL-3 action in vivo: wild-type IL-7Rα+ bone marrow cells, which were enriched by culture in IL-7, expressed the receptor for IL-3, and both proliferated in response to IL-3 and activated Stat5 comparably to stimulation with IL-7. In support of our findings, Winkler et al27 found that IL-3 and IL-7 were interchangeable in their proliferative effects on purified B220+, c-kit+bone marrow-derived pre-B cells in the presence of stromal cells. We note that our results differ from the inhibitory effects of IL-3 on lymphoid progenitors previously observed by Ogawa et al.28-30 Our experimental system differs significantly in that IL-3 was used in vivo on unselected target cells in neonatal mice that lack IL-7–dependent lymphopoiesis. It is possible that, among other factors, the inhibitory effects of IL-3 on lymphoid progenitors may depend on the target cell type. Alternatively, IL-3–induced lymphoid expansion may depend critically on the hematopoietic microenvironment.
In addition to Stat5, IL-3 and IL-7 activate pathways involving ras and the expression of the antiapoptotic genes for Bcl-2 and Bcl-XL. The importance of the activation of Bcl-2 was elegantly illustrated by the observation that transgenic expression of Bcl-2 rescued the T-cell lymphopenia of mice deficient in the IL-7 receptor α or γc chains.31-33 However, the B-cell defect was not rescued, demonstrating the requirement for additional signaling events in some cell populations.
In contrast to IL-3, SCF was unable to rescue the lymphoid populations in Jak3−/− mice. We propose that SCF regulates an earlier precursor than IL-7/IL-3–responsive lymphoid progenitors, which may be multilineage precursors.34,35 It should be noted that SCF stimulation of hematopoietic cells does not result in the detectable activation of Jak3 and thus Jak3 would not be predicted to be required for SCF function. Furthermore, whereas a deficiency of SCF or its receptor reduces thymic cellularity, c-kit deficiency combined with a γc deficiency abrogates thymocyte development.36-38
IL-3 is produced predominantly by activated T cells and was therefore envisioned to be an immune response mediator of early hematopoietic function. Whereas mutant mice lacking IL-3 clearly demonstrate that IL-3 does not have an essential role in early lymphoid expansion, other data indicate that IL-3 may influence the generation of immune responses. For example, in comparison with laboratory strains, wild strains and species of mice preferentially retain an intact IL-3 receptor system. This observation suggests that retention of the IL-3 receptor system confers an evolutionary advantage upon wild mice, which are exposed to more vigorous infectious challenges.39,40Furthermore, in IL-3–deficient mice, impaired mast cell effector functions reduced host defenses against pathogenic parasites.41 Our results lead us to suggest that, in addition, one of the physiological functions of IL-3 may be to expand early lymphoid progenitors during the course of an immune response. Specifically, after the initial differentiation of the immune system, IL-7 may become limiting and any subsequent expansions may require other cytokines, including IL-3.
Finally, we believe that our findings may have clinical relevance. In Jak3−/− mice, IL-3 expanded the numbers of peripheral B and T cells, the latter ostensibly via a thymic pathway, and enhanced the cytolytic function of T cells against allogeneic targets. The cytolytic activity itself does not depend on Jak3, and we propose that, after IL-3 treatment, increased numbers of CD8+ T cells generated within a large pool of alloreactive CTL precursors underwent an initial Jak3-independent phase of limited clonal expansion.42,43 Also, IL-3 may promote survival of apoptosis-prone Jak3-deficient T cells, as it does for other cell types,44 and IL-3 may enhance antigen presentation via effects on myeloid lineage cells.
In addition, we predict that our results would extend to γc-deficient mice, because γc signaling depends on intact Jak3 function. Thus, IL-3 treatment may be of value in SCID patients who have either X-linked or Jak3-deficient SCID. Although allogeneic bone marrow transplantation is the only treatment that is potentially curative, it is only available to approximately 60% of patients who have histocompatible donors.45 For patients who lack donors or who are otherwise unsuited to bone marrow transplantation, our data imply that administration of IL-3 might help to alleviate the infectious complications of SCID. Other cytokines have shown promise in the treatment of immunodeficiency. For example, IL-7 hastened the reconstitution of a functional immune system in mice after syngeneic bone marrow transplantation.46,47 However, in human immunodeficiency disorders, the potential role of IL-7 in enhancing lymphopoiesis is less certain. IL-7 will not be effective in primary immunodeficiencies caused by defects of γc or Jak3 and, unlike murine B lymphopoiesis, IL-7 was not required for human B lymphopoiesis in vitro.27,48 On the other hand, IL-3 has been widely tested in clinical trials for various hematologic disorders.49
ACKNOWLEDGMENT
The authors thank D. Wang for the oligonucleotide probe, O.N. Witte and M.F. Roussel for T220-29 cells, S. Nishikawa and T. Sudo for anti–IL-7Rα antibody, and D. Gorman for the anti–IL-3Rα antibody. M. Holladay, K. Farris, and R. Cross assisted with the FACS analysis. M.F. Roussel, W.E. Thierfelder, and D. Stravopodis gave technical advice and Y. Minegishi provided valuable discussions. Lastly, L. Snyder, C. J. Nagy, and J. H. Swift provided technical assistance throughout.
M.P.B. and T.N. contributed equally to the studies.
Supported in part by Cancer Center CORE Grant No. CA21765 and Grant No. DK42932 to J.N.I., Grant No. AI-29579 to P.C.D., Grants No. CA 78792 and CA 75014 to M.K.B., the Assisi Foundation, and the American Lebanese Syrian Associated Charities (ALSAC).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to James N. Ihle, PhD, Howard Hughes Medical Institute, St. Jude Children’s Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: james.ihle@stjude.org.


![Fig. 2. IL-7–responsive bone marrow cells are pro-B cells and early pre-B cells that express IL-7R, IL-3R, and c-kit. (A) Flow cytometric analysis of IL-7R, IL-3R, B220, and c-kit expression of IL-7–responsive normal bone marrow cells. The cells are IL-7R+, predominantly CD19+ and c-kit+, and B220+, CD43+. They also express IL-3R at low density, and after gating, these cells were found to coexpress IL-7R and B220 (lower histograms). Bone marrow cells from a 7-week-old B6J129SVF2 mouse were cultured for 10 days on NIH-3T3 cells that secrete mIL-7 (T220-29 cells) and then stained, fixed, and analyzed by flow cytometry. Fifty thousand cells were analyzed without gating for size. The percentage of positive cells is shown in the quadrant. Histograms: dotted line, isotype-matched control antibody; heavy line, antibody. (B) IL-3 and SCF induce the proliferation of IL-7–responsive cells. IL-7–responsive cells (wild-type bone marrow cells cultured for 9 days on T220-29 cells) were washed and cultured without feeder cells in the presence of rmIL-7 (5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). The cells were pulse-labeled with [3H]-thymidine and the incorporation of [3H]-thymidine was measured as counts per minute (cpm). Error bars show the standard deviation of 12 replicates. (C) IL-3 and IL-7 both activate STAT5 in IL-7R+ B-lymphoid progenitors from normal bone marrow. A gel mobility shift assay was performed after 10 hours of IL-7 starvation by using +/+ bone marrow cells that had been cultured for 14 days on T220-29 cells. The addition of anti-Stat5A antiserum50 reduced STAT5-DNA complex formation, whereas control serum did not. The arrowhead indicates the position of the Stat5-DNA complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.1906/5/m_blod41832002ax.jpeg?Expires=1765887261&Signature=Sh1WrMDnq12bYsUsJyyWMW~yxMLN4vZA3g4UF-H2H5NFOZO1P7LNf5APfRHnn~O-ejvOlJJGbOZb6kF8u5jW18cDeg-vIylf6QM56qMh8Fey4gQgmk7d2uDP4Xwmt8qSWT83YHKmZjA2mhCgC4bMtxmh2~q84mkuyYg8PCvzI1V0SnDSV5YkoI-WWViBHG2BF5UIK7yJf2oeHhe5Mr8yCcrsWvl1JMYWzNOJZQd~hHLmFl~aJkpS3WYXEoepPaa3FyGsPX7BABb--Kti-bb1ZF6VqdikrcBKMjtWIejpDdUpA4I-bjy7qNCZ4SZ~7sJY1UKKpRkg~q70JC5v24OnCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. IL-7–responsive bone marrow cells are pro-B cells and early pre-B cells that express IL-7R, IL-3R, and c-kit. (A) Flow cytometric analysis of IL-7R, IL-3R, B220, and c-kit expression of IL-7–responsive normal bone marrow cells. The cells are IL-7R+, predominantly CD19+ and c-kit+, and B220+, CD43+. They also express IL-3R at low density, and after gating, these cells were found to coexpress IL-7R and B220 (lower histograms). Bone marrow cells from a 7-week-old B6J129SVF2 mouse were cultured for 10 days on NIH-3T3 cells that secrete mIL-7 (T220-29 cells) and then stained, fixed, and analyzed by flow cytometry. Fifty thousand cells were analyzed without gating for size. The percentage of positive cells is shown in the quadrant. Histograms: dotted line, isotype-matched control antibody; heavy line, antibody. (B) IL-3 and SCF induce the proliferation of IL-7–responsive cells. IL-7–responsive cells (wild-type bone marrow cells cultured for 9 days on T220-29 cells) were washed and cultured without feeder cells in the presence of rmIL-7 (5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). The cells were pulse-labeled with [3H]-thymidine and the incorporation of [3H]-thymidine was measured as counts per minute (cpm). Error bars show the standard deviation of 12 replicates. (C) IL-3 and IL-7 both activate STAT5 in IL-7R+ B-lymphoid progenitors from normal bone marrow. A gel mobility shift assay was performed after 10 hours of IL-7 starvation by using +/+ bone marrow cells that had been cultured for 14 days on T220-29 cells. The addition of anti-Stat5A antiserum50 reduced STAT5-DNA complex formation, whereas control serum did not. The arrowhead indicates the position of the Stat5-DNA complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.1906/5/m_blod41832002bx.jpeg?Expires=1765887261&Signature=ji4yfCurnhhn4QU5t8QPyfnloxJimMLh2K59MhbrRqz7JbnU88UXcvylvEcKKJj9si5Zu09Mx0YSGBakBYlfSUgygdUNbRWo2mHQUrG6mvi4E7znnMrGy7sinrFnbkmlhoWsx9SaZLuYPSsQb9qUZzK3RfrT~45yCOsy-tYb-HEpLRLHcs3crA1pSXAS4DI~XUle9Ook0CeSgI4q5kSAOovyi7ERAhBid93od1Uqygac7f3pZ3bhJD-nCwgEV48hGltEe6G~vGy7h1weh1DAWoWrBnGGWbW5GuGJ7ldVz5LMqHraYQVvVY8Dp1Uxdwyodlnlri-epI~wAGwetfW8eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. IL-7–responsive bone marrow cells are pro-B cells and early pre-B cells that express IL-7R, IL-3R, and c-kit. (A) Flow cytometric analysis of IL-7R, IL-3R, B220, and c-kit expression of IL-7–responsive normal bone marrow cells. The cells are IL-7R+, predominantly CD19+ and c-kit+, and B220+, CD43+. They also express IL-3R at low density, and after gating, these cells were found to coexpress IL-7R and B220 (lower histograms). Bone marrow cells from a 7-week-old B6J129SVF2 mouse were cultured for 10 days on NIH-3T3 cells that secrete mIL-7 (T220-29 cells) and then stained, fixed, and analyzed by flow cytometry. Fifty thousand cells were analyzed without gating for size. The percentage of positive cells is shown in the quadrant. Histograms: dotted line, isotype-matched control antibody; heavy line, antibody. (B) IL-3 and SCF induce the proliferation of IL-7–responsive cells. IL-7–responsive cells (wild-type bone marrow cells cultured for 9 days on T220-29 cells) were washed and cultured without feeder cells in the presence of rmIL-7 (5 ng/mL), rmIL-3 (10 ng/mL), or rmSCF (100 ng/mL). The cells were pulse-labeled with [3H]-thymidine and the incorporation of [3H]-thymidine was measured as counts per minute (cpm). Error bars show the standard deviation of 12 replicates. (C) IL-3 and IL-7 both activate STAT5 in IL-7R+ B-lymphoid progenitors from normal bone marrow. A gel mobility shift assay was performed after 10 hours of IL-7 starvation by using +/+ bone marrow cells that had been cultured for 14 days on T220-29 cells. The addition of anti-Stat5A antiserum50 reduced STAT5-DNA complex formation, whereas control serum did not. The arrowhead indicates the position of the Stat5-DNA complex.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.1906/5/m_blod41832002cw.jpeg?Expires=1765887261&Signature=DDw8Iw7X1~f9T02hG4GrmvaxWvl5Dc5ndqghaAWCFEruUsm4veng-Snjx~2SmXjma64r4T0AheR1DOCdL1akhiWC93M7~UKIfgtQdXtQcWx3~FXn11PCx9DgL5aSygtvY6B8qH22grAgtKYxYSkQTi2uwwNUEjcYpJWKRr8Cr8FA4vgdc5NBdGJ8suoQZzstGxnbzuRiYNf-F6x1DSg5OOI0JzT9OCuLJjZVc536fs3mY-qKJ3X3Vzuyg8WtwZbxET7pXL~KRfQCrRF8UsHPBAVCYuHOb3HrVA-sGxL03YKyMcoyGT3zi8UK6gV8OeCCsm8wKKMijv73s6qic0mtcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. Proliferative responses of thymocytes from Jak3+/+ mice (+/+), control-treated Jak3−/− mice (−/−), and IL-3–treated Jak3−/− littermates (−/− IL-3). Jak3−/− thymocytes do not respond to T-cell mitogenic and Jak3-dependent cytokine stimuli. Thymocytes were stimulated with mitogen/cytokine combinations as described in Materials and Methods, and their proliferation was measured by incorporation of [3H]-thymidine. Values are average stimulation indices ± SD (n = 2 in each group).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/6/10.1182_blood.v94.6.1906/5/m_blod41832004x.jpeg?Expires=1765887261&Signature=zo0q8YNNggjbdAW2tL~zohJAgN7Jyc1u99kouN~UUQoozu07SleVA0hKw4vz1v7UUxHoIi2HX3ZFaEDYSnzbGJQTA67xa1eh0NeO~PgJDpl1U1cUu1N0hTpxUzeSrNkO~OPVSjKuDHWiZX6gLcnPuYJ6XhJ~XDxmC7jAhMQuPrgpmJMWgm73z1iiJJER2-IcTbsJyGJi9~Ha2cfrf4ewGMBhebkYAaCdmUdzPGWwOPw1UtoAkHlnV~DBtOCp27NPNqOeDOlvTe7tenQG9I3T6IYIIQMq4U72yKs5583gfdk7FBJxN-PVRWOkUghHill8c9aESRHSReGjzf06Qp8ZvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal