Stable mixed chimerism can be established in dogs given a sublethal dose of 200 cGy total body irradiation (TBI) before and immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP) for 28 and 35 days, respectively, after dog leukocyte antigen-identical marrow transplantation. Most likely, the role of pretransplant TBI was to provide host immunosuppression, since stable mixed chimerism was also achieved in MMF/CSP-treated dogs when 450 cGy irradiation, targeted to cervical, thoracic, and upper abdominal lymph nodes, was substituted for TBI. When TBI was reduced from 200 to 100 cGy, all grafts were rejected within 3 to 12 weeks. Here, we asked whether stable engraftment after 100 cGy TBI could be accomplished by first reducing the intensity of host immune responsiveness with help of the fusion peptide CTLA4Ig, which blocks T-cell costimulation through the B7-CD28 signal pathway. Accordingly, recipient T cells were activated with intravenous (IV) injections of 106 donor peripheral blood mononuclear cells (PBMC)/kg per day on days −7 to −1 before 100 cGy TBI, with concurrent administration of CTLA4Ig 4 mg/kg/d IV. All 7 dogs so treated showed initial mixed chimerism. Two rejected their allografts after 8 and 20 weeks, respectively, and survived with autologous marrow recovery; 1 mixed chimera was unevaluable because of death at 3 weeks from intussusception; and 4 showed persisting mixed chimerism, including unirradiated marrow and lymph node spaces, for now more than 46 to 70 weeks after transplant. Data support the hypothesis that stable marrow allografts can be established by combining nonmyeloablative pretransplant host immunosuppression with posttransplant host and donor cell immunosuppression using MMF/CSP.
WE RECENTLY PROPOSED a new concept for performing marrow allografts which was founded on the knowledge that both host-versus-graft (HVG) and graft-versus-host (GVH) reactions are effected by T cells in the major histocompatibility complex (MHC)-identical setting.1 Accordingly, we designed a regimen of posttransplant immunosuppression that was capable of not only preventing graft-versus-host disease (GVHD),2 but also controlling HVG reactions.3 The regimen consisted of administration of the antimetabolite mycophenolate mofetil (MMF) combined with the T-cell activation blocker cyclosporine (CSP). Using MMF/CSP postgrafting enabled us to decrease the dose of total body irradiation (TBI) otherwise needed for successful engraftment of major histocompatibility complex (MHC)-identical littermate marrow in a canine model from a myeloablative and toxic dose of 920 cGy to a nonlethal dose of 200 cGy.3 Most likely, the major role of the residual pretransplant TBI was in providing host immunosuppression and not in creating “marrow space,” since stable donor engraftment was also achieved in MMF/CSP-treated dogs when 450 cGy local irradiation to cervical, thoracic, and upper abdominal lymph nodes was substituted for 200 cGy TBI.4 This finding raised the question whether pretransplant TBI in the model could, in part or completely, be replaced by noncytotoxic immunosuppression.
A number of biologic reagents are potential candidates for targeting host immune responses, including those that modify first and second signals of T-cell activation. Some of these have been used successfully both in murine and canine models of hematopoietic stem-cell transplantation.5-12 Specifically, sustained T-cell activation is likely to require signal amplification. An important component of this amplification is provided by the costimulatory molecule CD28 on T cells. Wülfing and Davis13 suggest that CD28 costimulation brings about an active accumulation of molecules at the interface of the T cell and the antigen-presenting cell, thereby building an immunologic synapse that increases the overall amplitude and duration of T-cell signaling. Here, we sought to block the costimulatory signal using CTLA4Ig, a soluble, immunosuppressive fusion peptide that consists of the extracellular domain of CTLA4 and the Fc portion of the IgG1 heavy chain and binds to B7.1 and 7.2 (CD80 and CD86) with high avidity.14-17 Antigen recognition through the T-cell receptor, while CD28 costimulation is blocked by CTLA4Ig, resulted in T-cell hyporesponsiveness as tested in both in vitro and in vivo studies.18-24 Accordingly, in the present study, the marrow recipients’ T cells were activated by intravenous (IV) injections of donor antigen in the form of peripheral blood mononuclear cells (PBMC) for 7 days before conditioning with 100 cGy TBI while concurrently administering CTLA4Ig. While a previous study had shown only transient allogeneic engraftment following 100 cGy TBI when no pretransplant CTLA4Ig was administered,3 most current dogs achieved sustained engraftment of dog leukocyte antigen (DLA)-identical littermate marrow.
MATERIALS AND METHODS
Litters of beagles, harriers, pit bull-beagle crossbreeds, and other mixed breeds were either raised at the Fred Hutchinson Cancer Research Center (Seattle, WA) or purchased from commercial kennels. The dogs weighed from 7.2 to 11.1 (median, 10.5) kg and were 7 to 14 (median, 8) months old. They were observed for disease for at least 2 months before study. All were immunized for papillomavirus, leptospirosis, distemper, hepatitis, and parvovirus. The research protocols were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center. Research was conducted according to the principles outlined in the Guide for Laboratory Animal Facilities and Care prepared by the National Academy of Sciences, National Research Council. The kennels were certified by the American Association for Accreditation of Laboratory Animal Care.
DLA-identical littermate donor/recipient pairs were chosen based on the identity for highly polymorphic MHC class I and class II microsatellite marker polymorphisms.25 Specific DLA-DRB1 allelic identity was determined by direct sequencing.26
The day of marrow grafting was designated as day 0. Recipients were injected IV with 106 marrow donor PBMC/kg/d on days −7 to −1. Additionally, they received IV CTLA4Ig 4 mg/kg/d on days −7 to −1. On day 0, they were given a single dose of TBI 100 cGy delivered at 7 cGy/min from 2 opposing cobalt-60 sources.27 Marrow was harvested from the donors under general anesthesia through needles inserted into humeri and femora,27 and infused IV into the recipients at doses of 3.8 to 4.7 (median, 4.0) × 108 nucleated cells/kg within 4 hours of TBI. Recipients were given postgrafting immunosuppression that consisted of CSP 15 mg/kg twice daily orally from day −1 to day 35, and MMF 10 mg/kg twice daily subcutaneously from day 0 to day 27.3 Standard postgrafting care27 included twice-daily oral nonabsorbable antibiotics (neomycin sulfate and polymyxin sulfate) beginning on day −5 until day 14 after transplant. Dogs did not require transfusions. Their clinical status was checked twice daily. Upon completion of the studies, dogs were euthanized and underwent complete autopsies including histopathologic examinations of autopsy specimens.
Hematopoietic engraftment was assessed by sustained recoveries of peripheral blood granulocyte and platelet counts after the postirradiation nadirs, histologic features of the marrow from biopsy or autopsy specimens, and documentation of donor microsatellite marker polymorphisms in nucleated cells from blood and marrow. Conversely, graft rejection was defined as complete repopulation of the hematopoietic system with cells of host type. Posttransplant marrow aspirates from the humoral head were done under general anesthesia. Donor and host hematopoietic cells were distinguished by microsatellite marker polymorphisms as assessed in a polymerase chain reaction (PCR)-based assay.28 The technique detected between 2.5% and 97.5% mixtures of donor and host cells. Mixed hematopoietic chimerism was quantified by estimating the proportion of donor-specific DNA among host DNA using the storage phosphorimaging technique.29
Results in current dogs were compared with those in 6 previously reported dogs given the same treatment except for omission of pretransplant donor PBMC and CTLA4Ig injections.3Additionally, peripheral blood count changes in transplanted dogs were compared to those of 12 dogs given 100 cGy TBI and no subsequent marrow transplant (unpublished observations, 1999).
Mixed leukocyte cultures (MLC)30 were performed to assess the immunosuppressive effectiveness of CTLA4Ig on dog cells in vitro. To this purpose, PBMC from healthy dogs were separated from heparinized whole blood using a Ficoll-Hypaque gradient (density = 1.074). Cells were washed and then resuspended in Waymoth’s medium supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 1% L-glutamine, and 20% heat-inactivated pooled, normal dog serum. A total of 105 responder cells/well and 105 irradiated (2,200 cGy in vitro irradiation from a cesium source) stimulator cells/well obtained from DLA-nonidentical unrelated donors were cultured together in the presence of increasing concentrations of CTLA4Ig or a control peptide, L-6, in round-bottom, 96-well plates for 6 days at 37°C in a humidified 5% CO2 air atmosphere. On day 6, cultures were pulsed with 1 μCi of 3H-thymidine (3H-Td) for 18 hours before harvest. 3H-Td incorporation was determined using a β-scintillation counter (Beckman Instruments, Fullerton, CA). Data were analyzed as mean counts per minute (cpm) of 3 replicates.
Samples for serum levels of CTLA4Ig were collected from 3 dogs before and at various time intervals after a single IV administration of CTLA4Ig 4 mg/kg/d. Serum CTLA4Ig levels were measured using a sandwich enzyme-linked immunoadsorbent assay (ELISA).31 All tests were performed in triplicate.
RESULTS
Table 1 lists data on the immunosuppressive effectiveness of CTLA4Ig in an in vitro MLC assay. At CTLA4Ig concentrations ranging from 0.625 to 10 μg/mL, 90% to 95% inhibition of MLC reactivities was seen. At 0.3 μg/mL, inhibition ranged from 55% to 90%, and at 0.1 μg/mL, it ranged from 35% to 60%. No significant suppression of MLC reactivity was seen with comparable concentrations of the control peptide, L-6.
Immunosuppressive Effect of CTLA4Ig in MLC
| . | Protein (μg/mL) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 0.15 . | 0.31 . | 0.625 . | 1.25 . | 2.50 . | 5.00 . | 10.00 . | |
| CTLA4-Ig (cpm) | ||||||||
| Experiment 1 | 25,346 | 10,254 | 2,536 | 1,181 | 1,333 | 1,193 | 1,126 | 700 |
| Experiment 2 | 8,091 | 5,190 | 3,331 | 1,182 | 473 | 328 | 179 | 334 |
| L-6 (cpm) | 14,607 | 20,956 | 19,360 | 14,265 | 19,568 | 20,332 | 13,393 | 13,326 |
| . | Protein (μg/mL) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 0.15 . | 0.31 . | 0.625 . | 1.25 . | 2.50 . | 5.00 . | 10.00 . | |
| CTLA4-Ig (cpm) | ||||||||
| Experiment 1 | 25,346 | 10,254 | 2,536 | 1,181 | 1,333 | 1,193 | 1,126 | 700 |
| Experiment 2 | 8,091 | 5,190 | 3,331 | 1,182 | 473 | 328 | 179 | 334 |
| L-6 (cpm) | 14,607 | 20,956 | 19,360 | 14,265 | 19,568 | 20,332 | 13,393 | 13,326 |
A total of 105 responder cells and 105irradiated (2,000 cGy) stimulator cells obtained from DLA-nonidentical unrelated dogs were cultured per well either in the absence or the presence of increasing concentrations of CTLA4Ig or the control peptide L-6. 3H-thymidine incorporation was determined after 6 days of culture. Responses are presented as the mean cpm of triplicate experiments. CTLA4Ig was tested in 2 separate experiments.
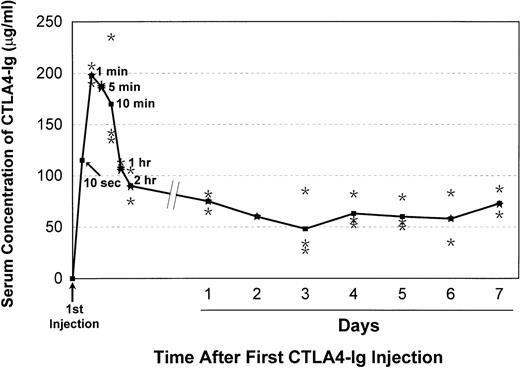
CTLA4Ig serum levels in 3 dogs given 7 daily injections of CTLA4Ig 4 mg/kg IV each are shown in Fig 1. Within 1 minute of the first injection, CTLA4Ig levels up to 200 μg/mL were observed, with subsequent declines over 2 hours to levels ranging from 60 to 70 μg/mL. Subsequent daily trough levels ranged from 25 to 80 μg/mL. These levels were well above the range at which virtually complete suppression of MLC reactivity was observed in vitro.
Mean CTLA4Ig serum concentrations (ELISA)31in 3 dogs given daily injections of 4 mg of CTLA4Ig/kg. Dots indicate data points for individual dogs.
Mean CTLA4Ig serum concentrations (ELISA)31in 3 dogs given daily injections of 4 mg of CTLA4Ig/kg. Dots indicate data points for individual dogs.
Table 2 summarizes the results of the transplant studies. The 6 previously reported dogs not given pretransplant donor PBMC and CTLA4Ig showed initial allogeneic engraftment that lasted for 3 to 12 weeks with subsequent graft rejection, and they survived with complete autologous recovery.3 The 7 dogs given pretransplant PBMC and CTLA4Ig also engrafted. One of the 7 died from an intussusception, a CSP-associated toxicity in dogs, at 3 weeks with mixed donor/host hematopoietic chimerism present. This dog’s early death precluded final evaluation of the fate of the allograft. Two dogs rejected their grafts after 8 and 20 weeks, respectively, and survived with autologous marrow recovery. Four dogs have remained stable mixed donor/host chimeras for now more than 46 to 70 weeks after transplant.
Marrow Grafts From DLA-Identical Littermates After Conditioning With 100 cGy TBI Delivered as a Single Dose at 7 cGy/min
| Pretransplant Donor PBMC + CTLA4Ig* . | Recipient No. . | Sustained Allograft . | Acute or Chronic GVHD . | Complete Autologous Recovery . | Duration of Mixed Chimerism (wk)† . | Cause of Death . |
|---|---|---|---|---|---|---|
| No‡ | n = 6 | 0/6 | — | 6/6 | 3-12 | ET |
| (median, 10) | ||||||
| Yes | E736 | NE | — | NE | >3 | Intussusception2-153 |
| E406 | No | — | Yes | 8 | ET | |
| E465 | No | — | Yes | 20 | ET | |
| E519 | Yes | No | — | >70 | ||
| E520 | Yes | No | — | >70 | ||
| E606 | Yes | No | — | >48 | ||
| E633 | Yes | No | — | >46 |
| Pretransplant Donor PBMC + CTLA4Ig* . | Recipient No. . | Sustained Allograft . | Acute or Chronic GVHD . | Complete Autologous Recovery . | Duration of Mixed Chimerism (wk)† . | Cause of Death . |
|---|---|---|---|---|---|---|
| No‡ | n = 6 | 0/6 | — | 6/6 | 3-12 | ET |
| (median, 10) | ||||||
| Yes | E736 | NE | — | NE | >3 | Intussusception2-153 |
| E406 | No | — | Yes | 8 | ET | |
| E465 | No | — | Yes | 20 | ET | |
| E519 | Yes | No | — | >70 | ||
| E520 | Yes | No | — | >70 | ||
| E606 | Yes | No | — | >48 | ||
| E633 | Yes | No | — | >46 |
NOTE. All recipients were given MMF/CSP* after transplant: MMF 10 mg/kg/twice daily, subcutaneously days 0 to 27; CSP, 15 mg/kg/twice daily, orally days −1 to 35.
Abbreviations: ET, euthanized at completion of the study; NE, not evaluable.
106 PBMC/kg/day IV days −7 to −1; CTLA4Ig, 4 mg/kg/d IV days −7 to −1.
Assessed by weekly microsatellite marker studies of PBMC or marrow cells.
Data in these dogs were previously published.3
Dog was a mixed chimera at the time of death.
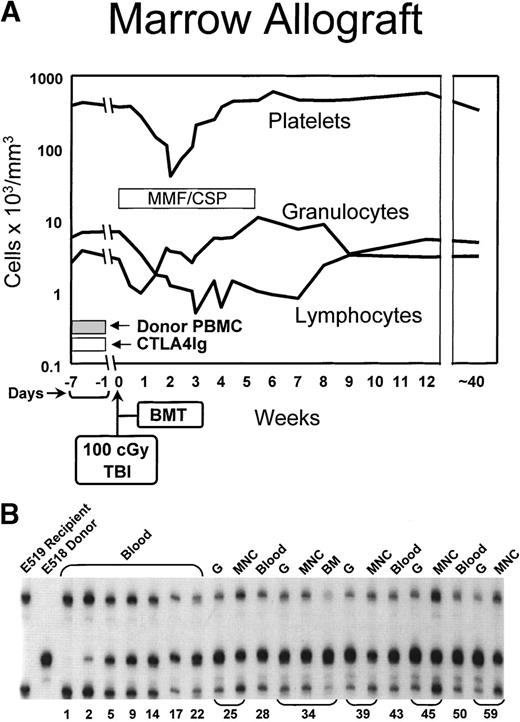
Figure 2 illustrates the hematologic changes and the results of microsatellite marker studies in one of the current dogs (E519). The granulocyte nadir occurred at approximately day 10 with a count of 3,000/μL followed by rapid recovery. The platelet count nadir occurred at 60,000/μL on day 14 followed by recovery, while the lymphocytopenia was more prolonged, and recovery did not occur until after week 7. The lowest lymphocyte counts were on the order of 600/μL. The microsatellite marker studies performed 41 to 44 weeks after transplant demonstrated the presence of donor cells among all nucleated peripheral blood cells, mononuclear cells, and granulocytes.
(A) Peripheral blood granulocyte, platelet, and lymphocyte changes in a dog conditioned with 100 cGy TBI before and MMF/CSP after transplantation of DLA-identical littermate marrow (BMT). In addition, the dog was pretreated with donor PBMC and IV CTLA4Ig. (B) Results of testing for microsatellite markers of donor and recipient cells before transplant (lanes 1 and 2) and recipient cells up to 59 weeks after transplantation (lanes 3 through 21). “Blood” indicates all nucleated peripheral blood cells; MNC, mononuclear cells; G, granulocytes.
(A) Peripheral blood granulocyte, platelet, and lymphocyte changes in a dog conditioned with 100 cGy TBI before and MMF/CSP after transplantation of DLA-identical littermate marrow (BMT). In addition, the dog was pretreated with donor PBMC and IV CTLA4Ig. (B) Results of testing for microsatellite markers of donor and recipient cells before transplant (lanes 1 and 2) and recipient cells up to 59 weeks after transplantation (lanes 3 through 21). “Blood” indicates all nucleated peripheral blood cells; MNC, mononuclear cells; G, granulocytes.
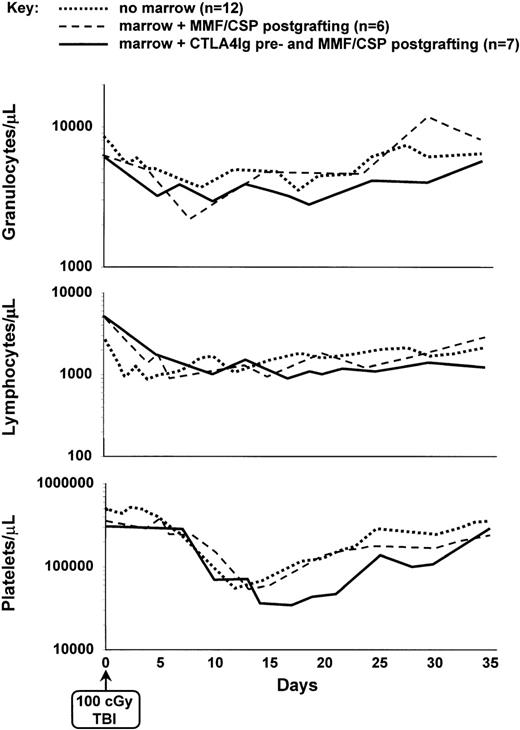
Figure 3 illustrates the median peripheral blood granulocyte, lymphocyte and platelet changes in all current dogs. During the first 2 weeks after TBI, no obvious differences were seen between their lymphocyte counts and those of the previously transplanted dogs not given pretransplant CTLA4Ig and of dogs not given marrow grafts after exposure to 100 cGy TBI. The speed of recovery of counts between days 15 and 35 was marginally but not significantly slower in CTLA4Ig-treated dogs compared with dogs of the 2 other groups.
Median peripheral blood platelet, granulocyte, and lymphocyte changes in dogs given either 100 cGy TBI and no marrow grafts (n = 12); 100 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 6), or pretransplant PBMC plus CTLA4Ig, 100 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 7).
Median peripheral blood platelet, granulocyte, and lymphocyte changes in dogs given either 100 cGy TBI and no marrow grafts (n = 12); 100 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 6), or pretransplant PBMC plus CTLA4Ig, 100 cGy TBI, marrow grafts, and postgrafting MMF/CSP (n = 7).
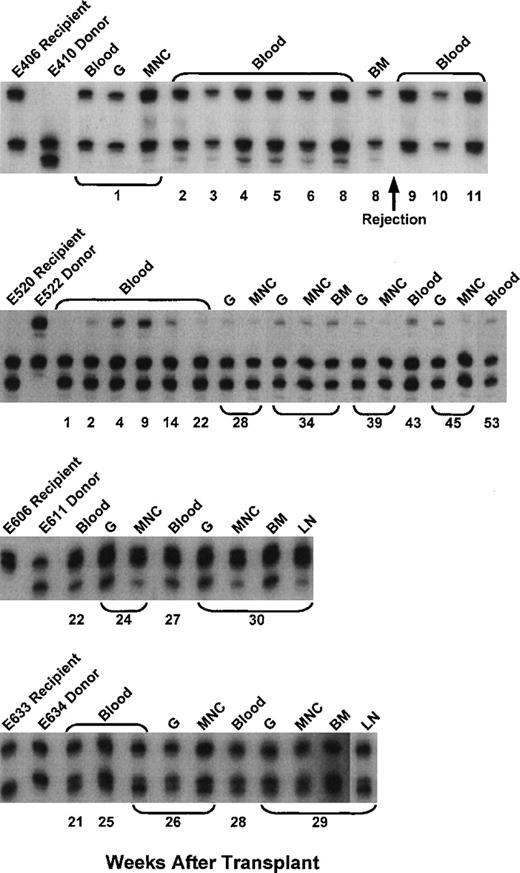
The complete results of microsatellite marker studies in 5 of the evaluable current dogs are shown in Fig 4. Continued allogeneic engraftment was seen in 4 and graft rejection in 1 of the 5 dogs. The degree of stable donor chimerism ranged from 10% to 60%.
Microsatellite marker studies of donor and recipient cells before transplantation and recipient cells after marrow transplantation in 4 dogs given pretransplant PBMC plus CTLA4Ig, 100 cGy TBI, marrow grafts from DLA-identical littermates on day 0, and postgrafting MMF/CSP for 4 and 5 weeks, respectively. BM, bone marrow cells.
Microsatellite marker studies of donor and recipient cells before transplantation and recipient cells after marrow transplantation in 4 dogs given pretransplant PBMC plus CTLA4Ig, 100 cGy TBI, marrow grafts from DLA-identical littermates on day 0, and postgrafting MMF/CSP for 4 and 5 weeks, respectively. BM, bone marrow cells.
Comparing the duration of mixed chimerism in the 6 current evaluable CTLA4Ig-treated dogs (not included was dog E736 that died from intussusception) with that among previously reported controls not given CTLA4Ig by a 2-sided Mann-Whitney U test showed a significant prolongation in current dogs (P = .02).
DISCUSSION
The present study is based on the assumption that allografts can create their own space in the host’s marrow through GVH reactions and, consequently, that intensive cytoreductive and toxic conditioning regimens conventionally used for hematopoietic stem-cell transplants can be replaced by nonmyelotoxic immunosuppression capable of promoting acceptance of grafts.1 Specifically, immunosuppressive agents delivered before transplant would be applied to blunt host T-cell responses, while immunosuppression delivered after transplant would serve to modify both host and donor immune reactivities, with resulting mutual graft/host tolerance in the form of stable mixed donor/host hematopoietic chimerism. Ideally, agents administered before transplant would suppress only host cells with the potential to react to donor antigens while other, say anti-viral, immune responses would be left intact. Experimental evidence in support of this novel transplant approach was provided by a previous canine study in which pretransplant TBI was replaced by 450 cGy irradiation targeted to cervical, thoracic, and upper abdominal lymph nodes.4 When combined with posttransplant immunosuppression by MMF/CSP, pretransplant lymph node radiation resulted in stable, long-term mixed chimerism also in those marrow and lymph node spaces that were shielded from irradiation. Further evidence has come from successful clinical studies in 2 patients with inherited T-cell deficiencies other than severe combined immunodeficiency disease (SCID), in whom pretransplant immunosuppression was omitted and stable grafts were established solely with posttransplant MMF/CSP.32
The current study was a first step toward the ideal transplant program in patients with malignant and nonmalignant hematologic diseases that would rely entirely on nonmyelotoxic immunosuppression. The dose of TBI used here, 100 cGy delivered at 7 cGy/min, was small and resulted only in moderate and transient declines of peripheral blood cell counts, even in dogs not rescued by subsequent marrow transplants. Previous studies had shown 100 cGy TBI alone to be insufficient to assure stable allografts in this model.3 Adding pretransplant activation of host T-cell receptors through daily injections of donor PBMC along with blockade of the second T-cell activation signal through B7→CD28 allowed stable engraftment in 4 of 6 evaluable dogs conditioned with 100 cGy TBI. The success with this approach is all the more impressive since extensive previous studies had shown exposure of canine recipients to donor PBMC or whole blood transfusions before high-dose TBI (920 cGy single dose) to result in a high incidence of graft rejection in the absence of concurrently administered CTLA4Ig.33
Current CTLA4Ig-treated marrow transplant dogs had slower peripheral blood count recoveries than either radiation controls or marrow grafted dogs not given CTLA4Ig. The marginal differences in blood count recoveries could be explained as follows. Recoveries in radiation controls and in previous marrow transplant recipients were largely or entirely derived from the considerable numbers of autologous progenitors which had survived 100 cGy TBI—the donor contributions in the transplanted dogs were not only transient, but also weak and barely above the levels of detection by PCR (data not shown). In contrast, the donor contribution in current CTLA4Ig-treated dogs was strong in at least 4 of the 6 evaluable recipients. This would imply the elimination of a proportion of host progenitors via the graft and, accordingly, a shift in the dependence of blood count recoveries from the relatively large autologous toward the smaller pool of transplanted allogeneic progenitors. Given the differences in stem-cell pool sizes, slower recoveries would be anticipated in the allogeneically engrafted dogs.
A study by Wekerle et al34 in mice conditioned with 300 cGy TBI (dose rate not given) has explored blockade of costimulatory signals to establish mixed hematopoietic chimerism. They found that monotherapy with either an injection of CTLA4Ig on day 2 or a monoclonal antibody to CD40L on day 0 failed to induce stable mixed chimerism. However, combining injections of CTLA4Ig and antibody to CD40L and, thereby, blocking 2 costimulatory signals, resulted in “high levels (>40%) of stable (>8 months) multilineage donor hematopoiesis.” Improved cardiac and skin xenograft survivals were also observed when both CLTA4Ig and antibody to CD40L were combined in murine hosts.18 The combination of the 2 agents also prolonged kidney grafts in primates better than either agent alone.22
There are at least 2 reasons why pretransplant CTLA4Ig was not uniformly successful in the current model. First, the fusion peptide not only blocks the positive signal from B7→CD28, but it also interrupts the desirable negative signal from B7→CTLA4Ig, which serves to turn off T-cell activation.35,36 Second, it is known from studies in mice lacking CD28 that they still could mount immune responses, although mice required repeated stimulations with high doses of antigen.37-39 Also, one study has shown the development of acute GVHD in TBI-treated mice given hematopoietic grafts from CD28− donors.40 Another study, however, failed to confirm that result.41 These contrasting results were explained by the differences in the mouse strain combinations used.
Nevertheless, taken together, these studies suggest that either selective blockade of the B7→CD28 pathway with intact signaling through CTLA4 or the additional blockade of other costimulatory signals, may permit to lower the TBI dose further or even completely eliminate TBI in the current canine model of stem-cell transplantation.
ACKNOWLEDGMENT
The authors are grateful to the technicians of the Shared Canine Resource and the Hematology and Transplantation Biology Laboratories. Barbara Johnston, DVM, provided veterinary support. We thank Dr Peter Linsley, formerly of Bristol Myers Squibb Research, Seattle, WA, and now Rosetta Inphamatics, Kirkland, WA, for the gift of CTLA4IG; Dr Wen Chyi Shyu, Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, NJ, for measuring CTLA4Ig serum levels; Sabine Hadulco, Roche Bioscience-Nutley, Nutley, NJ, for the gift of MMF; and Dr Elizabeth C. Squiers, Sangstat Medical Corp, Menlo Park, CA, for the gift of CSP. We are very grateful to Bonnie Larson, Helen Crawford, Lori Ausburn, and Sue Carbonneau for their outstanding secretarial support.
Supported in part by Grants No. CA78902, CA15704, HL36444, HL03701, and DK42716 from the National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, MD. P.M. and H.J.D. are also supported by grants from the Gabriella Rich Leukemia Foundation. G.G. received support from NIH Grant No. DK09718. J.L.W. and C.Y. received support from NIH Grant No. RR12558. R.S. also received support through a prize from the Josef Steiner Krebsstiftung, Bern, Switzerland, and the Laura Landro Salomon Endowment Fund. H.P.K. is a Markey Molecular Medicine Investigator.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal