We conducted a phase I-II clinical trial to test the hypothesis that removal of CD4 cells from an HLA-mismatched unrelated marrow graft would substantially reduce the risk of grades III-IV graft-versus-host disease (GVHD) and that retention of a specified number of CD8 cells in the graft would be sufficient to prevent rejection. Patients were eligible for this study when an HLA-A, -B, or -DRB1–matched unrelated donor could not be identified. HLA matching of the donor and recipient was based on typing of HLA-A and -B antigens by serologic methods and by typing of HLA-DRB1 alleles by molecular methods, and donors were selected when disparity was limited to a single HLA-DRB1 allele or a single HLA-A or -B antigen. Twenty-seven patients with hematologic malignancy or aplastic anemia were prepared to receive a transplant with conventional regimens of cyclophosphamide and fractionated total body irradiation, and a standard regimen of methotrexate and cyclosporine was given for GVHD prophylaxis. CD4 cells were removed from the donor marrow, and the numbers of CD8 cells were adjusted systematically in graded steps for successive patients, depending on the occurrence of grades III-IV GVHD or graft failure in previously enrolled patients. Removal of CD4 cells did not cause graft rejection or appreciably decrease the risk of grades III-IV GVHD. Depletion of CD8 cells was associated with an increased risk of rejection with either HLA-DRB1 disparity or with HLA-A or -B disparity. With either type of disparity, the risk of grades III-IV GVHD is likely to be higher than 15% at any dose of CD8 cells associated with less than 5% risk of graft failure. The absence of graft failure associated with CD4 depletion supports the hypothesis that donor CD4 cells are not essential for preventing marrow graft rejection in humans. The correlation between graft failure and the number of CD8 cells in the donor marrow supports the hypothesis that donor CD8 cells help to prevent marrow graft rejection.
CURRENTLY AVAILABLE posttransplant immunosuppressive regimens do not provide optimal protection against graft-versus-host disease (GVHD) after marrow transplantation from an HLA-nonidentical family member or from an unrelated donor.1,2 Clinical studies have shown that GVHD can be prevented by removing T cells from the donor marrow, but this approach has been associated with an increased risk of graft failure, among other significant complications affecting survival.3Immunologic rejection mediated by small numbers of recipient lymphoid cells that survive the conditioning regimen is a likely cause of graft failure associated with removal of T cells from the donor marrow.3 Laboratory studies have shown that donor T cells prevent rejection by eliminating or inactivating recipient T cells that survive the conditioning regimen.4,5 This occurs primarily through the generation of cytotoxic effectors that recognize alloantigens expressed by recipient T cells.6
In murine marrow transplant models, both CD4 cells and CD8 cells of the donor can cause GVHD, but donor CD8 cells were at least 5-fold more effective than donor CD4 cells for preventing marrow graft rejection mediated by recipient T cells.4 In humans, removal of CD8 cells from the donor marrow is associated with an increased risk of graft failure in HLA-identical sibling recipients.7 Taken together, these studies suggest that donor CD8 cells play a critically important role in preventing allogeneic marrow graft rejection. Based on these considerations, we designed a clinical trial to determine whether removal of CD4 cells from HLA-mismatched unrelated donor marrow could safely and substantially reduce the risk of grades III-IV GVHD when a specified number of CD8 cells is retained in the graft to prevent rejection.
MATERIALS AND METHODS
Patient and donor selection.
Patients were eligible for this study if they had a hematologic malignancy deemed to have less than 5% chance of cure by conventional therapy but potentially curable by treatment with high-dose cyclophosphamide and total body irradiation followed by allogeneic marrow transplantation. Patients with severe aplastic anemia were also eligible. Patients were ineligible if they were older than 55 years of age or had previously received greater than 3,000 cGy whole brain irradiation or greater than 1,500 cGy to the chest or abdomen, or any involved field irradiation to these areas within 6 months before transplantation.
HLA matching of the donor and recipient was based on typing of HLA-A and -B antigens by serologic methods and by typing of DRB1 alleles by DNA hybridization with sequence-specific oligonucleotide probes.8 Patients with an HLA-identical sibling, an HLA-haploidentical relative with no HLA-A, -B, or -DRB1 disparity, an HLA-haploidentical relative with disparity for a single HLA-A, -B or -DR antigen, or an HLA-A, -B, or -DRB1-matched unrelated donor were eligible for other protocols and were excluded from this study. Unrelated donors were selected for patients in this study if disparity was limited to a single HLA-DRB1 allele or a single HLA-A or -B antigen. To minimize the risk of rejection, donor disparity was not allowed at any HLA-A, -B or -DRB1 locus for which the patient was homozygous. Donors with positive lymphocyte crossmatching tests against patient serum were excluded. Our institutional review board approved the protocols and consent forms used for this study, and all patients provided written informed consent. The study enrolled 22 men, 4 women, and 1 child with a median age of 42 years (range, 9 to 55 years).
The study enrolled 14 patients with disparity for an HLA-DRB1 allele. Nine of these patients also had recipient HLA-DQB1 disparity, which is known to increase the risk of acute GVHD.9 Three had acute myeloid leukemia (AML) in relapse, 1 had myelodysplasia, 1 had acute lymphoblastic leukemia (ALL) in relapse, 1 had lymphoma in relapse, and 8 had chronic myeloid leukemia (CML), 3 in chronic phase, 3 in accelerated phase, 1 in blast crisis and 1 in chronic phase after blast crisis. For patients with CML, as opposed to other diseases, the risk of rejection with T-cell–replete marrow is increased when an unrelated donor has disparity for more than one HLA-A, -C, or -B allele.10 Posttransplant typing of HLA-A, -C, and -B alleles by DNA sequencing10 showed that 7 of the 8 donors for patients with CML had no HLA-A, -C, or -B disparity, and 1 had disparity at both HLA-A and -C. HLA class I sequences were not analyzed when patients had diseases other than CML.
The study enrolled 13 patients with disparity for an HLA-A or -B antigen. Two of these patients also had recipient HLA-DQB1 disparity. Three had AML (2 in relapse and 1 in second remission), 2 had ALL (1 in relapse and 1 in third remission), 1 had chronic lymphocytic leukemia, 1 had aplastic anemia, and 6 had CML, 1 in chronic phase, 4 in accelerated phase, and 1 in blast crisis. Posttransplant typing of HLA-A, C and B alleles by DNA sequencing showed that 2 of the 6 donors for patients with CML had a single HLA-A, -C or -B disparity, and 3 had multiple class I disparities. One donor was homozygous at HLA-A, resulting in recipient disparity without donor disparity.
Transplantation procedures and supportive care.
Patients were prepared to receive a transplant with 60 mg of cyclophosphamide per kg body weight for 2 days and either 13.2 Gy total body irradiation (TBI) given in 1.2 Gy fractions 3 times daily (n = 12) or 13.5 Gy TBI given in 1.5 Gy fractions twice daily (n = 15). Intrathecal methotrexate was given for prevention of central nervous system (CNS) malignancy and local field irradiation was given for prevention of testicular relapse in patients who were at risk of these complications. Cyclosporine and methotrexate were given for GVHD prophylaxis.11 Trimethoprim-sulfamethoxazole was given before transplantation and after engraftment for prophylaxis againstPneumocystis carinii pneumonia. Nineteen patients were hospitalized in isolation rooms with laminar airflow and received oral nonabsorable antibiotics for prevention of bacterial and fungal infection. Six of the remaining eight patients were hospitalized in rooms with high-efficiency particulate air–filtered air and positive-pressure ventilation. Antibiotics were given intravenously for prevention of bacterial infection whenever the absolute neutrophil count was less than 500/μL. All patients received fluconazole for prevention of fungal infection.12 Amphotericin was given whenever fungal infection was suspected. Acyclovir was given during the first 30 days after transplantation to prevent herpes simplex reactivation in seropositive patients.13 Patients who were seronegative for cytomegalovirus (CMV) received leukopoor filtered blood products or blood products from donors who were CMV-seronegative.14 CMV-seropositive patients were treated with ganciclovir whenever weekly blood tests for CMV pp65 antigen were positive.15 Gamma globulin was given intravenously at weekly intervals for the first 90 days after transplantation whenever the serum immunoglobulin G (IgG) level was less than 400 mg/dL. Recombinant human granulocyte colony-stimulating factor (G-CSF) was given at the discretion of the attending physician if the absolute neutrophil count did not surpass 100/μL by day 21 after transplantation.
Marrow treatment.
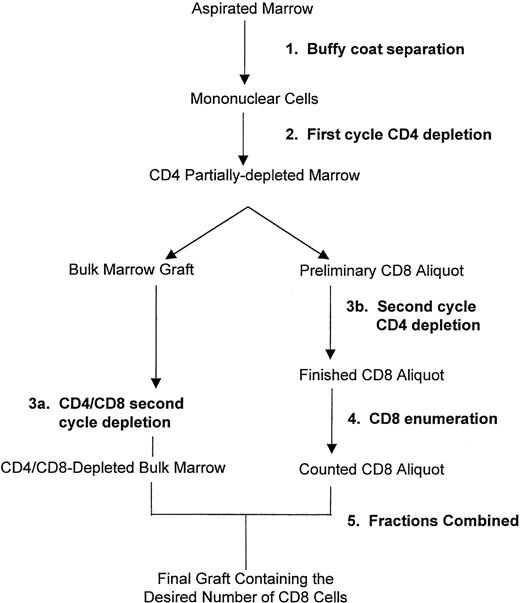
Marrow was aspirated by conventional methods at the donor center and transported to the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA) according to procedures of the National Marrow Donor Program (Minneapolis, MN). At the time of arrival, red blood cells were depleted and mononuclear cells were enriched by centrifugation using a COBE Spectra machine (Cobe, Lakewood, CO). CD4 cells and CD8 cells were depleted by an immunomagnetic procedure using clinical grade monoclonal antibodies prepared in the FHCRC Biologics Production Shared Resource. Antibodies were used with paramagnetic polystyrene beads (Dynal, Lake Success, NY) and the MaxSep device (kindly donated by Baxter Healthcare Corporation, Irvine, CA) under an Investigational Device Exemption from the Food and Drug Administration. The CD4-specific murine IgM antibody 66.116 was conjugated to Dynabeads M-450 (Dynal) by overnight incubation at pH 9.5. The CD8-specific murine IgG2a antibody 51.117 was bound to Dynabeads M-450 coated with sheep antimouse IgG. The immunomagnetic separation steps are summarized in Fig 1. CD34 cells, CD4-bright cells, and CD8-bright cells were enumerated before and after the immunomagnetic separation by flow cytometry after direct immunofluorescent staining with commercially available antibodies (Becton Dickinson, San Jose, CA). For enumeration of CD4-bright cells, 100,000 events were analyzed with subtraction for 0% to 0.021% (mean, 0.0048%) background after staining with an isotype-matched irrelevant control antibody.
Procedure for immunomagnetic removal of CD4 cells and partial depletion of CD8 cells from the marrow. Erythrocytes were removed and mononuclear cells were enriched by centrifugation using a Cobe Spectra (Step 1). Cells were incubated with CD4-specific immunomagnetic beads and exposed to a magnetic field in a first cycle of CD4 depletion (Step 2). A preliminary aliquot containing the desired number of CD8 cells was removed from the CD4 partially depleted marrow and subjected to a second cycle of CD4 depletion (Step 3b), and the remaining CD8 cells were enumerated (Step 4). The bulk marrow graft remaining after removal of the preliminary CD8 aliquot was subjected to a second cycle of CD4 depletion combined with CD8 depletion (Step 3a). The CD4/CD8-depleted bulk marrow was combined with the counted CD8 aliquot (Step 5) to yield the final graft containing the desired number of CD8 cells. For dose level 0, the entire marrow was subjected to 2 cycles of CD4 depletion with no removal of CD8 cells.
Procedure for immunomagnetic removal of CD4 cells and partial depletion of CD8 cells from the marrow. Erythrocytes were removed and mononuclear cells were enriched by centrifugation using a Cobe Spectra (Step 1). Cells were incubated with CD4-specific immunomagnetic beads and exposed to a magnetic field in a first cycle of CD4 depletion (Step 2). A preliminary aliquot containing the desired number of CD8 cells was removed from the CD4 partially depleted marrow and subjected to a second cycle of CD4 depletion (Step 3b), and the remaining CD8 cells were enumerated (Step 4). The bulk marrow graft remaining after removal of the preliminary CD8 aliquot was subjected to a second cycle of CD4 depletion combined with CD8 depletion (Step 3a). The CD4/CD8-depleted bulk marrow was combined with the counted CD8 aliquot (Step 5) to yield the final graft containing the desired number of CD8 cells. For dose level 0, the entire marrow was subjected to 2 cycles of CD4 depletion with no removal of CD8 cells.
Study design, endpoints, and statistical analysis.
This phase I-II clinical trial was undertaken to determine whether removal of CD4 cells from the marrow graft can substantially reduce the risk of grades III-IV acute GVHD after transplantation from an unrelated donor with MHC-class II (HLA-DRB1) or MHC-class I (HLA-A or -B) disparity. Because we anticipated that removal of CD4 cells might not be sufficient to prevent grades III-IV GVHD, the study was further designed to determine whether the number of CD8 cells in the graft could be adjusted in a way that reduced the risk of grades III-IV GVHD to 15% or less (equivalent to results with HLA-identical sibling donors) without increasing the risk of rejection to more than 5% in patients given conventional regimens of cyclophosphamide and fractionated TBI before transplantation together with methotrexate and cyclosporine after transplantation.
Patients with HLA-DRB1 disparity and those with HLA-A or -B disparity were enrolled in 2 separate, parallel phase I-II trials based on the supposition that donor CD8 cells might have different biologic effects in the 2 groups. Briefly summarized, patients initially enrolled in each group during phase I received marrow depleted of CD4 cells with no depletion of CD8 cells (dose level “0”). For patients with HLA-DRB1 disparity, dose levels “−1” and “−2” were, respectively, defined as 5.0 × 106 and 1.25 × 106 CD8 cells/kg recipient body weight. For patients with HLA-A or -B disparity, dose level “−1” was defined as 2.5 × 106 CD8 cells/kg recipient body weight. During phase I, the number of CD8 cells in the graft was decreased by 1 dose level whenever at least 1 patient developed grades III-IV GVHD. De-escalation was stopped whenever any 2 of 2 to 8 patients at any given dose of CD8 cells developed graft failure, and phase II was initiated with marrow containing twice the number of CD8 cells at which graft failure occurred. During phase II, the number of CD8 cells in the graft was doubled whenever any 2 of 2 to 8 patients at any given dose of CD8 cells developed graft failure. Enrollment during phase II was stopped when 3 of 3 to 12 patients at any given dose of CD8 cells developed grades III-IV GVHD. Decisions regarding CD8 dose were made on the basis of information available at the time with no requirement for waiting to determine outcome in previously enrolled patients. A complete description of a similar study design has been published previously.18 With 4 different hypothetical scenarios concerning the relationship between the number of CD8 cells and the risks of rejection and grades III-IV GVHD, we estimated that the stopping rules had less than 20% probability of leading to an erroneous conclusion that no satisfactory dose of CD8 cells could be found when one does exist. For the scenarios tested, there was a 67% to 88% chance that the study design would correctly identify a dose of CD8 cells where the risks of rejection and grades III-IV GVHD met the desired specifications.
Primary endpoints were graft failure and grades III-IV GVHD. Patients who died without evidence of rising granulocyte counts and no evidence of engraftment in the marrow were considered as having graft failure. Two patients who died, respectively, on days 4 and 12 after transplantation were not evaluated for rejection. Other manifestations of graft failure were autologous reconstitution with no donor cells, or the disappearance of myeloid cells in the blood with marrow aplasia after evidence of initial engraftment in the absence of any other obvious causes of pancytopenia such as viral infection or drug toxicity. The severity of acute GVHD was judged according to previously published criteria19 with allowances made for hepatic and gastrointestinal abnormalities caused by complications other than GVHD. Secondary endpoints were persistence of recipient-derived cells in the blood or marrow as detected by in situ hybridization or variable number tandem repeat assays with approximately 1% sensitivity,20platelet reconstitution in engrafted patients, the development of chronic GVHD in patients who survived for at least 100 days, development of recurrent malignancy, and immune deficiency manifested by the development of opportunistic infections or Epstein-Barr virus–induced lymphoproliferative disorders.
Correlations between outcome after transplantation and the numbers of nucleated cells, CD34 cells, CD4 cells, and CD8 cells in the marrow were evaluated by Wilcoxon rank-sum tests. No correction was made for multiple comparisons. An exact logistic regression model was used to test correlation between graft failure and the numbers of CD8 cells in the donor marrow. Odds ratios were estimated with exact methods to evaluate correlation between T-cell depletion and graft failure with stratification according to pretransplant diagnosis and donor HLA allele disparity. Inferences with single proportions were evaluated according to the binomial distribution.
RESULTS
Results of marrow treatment.
From the immunomagnetic separation procedure, the mean recovery of nucleated cells was 46.8% (range, 17% to 73%), and the mean recovery of CD34-positive cells was 67.8% (range, 34% to 110%). The final processed grafts contained 5.88 (range, 1.3 to 28.3) × 107 nucleated cells and 2.75 (range, 0.4 to 6.3) × 106 CD34-positive cells per kg recipient body weight. Before the immunomagnetic separation, the grafts contained 16.6 (range, 5.4 to 41.1) × 106 CD4-bright cells per kg recipient body weight. In 7 of the processed marrows, no CD4-bright cells were detected by flow cytometric analysis of 1.0 × 105 events (Tables 1 and2). In the remaining 20 marrows, the immunomagnetic separation procedure achieved an average 3.37 (range, 1.5 to 5.0) log depletion of CD4-bright cells. The median number of residual CD4-bright cells in the graft was 3.0 × 103/kg recipient body weight, and the highest number administered was 2.1 × 105/kg (Tables 1 and2).
Patients With Disparity for an HLA-DRB1 Allele
| UPN . | No. of CD4 Cells/kg (×10−4) . | No. of CD8 Cells/kg (×10−6) . | Graft Failure . | GVHD Grade . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Given . | Intended . | ||||||
| 8399 | BL | 13.0 | NA | No | IV | 118 | GVHD |
| 8343 | BL | 9.1 | NA | No | III | 39 | Bacterial infection |
| 8358 | 0.069 | 5.6 | 5.0 | No | III | 68 | Bacterial infection |
| 8634 | BL | 5.0 | 5.0 | No | III | 166 | CMV infection; chronic GVHD |
| 8650 | 0.35 | 4.6 | 5.0 | NE | NE | 4 | Cardiomyopathy |
| 9303 | 3.7 | 4.3 | 5.0 | No | III | 57 | CMV infection |
| 8441 | BL | 3.9 | 5.0 | No | III | 465 | P carinii infection; chronic GVHD |
| 9363 | 0.24 | 3.1 | 5.0 | No | III | 499 | Recurrent malignancy |
| 8670 | 0.49 | 2.8 | 2.5 | No | II | >988 | |
| 9202 | 2.0 | 2.7 | 2.5 | Yes | NE | >956 | |
| 8286 | BL | 2.6 | 5.0 | Yes | NE | 173 | Fungal infection |
| 8532 | 20 | 1.4 | 1.25 | No | I | >1,133 | |
| 8940 | 0.58 | 1.3 | 1.25 | Yes | NE | 26 | Bacterial infection; hemorrhage |
| 8371 | 0.046 | 1.1 | 1.25 | Yes | NE | 508 | Recurrent malignancy |
| UPN . | No. of CD4 Cells/kg (×10−4) . | No. of CD8 Cells/kg (×10−6) . | Graft Failure . | GVHD Grade . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Given . | Intended . | ||||||
| 8399 | BL | 13.0 | NA | No | IV | 118 | GVHD |
| 8343 | BL | 9.1 | NA | No | III | 39 | Bacterial infection |
| 8358 | 0.069 | 5.6 | 5.0 | No | III | 68 | Bacterial infection |
| 8634 | BL | 5.0 | 5.0 | No | III | 166 | CMV infection; chronic GVHD |
| 8650 | 0.35 | 4.6 | 5.0 | NE | NE | 4 | Cardiomyopathy |
| 9303 | 3.7 | 4.3 | 5.0 | No | III | 57 | CMV infection |
| 8441 | BL | 3.9 | 5.0 | No | III | 465 | P carinii infection; chronic GVHD |
| 9363 | 0.24 | 3.1 | 5.0 | No | III | 499 | Recurrent malignancy |
| 8670 | 0.49 | 2.8 | 2.5 | No | II | >988 | |
| 9202 | 2.0 | 2.7 | 2.5 | Yes | NE | >956 | |
| 8286 | BL | 2.6 | 5.0 | Yes | NE | 173 | Fungal infection |
| 8532 | 20 | 1.4 | 1.25 | No | I | >1,133 | |
| 8940 | 0.58 | 1.3 | 1.25 | Yes | NE | 26 | Bacterial infection; hemorrhage |
| 8371 | 0.046 | 1.1 | 1.25 | Yes | NE | 508 | Recurrent malignancy |
Patients are listed according to the number of CD8 cells in the graft.
Abbreviations: UPN, unique patient number; BL, below limits of detection; NA, not applicable because CD8 cells were not removed from the marrow for these patients; NE, not evaluable for graft failure because of early death, or not evaluable for acute GVHD because donor cells did not engraft.
Patients With Disparity for an HLA-A or -B Antigen
| UPN . | No. of CD4 Cells/kg (×10−4) . | No. of CD8 Cells/kg (×10−6) . | Graft Failure . | GVHD Grade . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Given . | Intended . | ||||||
| 8351 | BL | 17.1 | NA | No | II | 54 | Recurrent malignancy |
| 8268 | 0.13 | 8.7 | NA | No | II | 180 | Chronic GVHD |
| 7911 | 21 | 8.7 | NA | No | III | 48 | Fungal infection |
| 7850 | 1.9 | 5.9 | 5.0 | No | II | >896 | |
| 9478 | BL | 5.4 | 5.0 | No | III | 817 | Unknown |
| 8945 | 0.59 | 4.7 | 5.0 | No | II | 42 | Fungal infection |
| 9994 | 0.13 | 4.6 | 5.0 | No | II | 243 | Recurrent malignancy |
| 8125 | 0.45 | 4.6 | NA | NE | NE | 12 | Fungal infection |
| 9542 | 0.30 | 4.1 | 5.0 | No | II | 648 | Chronic GVHD |
| 8553 | 0.046 | 3.9 | NA | No | III | 77 | Bacterial infection |
| 7872 | 0.61 | 3.1 | 2.5 | Yes | NE | 111 | Fungal infection |
| 8765 | 1.6 | 2.4 | 2.5 | No | II | 156 | Recurrent malignancy |
| 9022 | 1.9 | 2.2 | 2.5 | Yes* | IV | 67 | Bacterial infection |
| UPN . | No. of CD4 Cells/kg (×10−4) . | No. of CD8 Cells/kg (×10−6) . | Graft Failure . | GVHD Grade . | Survival (d) . | Cause of Death . | |
|---|---|---|---|---|---|---|---|
| Given . | Intended . | ||||||
| 8351 | BL | 17.1 | NA | No | II | 54 | Recurrent malignancy |
| 8268 | 0.13 | 8.7 | NA | No | II | 180 | Chronic GVHD |
| 7911 | 21 | 8.7 | NA | No | III | 48 | Fungal infection |
| 7850 | 1.9 | 5.9 | 5.0 | No | II | >896 | |
| 9478 | BL | 5.4 | 5.0 | No | III | 817 | Unknown |
| 8945 | 0.59 | 4.7 | 5.0 | No | II | 42 | Fungal infection |
| 9994 | 0.13 | 4.6 | 5.0 | No | II | 243 | Recurrent malignancy |
| 8125 | 0.45 | 4.6 | NA | NE | NE | 12 | Fungal infection |
| 9542 | 0.30 | 4.1 | 5.0 | No | II | 648 | Chronic GVHD |
| 8553 | 0.046 | 3.9 | NA | No | III | 77 | Bacterial infection |
| 7872 | 0.61 | 3.1 | 2.5 | Yes | NE | 111 | Fungal infection |
| 8765 | 1.6 | 2.4 | 2.5 | No | II | 156 | Recurrent malignancy |
| 9022 | 1.9 | 2.2 | 2.5 | Yes* | IV | 67 | Bacterial infection |
Patients are listed according to the number of CD8 cells in the graft.
Abbreviations: UPN, unique patient number; BL, below limits of detection; NA, not applicable because CD8 cells were not removed from the marrow for these patients; NE, not evaluable for graft failure because of early death, or not evaluable for acute GVHD because donor cells did not engraft.
This patient had graft failure after initial engraftment.
Before the immunomagnetic separation, the grafts contained 12.5 (range, 2.7 to 32.3) × 106 CD8-bright cells per kg recipient body weight. In 2 of the 7 cases where no CD8 cells were removed from the marrow, the graft contained less than 5.0 × 106 CD8 cells/kg recipient body weight after processing (Tables 1 and 2). In addition, the marrow for unique patient number (UPN) 8286 was intended to contain 5.0 × 106 CD8-bright cells/kg but actually contained only 2.6 × 106 CD8-bright cells after the first cycle of CD4 depletion. In all other cases, the actual numbers of CD8-bright cells in the processed marrows corresponded closely with the intended numbers. For 11 grafts intended to contain 5.0 × 106 CD8-bright cells/kg (excluding UPN 8286), the mean number of CD8 cells was 4.65 (range, 3.1 to 5.9) × 106/kg. For 5 grafts intended to contain 2.5 × 106 CD8-bright cells/kg, the mean number of CD8 cells was 2.64 (range, 2.2 to 3.1) × 106/kg, and for 3 grafts intended to contain 1.25 × 106 CD8-bright cells/kg, the mean number of CD8 cells was 1.26 (range, 1.1 to 1.4) × 106/kg.
Incidence of graft failure and grades III-IV GVHD as correlated with the numbers of various cell types in the processed marrow.
Engraftment could not be evaluated in 2 patients (UPN 8650 and 8125) who died, respectively, on days 4 and 12 after transplantation. Among the 25 patients who could be evaluated, 5 had failure of initial engraftment, and 20 had evidence of initial engraftment manifested by an increase in absolute neutrophil counts to levels greater than 500/μL for at least 3 days (Tables 1 and 2). The median interval time from the marrow infusion to granulocyte recovery in these 20 patients was 23.5 days (range, 18 to 28 days). Five of these patients were treated with G-CSF to accelerate engraftment. One patient (UPN 9022) had initial engraftment followed by graft failure at 63 days after transplantation (Table 2).
Table 1 summarizes the incidence of graft failure and GVHD at each CD8 cell dose among patients with HLA-DRB1 disparity. All 6 patients who could be evaluated after transplantation with marrow containing 3.9 to 13.1 × 106 CD8 cells/kg had durable engraftment and developed grades III-IV GVHD, whereas 4 of 7 patients who were given marrow containing less than 3.9 × 106 CD8 cells/kg had graft failure. Among engrafted patients, the risk of GVHD appeared to correlate more closely with the number of CD8 cells in the marrow than with the number of CD4 cells. Four of the 7 engrafted patients with HLA-DRB1 disparity who developed grades III-IV GVHD had no CD4-bright cells detectable in the final processed marrow. The 2 engrafted patients who received the lowest doses of CD8 cells had grade I (UPN 8532) and grade II (UPN 8670) GVHD (Table 1), whereas all 7 engrafted patients who received higher doses of CD8 cells developed grades III-IV GVHD. The grafts for UPN 8532 and UPN 8670, respectively, contained 2.0 × 105 and 4.9 × 103 CD4 cells/kg recipient body weight.
Table 2 summarizes the incidence of graft failure and GVHD at each CD8 cell dose among patients with HLA-A or -B disparity. All 9 patients who could be evaluated after transplantation with marrow containing 3.9 to 17.1 × 106 CD8 cells/kg recipient body weight had durable engraftment, and 3 of the 9 had grade III GVHD, whereas 2 of 3 patients who were given marrow containing less than 3.9 × 106 CD8 cells/kg had graft failure. The occurrence of grades III-IV GVHD in engrafted patients with HLA-A or -B disparity showed no correlation with the numbers of CD4 cells or CD8 cells in the final processed marrow.
Among the 25 patients who could be evaluated, graft failure did not correlate with the number of nucleated cells, CD34 cells, or CD4 cells in the final processed marrows. In an exact univariate logistic regression model with log-transformed cell numbers, a lower CD8 dose was associated with a higher risk of graft failure (P = .0017), as might be expected from the design of the study. To determine whether graft failure in the present study might be explained by donor HLA disparity, we compared results with those for unrelated transplant recipients whose marrow grafts were not T-cell–depleted10 21 (and E.W.P. et al, unpublished observations, November 1998) (Table3). The common odds ratio for the association between T-cell depletion and graft failure across both risk groups in Table 3 is 8.45 (95% confidence interval 2.18 to 29.46; P = .002). Taken together, these results indicate that increased incidence of graft failure in the present study was caused by depletion of CD8 cells from the donor marrow and not by undetected disparity for HLA class I alleles.
Association of Graft Failure With T-Cell Depletion With Stratification According to Underlying Disease and Donor HLA Disparity
| Risk Group . | T-Cell Depletion . | Graft Failure . | |
|---|---|---|---|
| Yes . | No . | ||
| High | Yes | 2 | 2 |
| No | 11 | 41 | |
| Low | Yes | 4 | 17 |
| No | 10 | 501 | |
| Risk Group . | T-Cell Depletion . | Graft Failure . | |
|---|---|---|---|
| Yes . | No . | ||
| High | Yes | 2 | 2 |
| No | 11 | 41 | |
| Low | Yes | 4 | 17 |
| No | 10 | 501 | |
The high-risk group includes patients with CML who had a donor with disparity for multiple class I alleles. All other patients are categorized in the low-risk group. Results for marrow transplantation without T-cell depletion in patients with CML have been reported previously.10 Graft failure in controls was defined according to published criteria.21 All patients were prepared for transplantation with a conditioning regimen of cyclophosphamide and 12.0 to 15.75 Gy TBI and received methotrexate plus cyclosporine for GVHD prophylaxis.
Chimerism studies.
In 1 of the 5 patients with failure of initial engraftment, blood cells were predominantly of donor origin, and in the other 4, blood cells were predominantly of recipient origin. Chimerism studies documented the presence of donor cells in the peripheral blood or marrow during the first 100 days after transplantation in each of the 20 patients who had initial engraftment. In 7 of these cases, recipient cells were also detected on at least 1 occasion. In 2 of the 7, the presence of recipient cells was associated with evidence of recurrent malignancy after transplantation. In the other 5 cases, the persistence of recipient cells was not associated with either recurrent malignancy or graft failure. The patient with late graft failure had only donor cells detectable in the marrow 4 days before he died.
Platelet engraftment.
Nine of the 20 patients who had neutrophil engraftment also had recovery of self-sustained platelet counts at levels greater than 20,000/μL during the first 3 months after transplantation and were independent of platelet transfusion support. Five of these patients later developed thrombocytopenia, which could have been related to CMV infection or chronic GVHD. Eleven of the 20 patients who had neutrophil engraftment remained persistently thrombocytopenic and required continued platelet transfusion support. Seven of the 11 died between days 39 and 77 after transplantation without having recovered self-sustained platelet counts at levels greater than 20,000/μL, and 1 patient with recurrent malignancy remained thrombocytopenic until he died on day 243 after transplantation. In the remaining 3 patients, platelet counts eventually recovered to levels greater than 20,000 μL between 4 and 12 months after transplantation. Recovery of self-sustained platelet counts correlated strongly with higher numbers of nucleated cells in the final processed marrow (P = .009) and showed a trend for correlation with the number of CD34 cells (P = .12).
Infections.
No patient developed a lymphoproliferative disorder caused by Epstein-Barr virus. Fungal infections were notably frequent among patients in this study. One of the 2 patients who died during the first 2 weeks after transplantation had pulmonary infection with Candida glabrata and Aspergillus with no pretransplant history of fungal infection. Three of the 5 patients with failure of initial engraftment had Aspergillus or Fusariuminfection. One of these patients had a pretransplant history of pulmonary Aspergillus infection. Eight of the 20 patients with initial engraftment had documented fungal infection. Three hadCandida infection, 3 had Aspergillus infection, 1 had both Candida and Aspergillus infection, and 1 had bothCandida and Nocardia infection. Only 1 of these 8 patients had a pretransplant history of suspected fungal infection. Two of the cases with isolated Candida infection were related to a nosocomial outbreak caused by contamination of parenteral fluids withC parapsilosis. Development of fungal infection in engrafted patients showed a trend for correlation with lower numbers of CD4 cells in the final processed marrow (P = .14).
Recurrent malignancy.
Six of the 20 patients with initial engraftment had recurrent malignancy after transplantation. Two patients with acute leukemia in relapse at the time of transplantation had recurrent disease diagnosed on days 47 and 145 after transplantation. Among the other 7 patients with acute leukemia, 6 died without recurrent malignancy between 4 and 118 days after transplantation, and 1 died without recurrent malignancy on day 817. The other 4 patients with recurrent malignancy after transplantation had CML. One was in first chronic phase at the time of transplantation, 2 were in accelerated phase, and 1 was in chronic phase after blast crisis. In 2 of these 4 patients, the disease progressed despite discontinuation of immunosuppressive treatment. In 1 patient, the disease resolved after immunosuppressive treatment was stopped, and in 1 patient the disease was controlled by treatment with interferon. Among the other 7 patients with CML who engrafted, 6 died without recurrent malignancy between 39 and 180 days after transplantation, and 1 remains alive and well without recurrent malignancy nearly 3 years after transplantation.
Causes of death, chronic GVHD, and current status.
Four of the 5 patients with failure of initial engraftment died: 1 with fungal infection after a successful second transplant; 1 with fungal infection after unsuccessful second and third transplants; 1 with recurrent CML after spontaneous autologous reconstitution; and 1 with hemorrhage and bacterial enteritis. The patient with late graft failure died with systemic Pseudomonas infection. Sixteen of the 19 durably engrafted patients died: 8 with infection (3 bacterial, 2 fungal, 2 CMV, and 1 P carinii); 1 with acute GVHD; 2 with chronic GVHD (and 2 with chronic GVHD as a contributing cause of death in patients who died with infection); 4 with recurrent malignancy (2 CML, 1 ALL, and 1 AML); and 1 with unknown causes. Excluding UPN 9994 who was diagnosed with recurrent malignancy on day 82, 8 (73%) of 11 engrafted patients who survived for at least 100 days after transplantation developed clinical extensive chronic GVHD. Four patients remain alive: 1 with donor engraftment and chronic GVHD after a second transplant following rejection of the first transplant (UPN 9202); 1 with donor engraftment and resolved GVHD without recurrent CML (UPN 8670); 1 with donor engraftment and cytogenetic recurrence of CML, which resolved after immunosuppressive treatment was discontinued (UPN 8532); and 1 with donor engraftment and cytogenetic evidence of recurrent CML currently being treated with interferon (UPN 7850).
DISCUSSION
Results of this study suggest that with the use of conventional pretransplant and posttransplant immunosuppressive regimens, removal of CD4 cells from the graft does not cause rejection but also does not appreciably decrease the risk of grades III-IV GVHD after HLA-mismatched unrelated marrow transplantation. Depletion of CD8 cells was associated with an increased risk of rejection with either DRB1 disparity or with HLA-A or -B disparity. In both groups, the risk of grades III-IV GVHD is likely to be higher than 15% at any dose of CD8 cells associated with less than 5% risk of graft failure. The correlation between graft failure and the number of CD8 cells in the donor marrow supports the hypothesis that donor CD8 cells help to prevent marrow graft rejection. With the conditioning regimens of cyclophosphamide and 13.2 to 13.5 Gy TBI and the posttransplant immunosuppressive regimen of methotrexate and cyclosporine used for patients enrolled in this study, however, at least 5.0 × 106 donor CD8 cells/kg recipient body weight were needed to prevent rejection of a marrow graft with either HLA-DRB1 or HLA-A or -B disparity. With the use of more intensive conditioning regimens, more effective posttransplant immunosuppression, or possibly with larger numbers of stem cells in the graft, lower numbers of donor CD8 cells might be sufficient to prevent rejection without causing acute GVHD.
In unrelated marrow transplantation for treatment of CML, the risk of grades III-IV GVHD is highest in recipients with MHC-class II disparity and lowest in recipients with no HLA-A, -C, -B, -DRB1, or -DQB1 allele disparity and in recipients with disparity limited to a single HLA-A, -C, or -B allele.10 Because unprimed CD4 cells are activated by recipient MHC class II alloantigens and by minor antigens presented in the context of MHC class II molecules, we expected that depletion of donor CD4 cells would greatly diminish the risk of GVHD, especially in recipients with MHC class II disparity. Instead, we found that nearly complete removal of CD4 cells was not sufficient to prevent GVHD in MHC class II–disparate recipients when the graft contained large numbers of CD8 cells. Moreover, the risk of grades III-IV GVHD in MHC class II–disparate recipients appeared to correlate with the numbers of CD8 cells in the graft. The unexpected ability of CD8 cells to cause severe GVHD in MHC class II–disparate recipients might be explained in several ways. First, unprimed CD8 cells can generate in vitro cytotoxic responses against class II alloantigens.22,23 Second, MHC class I allospecific CD8 cells can crossreact with MHC class II alloantigens.24Third, the GVHD in these recipients might have been caused by recognition of minor histocompatibility antigens presented by MHC class I molecules. Because unprimed CD8 cells are activated by MHC class I alloantigens,22 we expected that CD8 cells would cause severe GVHD in recipients with MHC class I disparity. Instead, we found no correlation between the number of CD8 cells in the graft and the risk of grades III-IV GVHD, and the occurrence of grades III-IV GVHD in 4 of 11 engrafted patients in this group was similar to the 33% incidence observed without T-cell depletion in recipients with no HLA-A, -C, -B, -DRB1, or -DQB1 allele disparity.10 Taken together, these results suggest that the risk of GVHD is influenced more by the type of genetic disparity in the recipient than by the types of T cells in the graft.
We found no evidence that depletion of CD4 cells decreased the incidence of chronic GVHD. The 73% incidence of chronic GVHD observed in the present study is similar to the 67% cumulative incidence among patients who survived for at least 80 days after unrelated marrow transplantation without T-cell depletion for treatment of CML.25 The potential effect of CD4 depletion on risk of recurrent malignancy remains difficult to judge. Approximately 10% of patients develop recurrent CML by morphologic criteria within 2 years after unrelated marrow transplantation without T-cell depletion during chronic phase, accelerated phase, or chronic phase after blast crisis.26 By comparison, the recurrence of CML in 4 of 5 engrafted patients who survived for more than 6 months after transplantation in the present study is possibly higher than expected, suggesting that CD4 cells might be needed to mediate an optimal antileukemic effect. This suggestion is consistent with observations that CD8-depleted donor cells can induce remission in patients who have recurrent CML after T-cell–depleted marrow transplantation from an HLA-identical sibling.27
We did not examine immune reconstitution by measuring the number of CD4 cells, CD8 cells, and B cells after transplantation. In a similar study with HLA-identical sibling donors at another center, however, immune reconstitution after transplantation with CD4-depleted marrow was not notably different from the patterns reported with other methods of T-cell depletion.28 To some extent, T-cell reconstitution after marrow transplantation may depend on the presence of mature T cells in the graft, especially in older patients with impaired thymic function.29,30 The high incidence of fungal infections in the present study raises concern that CD4 depletion might have had a detrimental effect on immune reconstitution after transplantation. Approximately 10% of patients develop Aspergillus infection documented by microbiology or histopathology after unrelated marrow transplantation without T-cell depletion,31 and approximately 7% of marrow transplant recipients develop documentedCandida infection when fluconazole is used for prophylaxis during the first 75 days after transplantation.12 With an estimated overall 15% risk of fungal infection after unrelated marrow transplantation, the probability of 6 or more patients with this complication among a total of 18 engrafted patients at risk (excluding the 2 patients with nosocomial C parapsilosis) is 0.042. The association between T-cell depletion and fungal infection in the present study is consistent with results of an early study in which marrow was depleted of T cells with the use of a CD2-specific antibody.32 The observations from these and other studies30,33 34 highlight the importance of T-helper function in host defense against fungal organisms, especially in patients with other risk factors such as neutropenia and glucocorticoid treatment. More effective antifungal prophylaxis may be needed when T-cell depletion is used to prevent GVHD.
Our results show the importance of donor CD8 cells for preventing allogeneic marrow graft rejection in humans. Unfortunately, clinical outcome after HLA-mismatched marrow transplantation was not improved by removing CD4 cells from the graft. The number of CD8 cells needed to prevent rejection was sufficient to cause unacceptably high risks of acute and chronic GVHD. Exhaustive removal of CD4 cells from the graft may diminish graft-versus-leukemia effects in patients with CML and may impair immune reconstitution after transplantation. Alternative methods will be needed to improve results when optimal HLA matching of the donor and recipient is not possible.
ACKNOWLEDGMENT
We thank Kale Slechta and Dr Torstein Egeland for preclinical testing; Beth Macleod for processing the marrow grafts; Andrew Yamane and Mari Malkki for HLA class I allele typing; Lori Hubbard and Tracey Stevens for assistance with donor searches; Jennie Lorenz and Amy Mellon for data management; Alison Sell for assistance with Food and Drug Administration correspondence and preparation of the manuscript; and Dr Shelly Heimfeld and Jeanie Bjerke for critical review of the manuscript. Marrow from unrelated donors was procured with the assistance of the National Marrow Donor Program and other marrow donor registries.
Supported by Grant Nos. AI33484, CA15074, CA18029, HL36444, and CA18221 from the National Institutes of Health, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul J. Martin, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-100, Seattle, WA 98109-1024; e-mail: pmartin@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal