Abstract
With the aim of establishing whether the HR2 haplotype in factor V affects the risk of venous thromboembolism, a retrospective multicenter cohort study was performed in 810 family members identified through 174 probands who suffered from at least 1 episode of deep vein thrombosis and/or pulmonary embolism and had an inherited defect associated with thrombophilia (antithrombin, protein C, or protein S deficiency; factor V R506Q or prothrombin G20210A). Fifty-eight percent (468/810) of the family members had an inherited defect and 10% (47/468) were symptomatic. The HR2 haplotype was found in association with factor V R506Q more frequently in family members with venous thromboembolism (18%) than in those without (8%). Double heterozygosity for factor V R506Q and HR2 conferred a 3- to 4-fold increase in the relative risk of venous thromboembolism compared with factor V R506Q alone. The median age at first event was lower when the 2 defects were associated (46v 52 years). No increase in risk of venous thromboembolism could be demonstrated when the HR2 haplotype was associated with inherited thrombophilic defects other than factor V R506Q. Because both factor V R506Q and the HR2 haplotype are very frequent, the effect of their coinheritance on the risk of venous thromboembolism might represent a clinically relevant issue, and screening for HR2 in carriers of factor V R506Q should be considered.
THROMBOPHILIA results from the interaction of multiple genetic and environmental factors.1,2 Patients screened for thrombophilia after an episode of venous thromboembolism (VT) often have more than 1 genetic defect or at least 1 genetic defect and an acquired cause, such as cancer, antiphospholipid antibodies, or hyperhomocysteinemia, and/or circumstantial risk factors (oral contraceptive intake, pregnancy and puerperium, prolonged immobilization, or surgery). Some of the genetic defects associated with thrombophilia are shown to be independent risk factors for VT by large epidemiological studies (antithrombin deficiency,3,4 protein C deficiency,3,4 factor V R506Q,5 and prothrombin G20210A gene mutation6). For others, the data available are not conclusive, because not all studies concur (protein S deficiency3,4,7,8 mutations in the genes coding for enzymes that participate in the metabolism of homocysteine9). It has been shown that the association of 2 or more defects increases the global risk of developing VT (such is the case of factor V R506Q with either antithrombin, protein C or protein S deficiency, or prothrombin G20210A with any of the above10-14). Furthermore, the interaction of 2 defects, 1 of which confers no risk of VT when isolated, can be synergistic. An example is the association of the 4G/5G PAI-1 polymorphism with protein S deficiency15or of the homozygous C677T mutation in methylenetetrahydrofolate reductase with factor V R506Q, although not all investigators agree on the latter.9
The clinical importance of identifying all genetic defects in thrombophilic patients rests with the possibility of counseling them for primary prophylaxis in situations at risk for VT and of planning adequate secondary prophylaxis after thrombotic events have occurred. On the other hand, testing for the defects associated with thrombophilia is costly, so that it is necessary to establish which defects truly contribute to an increased thrombotic risk. We previously reported that the HR2 haplotype in the factor V gene, which includes 6 base substitutions in exons 13 and 16 predicting 2 amino acid changes, is associated with increased resistance to activated protein C (APC) both in normal subjects and in thrombophilic patients, independently of carriership of factor V R506Q.16 Because HR2 resides on the allele that does not carry the R506Q mutation, patients inherit each factor V gene defect independently.16 The coinheritance of HR2 and factor V R506Q determines a degree of APC resistance comparable to that observed in patients homozygous for factor V R506Q.16,17 HR2 by itself is not associated with VT, because a similar prevalence was found in normal individuals and in consecutive patients referred for a venous thromboembolic event.16 The aim of this study was to establish whether HR2 increases the risk of developing VT when coinherited with a defect known to be associated with thrombophilia.
PATIENTS AND METHODS
This is a retrospective multicenter cohort study of 839 individuals who are first and second degree family members of an index case (not included in the study to avoid selection bias) who was identified as follows: he/she had at least 1 episode of deep vein thrombosis or pulmonary embolism, underwent screening for thrombophilia between January 1994 and July 1998, and had 1 of the inherited defects associated with thrombophilia (antithrombin, protein C, protein S deficiency, factor V R506Q, or the G20210A mutation in the prothrombin gene). At least 1 additional family member had the same defect as the index case. Family members belonged to 174 unrelated kindreds (median number of family members per kindred, 4; range, 2 to 21). Of the 839 initially enrolled family members, 29 (3.4%) were not included in the study for various reasons (for 25, the DNA could not be amplified; for 4, the sample was not available). The final number of individuals included in the study was 810. All individuals gave informed consent to participate in the study.
Deep vein thrombosis was objectively diagnosed on the basis of the results of at least 1 of these tests: venography, compression ultrasonography, color-Doppler, or plethysmography. A diagnosis of pulmonary embolism was made on the basis of the results of ventilation-perfusion lung scan, pulmonary angiography, computerized tomography (CT) scan, or magnetic resonance imaging (MR). Deep vein thromboses diagnosed on the basis of clinical suspicion were considered as certain if patients subsequently developed another VT episode diagnosed by instrumental techniques or suffered from an objectively documented postphlebitic syndrome. Forty-six of the 52 venous thromboembolic events (88%) that occurred in the group of family members were objectively diagnosed or considered as certain because the aforementioned criteria were fulfilled. Only 6 of 52 VT episodes (12%) were diagnosed based on clinical suspicion only. Of these, 1 occurred in the factor V R506Q and HR2+group, 1 in the protein C deficiency and HR2+ group, and the remainder in the HR2− group (1 antithrombin deficiency, 1 protein S deficiency, and 2 factor V R506Q). Other thrombotic events (eg, superficial thrombophlebitis and arterial thrombosis) were not considered as an endpoint of the study.
Blood was collected from family members in sodium citrate (9 parts of blood, 1 part of 129 mmol/L trisodium citrate) and rapidly centrifuged at 4°C and 3,000g for 20 minutes. The plasma was separated and aliquoted for storage at −80°C, while the cells were used for DNA extraction by standard methods. Personal and family history of thrombosis and other pertinent information were obtained as well before the laboratory diagnosis was known. Besides a complete screening for thrombophilia (see below), the R2 polymorphism, which defines the HR2 haplotype, was identified. The R2 polymorphism is an A→G transition at position 4070 of the factor V gene (exon 13). The allele frequency has been previously estimated to be 0.08 to 0.11 in the Italian population.16 The whole haplotype comprises other polymorphic sites in exon 13, besides that in position 4070: 2298 C/T, 2325 T/C, 2379 A/G, and 2391 A/G. There is an additional polymorphic site in exon 16 (5380 A/G). Linkage studies have shown the latter to be in linkage disequilibrium with the other polymorphic sites. Because the presence of the G 4070 allele (R2) identifies with certainty the entire HR2 haplotype, in this study patients were screened for this polymorphic site only by polymerase chain reaction (PCR) and enzyme restriction analysis, as previously described.16
Thrombophilia screening was based on measurement of antithrombin (anti-Xa or anti-IIa function), protein C (amidolytic or clotting assay), protein S (free antigen), and search for factor V R506Q (by restriction fragment length polymorphism [RFLP])18 and the prothrombin G20210A mutation (by RFLP or by amplification refractory mutation system [ARMS]).19,20 When a defect was identified, further testing was performed to define the subtype of deficiency (antithrombin, protein C, and protein S), in accordance with current guidelines for diagnosis.21 Positive and negative controls were introduced in all test runs. Protein S functional tests were not run in the presence of the factor V R506Q to avoid artifacts induced by this abnormality on currently available assays.
The incidence of VT was estimated by dividing the number of episodes in each group by the total number of patient-years in that group. For each individual, the follow-up started from the date of birth to the first VT episode, if any, or to July 1998. Only the first thrombotic event of each subject was considered. Relative risks (RR) and their 95% confidence intervals (95% CI) were also calculated, taking the group with no inherited defects and no HR2 haplotype as reference. Survival analysis was performed with the Kaplan-Meier method and the log-rank test was used for comparison between curves. P values less than .05 were taken to indicate statistical significance. The Cox’s proportional-hazards model was also used to adjust the risk of VT for age and sex. The final hazard ratio (and its 95% CI) reflects the relative risk of VT for one group compared with another, adjusted for the other variables in the model. The SPSS for Windows package (release 7.5)22 (SPSS Inc, Chicago, IL) was used for the statistical analysis.
RESULTS
Clinical characteristics of the cohort.
The whole cohort consisted of 810 family members (445 women [55%] and 365 men [45%]; median age, 41 years; age range, 1 to 91 years). A total of 32,506 patient-years of follow-up were recorded. Distribution of defects is shown in Table1. The most frequent was factor V R506Q (32%), followed by prothrombin G20210A (15%). Among the 300 subjects with factor V R506Q, 296 (98.7%) were heterozygous and 4 (1.3%) were homozygous. Of the 156 prothrombin G20210A carriers, 151 (97%) were heterozygous and 5 (3%) were homozygous. Including the individuals with a double defect, there were 17 type I (94%) antithrombin deficiencies and 1 type II (6%), whereas all of the 10 protein C deficiencies were type I and all of the 25 protein S deficiencies were type I and/or III. There were 41 double defects (5%), the majority of which (n = 30) were factor V R506Q and prothrombin G20210A, 5 were factor V R506Q and antithrombin deficiency, 3 were factor V R506Q and protein C deficiency, 1 was factor V R506Q and protein S deficiency, 1 was prothrombin G20210A and antithrombin deficiency, 1 was prothrombin G20210A and protein S deficiency. The HR2 haplotype was detected in 94 of the 810 relatives (12%); 92 of them were heterozygous and 2 were homozygous. Homozygous individuals for any defect did not carry the HR2 haplotype.
Demographic and Clinical Characteristics of the 810 Members From the 174 Thrombophilic Families
| . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V R506Q . | FII G20210A . | Double Defect . | No Defect . |
|---|---|---|---|---|---|---|---|
| No. | 12 | 7 | 23 | 261 | 124 | 41 | 342 |
| % of cohort | 1.5 | 0.9 | 2.8 | 32.3 | 15.3 | 5.0 | 42.3 |
| Sex (M/F) | 8/4 | 5/2 | 11/12 | 109/152 | 54/70 | 19/22 | 159/183 |
| Current age (yr) | |||||||
| Median | 46 | 39 | 53 | 41 | 39 | 41 | 39 |
| Range | 18-76 | 15-67 | 12-74 | 4-91 | 8-78 | 14-83 | 1-84 |
| Prevalence of VT | |||||||
| No./total | 3/12 | 1/7 | 7/23 | 22/261 | 6/124 | 8/41 | 5/342 |
| (%) | (25) | (14) | (30) | (8.5) | (5) | (20) | (1.5) |
| Age at first event (yr) | |||||||
| Median | 29 | 45 | 36 | 51 | 46 | 22 | 45 |
| Range | (23-52) | — | (20-60) | (15-81) | (30-78) | (15-50) | (28-64) |
| Incidence of VT (% per year) | 0.63 | 0.38 | 0.81 | 0.20 | 0.12 | 0.50 | 0.04 |
| Types of VT (no.) | |||||||
| DVT | 1 | 1 | 7 | 22 | 3 | 7 | 3 |
| DVT + PE | 1 | — | — | — | 2 | 1 | 2 |
| PE | 1 | — | — | — | 1 | — | — |
| . | Antithrombin Deficiency . | Protein C Deficiency . | Protein S Deficiency . | Factor V R506Q . | FII G20210A . | Double Defect . | No Defect . |
|---|---|---|---|---|---|---|---|
| No. | 12 | 7 | 23 | 261 | 124 | 41 | 342 |
| % of cohort | 1.5 | 0.9 | 2.8 | 32.3 | 15.3 | 5.0 | 42.3 |
| Sex (M/F) | 8/4 | 5/2 | 11/12 | 109/152 | 54/70 | 19/22 | 159/183 |
| Current age (yr) | |||||||
| Median | 46 | 39 | 53 | 41 | 39 | 41 | 39 |
| Range | 18-76 | 15-67 | 12-74 | 4-91 | 8-78 | 14-83 | 1-84 |
| Prevalence of VT | |||||||
| No./total | 3/12 | 1/7 | 7/23 | 22/261 | 6/124 | 8/41 | 5/342 |
| (%) | (25) | (14) | (30) | (8.5) | (5) | (20) | (1.5) |
| Age at first event (yr) | |||||||
| Median | 29 | 45 | 36 | 51 | 46 | 22 | 45 |
| Range | (23-52) | — | (20-60) | (15-81) | (30-78) | (15-50) | (28-64) |
| Incidence of VT (% per year) | 0.63 | 0.38 | 0.81 | 0.20 | 0.12 | 0.50 | 0.04 |
| Types of VT (no.) | |||||||
| DVT | 1 | 1 | 7 | 22 | 3 | 7 | 3 |
| DVT + PE | 1 | — | — | — | 2 | 1 | 2 |
| PE | 1 | — | — | — | 1 | — | — |
Abbreviations: VT, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism.
Table 1 also shows the main demographic and clinical features of the family members divided according to the different subgroups of coagulation defects. Overall, 52 of 810 family members (6%) had VT. If only individuals with an inherited defect were considered, 47 of 468 (10%) had a VT episode. The lowest incidence of VT was observed in the prothrombin G20210A subgroup (0.12% per year), and the highest incidence of VT was observed in the protein S (0.81% per year) and double defect (0.50% per year) subgroups.
Relative risk of VT.
Forty-two subjects carried the HR2 haplotype alone, and none of them developed VT. Because the number of individuals in the subgroups with a deficiency of the naturally occurring anticoagulants (antithrombin, protein C, and protein S) was small, we chose to pool them in a single group and to analyze them together. Table 2shows the prevalence of the HR2 haplotype in family members with or without VT, according to their inherited thrombophilic defect. Only in the factor V R506Q subgroup and in that with a double defect was a higher prevalence of HR2 found in the VT-positive (4 of 22 [18%] and 1 of 8 [13%], respectively) than in the VT-negative individuals (19 of 239 [8%] and 1 of 32 [3%], respectively). Table 3 shows the impact of the HR2 haplotype on the risk of VT associated with factor V R506Q or a double defect. The relative risk for factor V R506Q alone, estimated at 4.2 (95% CI, 1.6 to 11.3), increased to 10.9 (95% CI, 2.9 to 40.6) when the HR2 haplotype was coinherited. By using Cox’s proportional-hazards model and taking the group with no inherited defects and no HR2 as reference, after adjustment for sex and age, the hazard ratio for individuals heterozygous for factor V R506Q not carriers of HR2 was 3.7 (95% CI, 1.4 to 10.1), whereas in the doubly heterozygous patients for factor V R506Q and HR2 it was 14.0 (95% CI, 3.7 to 53.4).
Prevalence of the HR2 Haplotype in Family Members With (DVT+) or Without (DVT−) VT, According to Their Inherited Thrombophilic Defect
| . | Naturally Occurring Anticoagulant Deficiency . | Factor V R506Q . | Prothrombin G20210A . | Double Defect . | ||||
|---|---|---|---|---|---|---|---|---|
| DVT+ . | DVT− . | DVT+ . | DVT− . | DVT+ . | DVT− . | DVT+ . | DVT− . | |
| HR2+ | 1/11 (9%) | 10/31 (32%) | 4/22 (18%) | 19/239 (8%) | 0/6 (0%) | 16/118 (14%) | 1/8 (13%) | 1/33 (3%) |
| . | Naturally Occurring Anticoagulant Deficiency . | Factor V R506Q . | Prothrombin G20210A . | Double Defect . | ||||
|---|---|---|---|---|---|---|---|---|
| DVT+ . | DVT− . | DVT+ . | DVT− . | DVT+ . | DVT− . | DVT+ . | DVT− . | |
| HR2+ | 1/11 (9%) | 10/31 (32%) | 4/22 (18%) | 19/239 (8%) | 0/6 (0%) | 16/118 (14%) | 1/8 (13%) | 1/33 (3%) |
Effect of the HR2 Haplotype on the Relative Risk of Thrombosis (RR) in Patients With Factor V R506Q or a Double Thrombophilic Defect
| . | Factor V R506Q . | Double Defect . | ||
|---|---|---|---|---|
| HR2− . | HR2+ . | HR2− . | HR2+ . | |
| No. of subjects | 238 | 23 | 39 | 2 |
| Sex (M/F) | 99/139 | 10/13 | 18/21 | 1/1 |
| Median age at first event (range) | 52 (20-80) | 46 (15-57) | 25 (15-50) | 19 (—) |
| No. with VT | 18 | 4 | 7 | 1 |
| Patient-years | 9909 | 861 | 1560 | 56 |
| Incidence of VT (%/yr) | 0.18 | 0.47 | 0.45 | 1.8 |
| RR (95% CI) | 4.2 (1.6-11.3) | 10.9 (2.9-40.6) | 10.5 (3.3-33.1) | 41.9 (4.9-359) |
| . | Factor V R506Q . | Double Defect . | ||
|---|---|---|---|---|
| HR2− . | HR2+ . | HR2− . | HR2+ . | |
| No. of subjects | 238 | 23 | 39 | 2 |
| Sex (M/F) | 99/139 | 10/13 | 18/21 | 1/1 |
| Median age at first event (range) | 52 (20-80) | 46 (15-57) | 25 (15-50) | 19 (—) |
| No. with VT | 18 | 4 | 7 | 1 |
| Patient-years | 9909 | 861 | 1560 | 56 |
| Incidence of VT (%/yr) | 0.18 | 0.47 | 0.45 | 1.8 |
| RR (95% CI) | 4.2 (1.6-11.3) | 10.9 (2.9-40.6) | 10.5 (3.3-33.1) | 41.9 (4.9-359) |
The reference group is that of individuals with no inherited defects and no HR2 haplotype (n = 300; I = 0.043%/year; RR = 1.0).
Abbreviations: HR2+, positive for HR2 haplotype; HR2−, negative for HR2 haplotype.
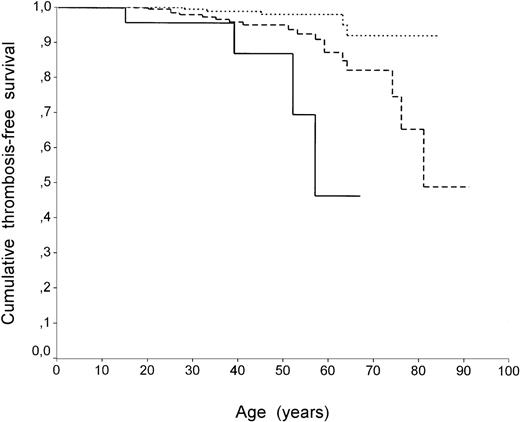
Survival analysis by the Kaplan-Meier method confirmed the different pattern between carriers of factor V R506Q with or without HR2 (P = .01 with log-rank test). Figure 1 shows thrombosis-free survival curves in patients with factor V R506Q with and without HR2 compared with individuals who did not carry any defect. The curve representing cumulative thrombosis-free survival of individuals with factor VR506Q and HR2 falls more sharply and earlier than that representing carriers of factor R506Q only. When homozygous individuals for factor V R506Q or prothrombin G20210A were excluded from the analysis, no differences in results were found (data not shown). The role of circumstantial risk factors associated with the first episode of VT was also analyzed. The prevalence of circumstantial risk factors (surgery, pregnancy/puerperium, oral contraceptive use, plaster, and/or immobilization) was similar in family members carrying factor V R506Q with and without HR2 (2 of 3 [67%] and 10 of 16 [62%], respectively).
Thrombosis-free survival curves (Kaplan-Meier method) of family members without defects (dotted line), with factor V R506Q alone (dashed line), and factor V R506Q with HR2 (continuous line). In all curves each step represents a VT event. Differences between the curves (factor V R506Q with HR2 v individuals with no defects orv factor V R506Q alone) were significant by log-rank test (P < .001 and P = .01, respectively).
Thrombosis-free survival curves (Kaplan-Meier method) of family members without defects (dotted line), with factor V R506Q alone (dashed line), and factor V R506Q with HR2 (continuous line). In all curves each step represents a VT event. Differences between the curves (factor V R506Q with HR2 v individuals with no defects orv factor V R506Q alone) were significant by log-rank test (P < .001 and P = .01, respectively).
DISCUSSION
A recent study by Alhenc-Gelas et al23 has shown that the HR2 haplotype of factor V is a mild risk factor for VT. This is in contrast with our previous findings16 and with the results of this study, in which none of the patients with HR2 alone had a thrombotic event. Although the different results of our previous study and that by Alhenc-Gelas et al23 could be due to the relatively small size of the sample originally studied by us, the discrepancy observed with the present study is most probably related to the different design and selection of patients (case-control, all venous thromboembolic events considered).23 However, we did find that HR2 increases the risk of VT by approximately 3-fold when associated with factor V R506Q. In the group of patients with double thrombophilic defects in our cohort, the majority of whom (39/41) carry factor V R506Q, the already high relative risk of thrombosis was approximately quadruplicated by the coexistence of HR2, bringing it to more than 40 times that of individuals with no known inherited defects. The coinheritance of HR2 also decreased the median age at which the first thrombotic event occurred. Our findings are in agreement with those of a preliminary report showing that the R2 polymorphism, although not significantly more prevalent in patients with thrombosis compared with patients without, increased the probability of developing thrombosis when in association with factor V R506Q.24
The conclusions relating to the effect of the coinheritance of HR2 on the relative risk of venous thromboembolism require a note of caution. Although the number of patients studied was globally large, the number of thrombotic events was low, as is generally the case in family studies, especially considering the restrictive criteria used. This applies especially to the double thrombophilic defect group, but it is also true for the association between HR2 and factor V R506Q. In addition, a possible limitation of this study is that index patients and their families were recruited in different centers and over a 4-year period. However, having selected family members from kindreds in whom probands had had at least 1 episode of VT makes us confident to have reduced the possible bias owing to different selection criteria. The design of the study (retrospective) leads also to the possibility of recall bias for thrombotic events, but we do not think that it could have affected our results, because most of the VT events in family members (46 of 52 [88%]) had an objective confirmation and only 1 event in the factor V R506Q with HR2 was diagnosed based on clinical criteria. Moreover, if the bias were present, it should be equally represented among the different coagulation defects.
The mechanism underlying the prothrombotic effect of the haplotype remains unclear. HR2 determines mild resistance to APC, which is evident especially when the haplotype is in the homozygous form.16 It also increases resistance to APC of patients that are heterozygous carriers of factor V R506Q, bringing it to a degree similar to that found in patients homozygous for factor V R506Q.16,17 Because resistance to APC in itself seems to be a risk factor for VT,25 26 the prothrombotic action of HR2 could be mediated through an enhancement of resistance to APC, synergistic with that determined by the factor V R506Q. However, other, as yet undetected functional effects on factor V are possible, because multiple amino acid substitutions are determined by HR2. One or more of these could impair, for example, the cofactor activity of factor V to APC. However, if it were so, one would expect to see an increased prothrombotic effect also when HR2 is associated with protein C or protein S deficiency. Because of the rarity of the defects of the naturally occurring anticoagulants compared with factor V R506Q and factor II G20210A, the number of family members in this group was small. However, the difference in number of individuals with VT in the naturally occurring anticoagulants deficiency HR2+ group (1/11) compared with that in the HR2− group (11/31) is striking. The factor II G20210A group was more abundant, so that the lack of interaction of HR2 with this thrombophilic mutation is very evident. It seems therefore that the HR2 haplotype is preferentially worsening the prothrombotic risk associated with factor V R506Q.
Because of the elevated allelic frequency of HR2 and factor V R506Q in the general and thrombophilic populations, the coinheritance of the 2 genetic variations is expected to be rather frequent (1 in 400 and 3 in 100, respectively). Accordingly, the impact of HR2 on the prothrombotic risk of factor V R506Q should be considered when evaluating the risk of VT of each individual patient, and the benefit of screening for the R2 allele in patients with factor V R506Q should be evaluated.
ACKNOWLEDGMENT
The precious contribution of A. Cappellari and I. Casorelli is gratefully acknowledged.
Supported by institutional grants from the IRCCS Maggiore Hospital (Milan, Italy).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to E.M. Faioni, MD, Hemophilia and Thrombosis Center, Via Pace 9, 20122 Milano, Italy; e-mail:elena.faioni@unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal