Abstract
Targeted mutation of the myeloid transcription factor C/EBPɛ in mice results in gram-negative septic death at 3 to 5 months of age. This study defines the underlying molecular defects in their terminal granulocytic differentiation. The mRNA for the precursor protein of the cathelin-related antimicrobial peptides was almost completely absent in the bone marrow cells of C/EBPɛ−/− mice. This finding may help explain their susceptibility to gram-negative sepsis, because both are bacteriocidal peptides with potent activity against gram-negative bacteria. Superoxide production was found to be reduced in both granulocytes and monocytes of C/EBPɛ−/− mice. While gp91 phox protein levels were normal, p47phox protein levels were considerably reduced in C/EBPɛ −/− granulocytes/monocytes, possibly limiting the assembly of the NADPH oxidase. In addition, expression of mRNA of the secondary and tertiary granule proteins, lactoferrin and gelatinase, were not detected, and levels of neutrophil collagenase mRNA were reduced in bone marrow cells of the knock-out mice. The murine lactoferrin promoter has a putative C/EBP site close to the transcription start site. C/EBPɛ bound to this site in electromobility shift assay studies and mutation of this site abrogated binding to it. A mutation in the C/EBP site reduced the activity of the promoter by 35%. Furthermore, overexpression of C/EBPɛ in U937 cells increased the activity of the wild-type lactoferrin promoter by 3-fold. In summary, our data implicate C/EBPɛ as a critical factor of host antimicrobial defense and suggests that it has a direct role as a positive regulator of expression of lactoferrin in vivo.
THE CCAAT ENHANCER binding protein (C/EBP) family of transcriptional factors contains 6 members. Two of these, C/EBPα and C/EBPε, are of critical importance for the development of normal neutrophils.1,2 All C/EBP proteins share a highly conserved, C-terminal leucine-zipper dimerization domain and a basic DNA binding domain.3-5 The DNA binding consensus site has been identified as TKNNGYAAK (Y = C or T, K = T or G).6 The aminoterminal transactivation domains are more diverse.
In the hematopoietic system, C/EBPα is expressed early in the granulocytic differentiation pathway, and its absence in C/EBPα knock-out mice leads to a complete lack of granulocytic differentiation with an arrest at the stage of immature myeloblasts. Myeloid cells of C/EBPα−/− mice lack granulocyte colony-stimulating factor (G-CSF) receptor mRNA, which may contribute to their defective differentiation.2 Recent findings place C/EBPα as a critical factor needed for granulocytic commitment of the myeloid progenitor cells.7 Expression of C/EBPε is strikingly restricted to the later stages of the granulocytic differentiation pathway and to the T-lymphoid lineage.8-11 More recently, expression has also been described in murine monocytes.12Consistent with the restricted expression pattern of C/EBPε, the C/EBPε knock-out mice morphologically display defects in terminal differentiation of neutrophils and decreased numbers of eosinophils. The G-CSF, macrophage (M)-CSF, and GM-CSF receptors are expressed in the myeloid cells of C/EBPε−/− mice.1Functional defects of C/EBPε−/− granulocytic cells include a significantly reduced capacity to produce superoxide in response to phorbol 12-myristate 13-acetate (PMA) as well as an impaired ability to migrate. The mice are born apparently healthy, but die 3 to 5 months after birth because of infectious complications, most notably infections with gram-negative bacteria like pseudomonas aeruginosa.1
To characterize further the differentiation defects in C/EBPε−/− mice and to determine potential target genes of C/EBPε, we examined the expression of various myeloid-specific genes in the bone marrow cells of C/EBPε knock-out mice. We focused on genes potentially involved in the phenotype of abnormal C/EBPε−/− granulocytes, eg, the NADPH oxidase complex, bacteriocidal peptides (cathelins), chemotaxis receptors, and the primary and secondary granule proteins. In addition, we provide evidence that the lactoferrin promoter is a direct target of the transcription factor C/EBPε.
MATERIALS AND METHODS
Expression vectors and promoter reporter constructs.
Eukaryotic expression vectors for the C/EBPε isoform p32 and murine C/EBPα have been described.1 The lactoferrin promoter (−750 to +39) was amplified using the polymerase chain reaction (PCR) with primers containing KpnI (5′) and BglII (3′) restriction sites. The PCR product was digested with KpnI and BgllI, agarose gel purified, and ligated into the KpnI/BglII predigested luciferase reporter plasmid pGL3 basic (Promega, Madison, WI). The construct was sequenced to confirm its identity to the published sequence (−750 Lac-Luc).13 A shorter construct −230 Lac-Luc was derived from this template by PCR using Pfu Polymerase. A mutation of the C/EBP site was introduced into the wild-type template by PCR mutagenesis with Pfu polymerase and the mutagenic primer: 5′ GGG TGT CTA TCT GAC GAC AGG GCG GG 3′ according to the method of Picard et al.14 Sequencing primers derived from pGL 3 basic were used as 5′ (pGL 2) and 3′ (RV3) primers for the PCR reaction. The PCR product was digested with KpnI andBglII and religated into the vector. A short construct only containing the proximal 87 bp of the lactoferrin promoter in theKpnI/BglII restriction sites of pGl3 basic was kindly provided by Dr Nancy Berliner (Division of Hematology, Yale School of Medicine, New Haven, CT).
Animals.
C/EBPε−/− mice and control 129/SvEv × NIH Black Swiss mice were bred under sterile conditions in the animal housing facility at the Burns and Allen Research Institute. Mice were killed by cervical neck dislocation. Bone marrow was flushed out of isolated femurs and tibiae with Iscove’s Modified Dulbecco’s Medium (IMDM) + 20% fetal calf serum (FCS) using a no. 26 gauge needle. After depletion of adherent cells by incubation on plastic dishes for 1 hour, cells were spun down and immediately dissolved in Trizol reagent. RNA was isolated according to the manufacturer’s protocol. The bone marrow cells taken for RNA isolation contained less than 1% monocytes and approximately 25% Gr-1 positive cells in wild-type and knock-out mice. Neutrophils were harvested 4 hours after intraperitoneal injection with 2 mL 4% thioglycollate by peritoneal lavage and dissolved in Trizol Reagent (GIBCO-BRL, Rockville, MD). The RNA was subsequently treated with DNAse I for 30 minutes at 37°C, phenol/chloroform extracted, precipitated with ice-cold ethanol, and resuspended in 50 μL DEPC-treated H2O.
Northern blot analysis.
Twenty micrograms of RNA of C/EBPε−/− bone marrow, control bone marrow, and NIH3T3 fibroblasts were run on a denaturing formaldehyde gel at 30 V for 3 hours. RNA was partially hydrolysed by soaking the gel in 0.05 mol/L NaOH/1.5 mol/L NaCl for 30 minutes followed by soaking 30 minutes in 0.5 mol/L TrisCl/(pH7.4) for pH neutralization. The gel was blotted in 20X sodium chloride sodium citrate solution (SSC) overnight on a nylon membrane (Magna Charge; Micron Separations Inc, Westborough, MA). The membrane was baked for 1 hour at 80°C in a vacuum oven and crosslinked in a UV crosslinker (Stratagene, La Jolla, CA).
Prehybridization and hybridization of the membrane were performed in Rapid Hyb hybridization buffer (Amersham, Arlington Heights, IL) at 65°C for 1 and 3 hours, respectively. Posthybridization washes were performed with 2X SSC, 0.1% sodium dodecyl sulfate (SDS) for 20 minutes at room temperature and twice with 0.1X SSC, 0.1% SDS at 65°C for 15 minutes each.
Plasmids containing the cDNAs for lactoferrin (probe:PvuII/SmaI fragment), neutrophil gelatinase (probe:EcoRI/BamHI fragment), and neutrophil collagenase (BglII/PstI fragment) were kindly provided by Dr Nancy Berliner. A plasmid encoding the cDNA for murine gp91 phox (probe:NcoI fragment) was kindly provided by Dr M. Dinauer (Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN). The probe for murine cathelin related protein (MCRP) was generated by PCR. The PCR product (200 bp) was gel purified and directly sequenced using the Perkin Elmer Applied Biosystems DNA sequencing kit (PE Applied Biosystems, Warrington, UK) to confirm its identity to the published sequence.15 All probes were randomly labeled before hybridization with 32P-dCTP using the Ready To Go DNA labeling Kit (Pharmacia Biotech, Piscataway, NJ).
Reverse transcriptase (RT)-PCR assay.
Five micrograms of DNAse I–treated RNA was reverse transcribed with avian myeloblastosis virus (AMV) RT (Promega, Madison, WI) for 30 minutes at 37°C. Untreated RNA was used as control for DNA contamination in the RT-PCR reactions. One microliter of cDNA of C/EBPε−/−, control bone marrow, and NIH3T3 fibroblasts were used as template. PCR was performed under the following conditions: an initial denaturation step at 94°C for 2 minutes was followed by 27 cycles, 94°C for 40 seconds, 55°C/58°C for 35 seconds, and 72°C for 35 seconds with the primer pairs listed in Table 1. The annealing temperature for rac-1 amplification was 52°C, and for rac-2 64°C. PCR products were blotted by alkaline transfer in 400 mmol/L NaOH on a nylon membrane and probed with 32P-γATP end-labeled internal oligonucleotides as indicated. In Table 1, primer sequences are given as cDNA sequence positions.
cDNA Positions of Primer Sequences Used for RT-PCR
| Gene . | Primer (Sense) . | Primer (Antisense) . | Internal Oligo . |
|---|---|---|---|
| MPO | 1027-1045 | 1410-1427 | 1218-1238 |
| Cathepsin G | 255-272 | 667-690 | 393-412 |
| Lactoferrin | 259-276 | 814-831 | 502-524 |
| CRAMP | 49-68 | 291-310 | |
| B9 | 190-209 | 411-430 | 282-302 |
| gp91phox | 313-331 | 682-699 | 501-533 |
| p22phox | 26-44 | 596-615 | 316-338 |
| p40phox | 262-282 | 786-808 | 561-584 |
| p47phox | 140-160 | 799-821 | 500-523 |
| p67phox | 328-349 | 919-939 | 560-578 |
| rac-1 | 201-220 | 421-440 | 301-320 |
| rac-2 | 187-208 | 377-398 | 305-324 |
| fMLP R | 44-63 | 235-255 | |
| Interleukin-8 R | 222-242 | 641-660 |
| Gene . | Primer (Sense) . | Primer (Antisense) . | Internal Oligo . |
|---|---|---|---|
| MPO | 1027-1045 | 1410-1427 | 1218-1238 |
| Cathepsin G | 255-272 | 667-690 | 393-412 |
| Lactoferrin | 259-276 | 814-831 | 502-524 |
| CRAMP | 49-68 | 291-310 | |
| B9 | 190-209 | 411-430 | 282-302 |
| gp91phox | 313-331 | 682-699 | 501-533 |
| p22phox | 26-44 | 596-615 | 316-338 |
| p40phox | 262-282 | 786-808 | 561-584 |
| p47phox | 140-160 | 799-821 | 500-523 |
| p67phox | 328-349 | 919-939 | 560-578 |
| rac-1 | 201-220 | 421-440 | 301-320 |
| rac-2 | 187-208 | 377-398 | 305-324 |
| fMLP R | 44-63 | 235-255 | |
| Interleukin-8 R | 222-242 | 641-660 |
Western blot.
107 cells were collected at either 12 or 72 hours after intraperitoneal injection of 4% thioglycollate into 3-week-old CEBPε−/− and wild-type mice. Cells were washed once in ice-cold phosphate-buffered saline (PBS) and then treated with 2.7 μmol/L DFP (diisopropyl fluorophosphate; Calbiochem, La Jolla, CA) for 10 minutes on ice, then washed 3 times in ice-cold PBS and lysed in Triton buffer (20 mmol/L TrisHCl, pH 8.0, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% TritonX-100, 2 mmol/L phenylmethylsulfonyl fluoride [PMSF], 20 μg/mL chymostatin, 10 μmol/L leupeptin, and 1 mmol/L AEBSF [4-(2-aminoethyl)benzene sulfonylfluoride]. All protease inhibitors were purchased from Calbiochem. Fifty micrograms of total cell lysates were loaded in each lane of a 12% polyacrylamide gel. After SDS polyacrylamide gel electrophoresis, the proteins were transferred to a nitrocellulose membrane overnight. Membranes were blocked with 5% nonfat dry milk in TBS-T buffer for 1 hour, then probed with gp91phox (dilution, 1:2,000) or p47 phox antiserum (dilution, 1:1,000). Antiserum to murine gp91 phox was kindly provided by Dr M. Dinauer.16 Antiserum to p47phox was described previously.17 Blots were then incubated with biotinylated anti-mouse/anti-rabbit IgG secondary antibody (dilution 1:3,000; Vector Laboratories Inc, Burlingame, CA) followed by peroxidase conjugated streptavidin (BioGenax, San Ramon, CA) as recommended by the manufacturers. All the blots were reprobed with mouse antirabbit GAPDH antibody (1:4,000 dilution) (Research Diagnostics Inc, Flanders, NJ) to ensure equal loading of samples. Signals were detected with Supersignal Blaze Chemiluminescent substrate (Pierce, Rockford, IL). Antibody incubations and wash steps were performed with TBS-T (0.05% Tween 20) + 5% nonfat dry milk.
Preparation of nuclear extracts.
For preparation of nuclear extracts, 5 × 106 cells were washed 3 times with ice-cold PBS. After the last wash, adherent cells were scraped off the dish with a rubber policeman and resuspended in 500 μL extraction buffer B (20 mmol/L HEPES, pH 7.9, 20% glycerol, 10 mmol/L NaCl, 0.2 mmol/L EDTA, 1.5 mmol/L MgCl2, 0.1% Triton X, 1 mmol/L dithiothreitol [DTT], 1 mmol/L PMSF, 40 μL/mL Complete [Boehringer, Indianapolis, IN]). After a 15-minute incubation on ice, the nuclei were pelleted at 250g for 10 minutes. Nuclei were resuspended in extraction buffer B, and NaCl was added dropwise with mixing to a final concentration of 300 mmol/L NaCl. Nuclei were rocked for 60 minutes at 4°C. Samples were microcentrifuged at 12,000 rpm and supernatants frozen at −80°C.
Electromobility shift assay (EMSA).
For protein expression, 10 μg of eukaryotic expression vector (C/EBPε, C/EBPα) was transfected into COS-1 cells (10-cm dish). Transfection was performed with Superfect reagent (Qiagen, Valencia, CA) over 3 hours according to the manufacturer’s protocol. Nuclear extracts were prepared after 48 hours. Alternatively, recombinant Maltose binding protein (MBP)-C/EBPε fusion protein was expressed in Bl 21 bacteria and purified with amylose resin as described previously.9 Two fragments of the cloned 5′, 750-bp lactoferrin promoter were generated by digestion withKpnI/Xba andXba/BglII. The fragments were gel purified, treated with calf intestinal phosphatase, and end-labeled with 32P-γATP. Double-stranded oligonucleotides (30 bp) containing the C/EBP consensus site of the lactoferrin promoter and adjacent sequences were end-labeled with 32P γATP. A standard reaction contained 1 ng labeled probe, 10 μg COS-1 nuclear extract expressing either C/EBPε or C/EBPα, 2 μg pdIdC, and 4.5 μg BSA in a 20-μL volume. Competing cold oligonucleotides (shown below at 10- and 100-fold molar excess) or antibodies (1 μg/μL) were added where indicated. Electrophoresis was performed on a 4% polyacrylamide gel at 30 mA.
Lactoferrin: 5′ GGGTGTCTATTGGGCAACAGGGCGGC 3′
Mutant Lactoferrin: 5′ GGGTGTCTATCTGACGACAGGGCGGC 3′
Transient transfection assays.
Cell line U937 was grown in RPMI 1640 + 10% fetal bovine serum (FBS) + penicillin/streptomycin. Approximately 2 × 107 U937 cells were transfected by electroporation with 1 pulse at 320 V, 30 ms with 25 μg of −230 Lac-Luc lactoferrin promoter reporter plasmid or C/EBP site mutant together with 2.5 μg of cytomegalovirus (CMV)–β-galactosidase vector in 500 μL RPMI + 10% FBS. Cells were harvested after 16 hours to measure luciferase and β-galactosidase activity. Transfection efficiency was normalized for all samples according to β-galactosidase activity.
Cell line U937 stably transfected with a zinc inducible C/EBPε (p32) expression vector or empty vector was grown in RPMI 1640 + 10% FBS + 800 μg/mL G418. Approximately 4 × 107 U937 (p32) or empty-vector (PMT) myeloid cells were transfected by electroporation with 1 pulse at 320 V, 30 ms with 40 μg (−87 Lac-Luc) lactoferrin promoter reporter plasmid and 4 μg of β-galactosidase vector in 500 μL RPMI + 10% FBS. After electroporation, cells were split in half and plated in 10 mL RPMI +10 FBS ± 100 μmol/L zinc-sulfate. Cells were harvested after 16 hours to measure luciferase activity. Aliquots were taken to monitor the induction of C/EBPε p32 by Western blot analysis.
Electron microscopy.
Peripheral blood of C/EBPε−/− and control mice was obtained from the retroocular venous plexus. A buffy coat was prepared in a 1-mL tuberculin syringe. The plasma was removed and the remaining cells were overlayered with 3% glutaraldehyde. The samples were fixed in 1% osmium-tetraoxide and embedded in epon for ultrathin sectioning. Sections were stained with uranylacetate and lead citrate and evaluated with a JEOL transmission electron microscope (JEOL, Peabody, MA).
Continuous ferricytochrome c assay.
Superoxide anion production was measured via superoxide dismutase-inhibitable reduction of ferricytochrome c18 on a UVIKON 941 spectrophotometer equipped with a temperature-controlled (37°C) cuvette holder. In brief, cells (2 × 106/mL) were incubated with cytochrome c (100 μmol/L) and PMA (100 ng/mL) in 1 mL of Hanks’ balanced salt solution (HBSS, pH 7.4). Reduced cytochrome c was measured on the basis of increase in absorbance at 550 nm, and O2−generation was calculated by using an absorption coefficient of 21 mmol/L−1 cm−1.
RESULTS
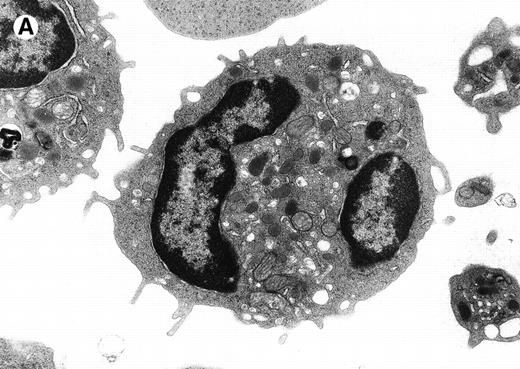
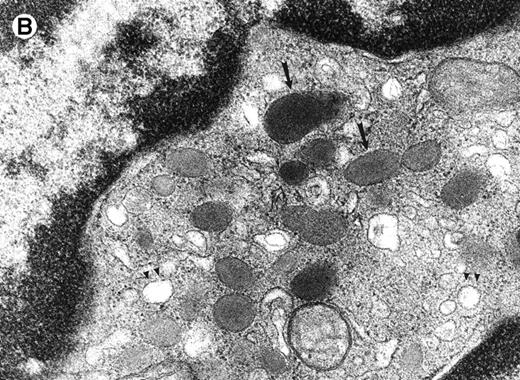
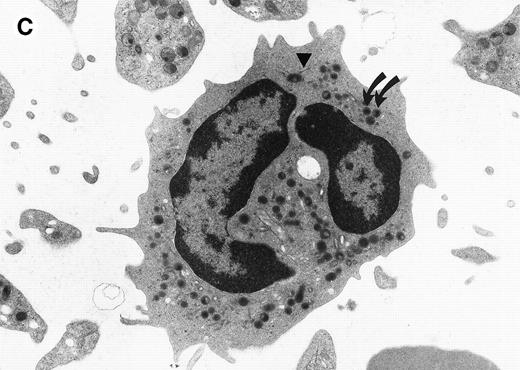
The morphological changes in the neutrophils of C/EBPε−/− mice suggest that C/EBPε transactivates target genes important in late myeloid differentiation.1Figure 1compares the ultrastructural morphology of peripheral blood granulocytes from C/EBPε−/− and wild-type mice. The EM study shows signs of cytoplasmic immaturity in C/EBPε−/− peripheral blood granulocytes. Rod-shaped tertiary granules are completely missing, the ratio of primary to secondary granules is increased, and the number of mitochondria/cell is higher in C/EBPε−/− granulocytic cells.
Electron micrograph of a peripheral blood neutrophil from a C/EBPɛ−/−mouse (A and B) and a wild-type mouse (C). (A) The C/EBPɛ−/− peripheral blood neutrophil shows signs of immaturity; the absolute number of granules is reduced and tertiary, bacilliform-shaped granules are missing. (B) Higher magnification of middle section of C/EBPɛ−/− neutrophil: The ratio of the larger, primary (electron-dense) granules (arrows) to the smaller less electron-dense, secondary granules is increased. In the less mature C/EBPɛ−/− neutrophils, most secondary granules appear electron-lucent, most likely due to extraction by glutaraldehyde fixation (arrowheads) as previously described.46 (C) For comparison, mature wild-type granulocyte has multiple, small secondary granules (arrows) and bacilliform tertiary granules (arrowhead).
Electron micrograph of a peripheral blood neutrophil from a C/EBPɛ−/−mouse (A and B) and a wild-type mouse (C). (A) The C/EBPɛ−/− peripheral blood neutrophil shows signs of immaturity; the absolute number of granules is reduced and tertiary, bacilliform-shaped granules are missing. (B) Higher magnification of middle section of C/EBPɛ−/− neutrophil: The ratio of the larger, primary (electron-dense) granules (arrows) to the smaller less electron-dense, secondary granules is increased. In the less mature C/EBPɛ−/− neutrophils, most secondary granules appear electron-lucent, most likely due to extraction by glutaraldehyde fixation (arrowheads) as previously described.46 (C) For comparison, mature wild-type granulocyte has multiple, small secondary granules (arrows) and bacilliform tertiary granules (arrowhead).
A striking feature of C/EBPε−/− granulocytic cells is their reduced superoxide production.1 To examine this observation further, we determined the degree of this defect by using a quantitative ferricytochrome c assay. We compared the superoxide production of normal mouse bone marrow cells with C/EBPε−/− bone marrow cells. Both samples contained approximately 20% Gr-1–positive granulocytic cells. 2 × 106 C/EBPε−/− bone marrow cells did not produce detectable superoxide anion levels. Under the same conditions, the identical number of normal mouse bone marrow cells produced 1.3 ± 1.27 nmol superoxide anions/min. Granulocytes and monocytes collected by peritoneal lavage at 12 and 72 hours after injection of 4% thioglycollate into the peritoneal cavity produced 5.95 and 6.5 nmol/min, respectively, in the wild-type and 1.09 and 0.38 nmol/min, respectively, in C/EBPε−/− mice. Due to a defect in the migratory function of C/EBPε−/− granulocytes, their percentage in the peritoneal cavity of these mice was lower than was found in the wild type (WT) at both 12 hours (C/EBPε−/−: 56% v WT: 70%) and 72 hours (C/EBPε−/−: 6%v WT: 75%) (Table2). Normal PMA activated monocytes are known to generate approximately 1/3 the amount of O2− compared with PMA-stimulated granulocytes.19 The difference in cell composition in the samples collected at 12 hours (56% v 70% granulocytes in intraperitoneal [i.p.] lavage from C/EBPε−/− v C/EBPε+/+, respectively) would not account for the difference in superoxide anion production between the cells of the knock-out and wild-type mice. Interestingly, the superoxide production of a population of 94% pure, C/EBPε−/− monocytes/macrophages (2 × 106 cells) was only 0.38 nmol/min, indicating that the defect may also affect C/EBPε−/− monocytes. (For comparison: O2− production of normal murine monocytes/macrophages [2 × 106]: 1 to 1.8 nmol/min20.)
Superoxide Anion Production by 2 × 106Wild-Type and C/EBPɛ−/− Cells Collected From Either the Bone Marrow or Peritoneal Cavity (12 or 72 hours after intraperitoneal injection of 4% thioglycollate)
| . | Granulocytes (%) . | Monocytes/ Macrophages (%) . | Production (nmol/min) . | ±SD . | n . |
|---|---|---|---|---|---|
| Bone marrow | |||||
| Wild type | 21 | 1 | 1.3 | 1.27 | 2 |
| C/EBPε−/− | 20 | 1 | 0* | — | 2 |
| Peritoneal phagocytes (12 h) | |||||
| Wild type | 70 | 30 | 5.95 | 0.08 | 2 |
| C/EBPε−/− | 56.5 | 43.5 | 1.09 | 0.01 | 2 |
| Peritoneal phagocytes (72 h) | |||||
| Wild type | 75 | 25 | 6.5 | 0.14 | 2 |
| C/EBPε−/− | 6 | 94 | 0.38 | 0.27 | 2 |
| . | Granulocytes (%) . | Monocytes/ Macrophages (%) . | Production (nmol/min) . | ±SD . | n . |
|---|---|---|---|---|---|
| Bone marrow | |||||
| Wild type | 21 | 1 | 1.3 | 1.27 | 2 |
| C/EBPε−/− | 20 | 1 | 0* | — | 2 |
| Peritoneal phagocytes (12 h) | |||||
| Wild type | 70 | 30 | 5.95 | 0.08 | 2 |
| C/EBPε−/− | 56.5 | 43.5 | 1.09 | 0.01 | 2 |
| Peritoneal phagocytes (72 h) | |||||
| Wild type | 75 | 25 | 6.5 | 0.14 | 2 |
| C/EBPε−/− | 6 | 94 | 0.38 | 0.27 | 2 |
production was measured by the continuous ferricytochrome c assay. Numbers represent mean and standard deviation of 2 separate experiments.
Undetectable.
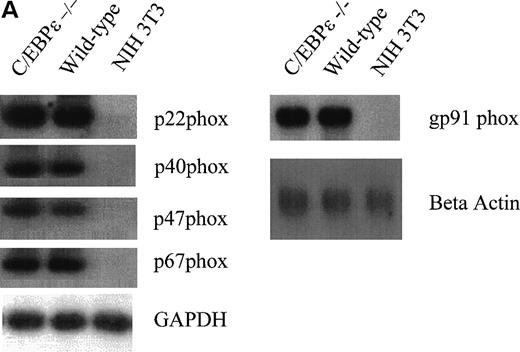
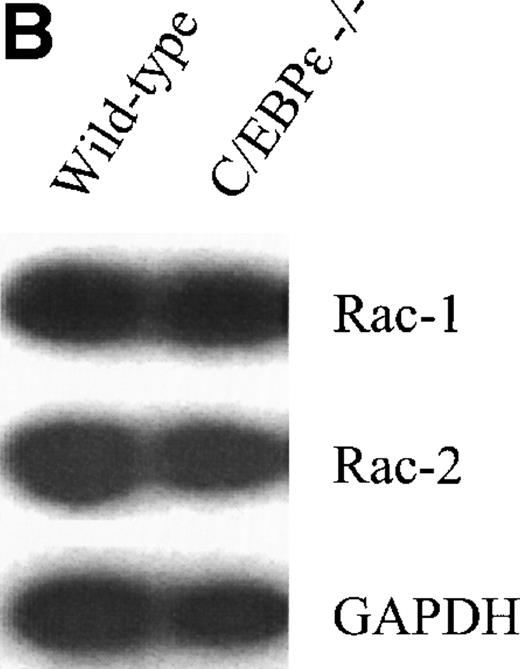
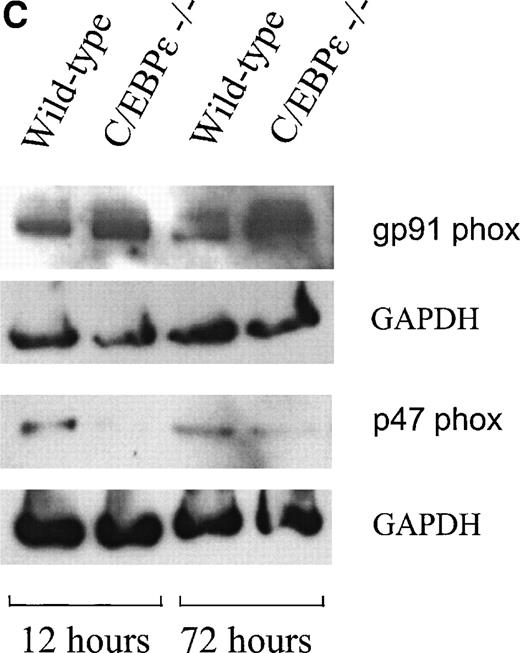
Because the NADPH oxidase system is mainly responsible for superoxide production in phagocytic cells, we examined the expression of its components. Northern blot analysis showed that the expression of the murine gp91 phox gene is not reduced in the monocyte-depleted bone marrow of C/EBPε−/− mice (Fig2A, right panel). Semiquantitative RT-PCR studies with bone marrow cells from mutant and wild-type animals also showed no difference for the other members constituting the NADPH oxidase complex: p22 phox, p40 phox, p47 phox, p67 phox (Fig 2A, left panel) as well as rac-1 and -2 (Fig 2B).21-26 Protein expression of gp91 phox and p47 phox was measured by Western blot analysis in wild-type and C/EBPε−/− granulocytes/monocytes (Fig 2C). Cells collected from the peritoneal cavity of 4 wild-type and 4 C/EBPε−/− mice at either 12 or 72 hours after the intraperitoneal injection of 4% thioglycollate were pooled at each time point to determine the mean number of granulocytes and monocytes and to extract protein. Gp 91 phox was found to be normally expressed in C/EBPε−/− granulocytes/monocytes collected at 12 hours. At 72 hours the level of gp 91phox expression was higher in the C/EBPε−/− sample. This sample contained 94% monocytes as opposed to only 6% in the wild type. The differences observed could result from a higher expression of gp91phox in monocytes/macrophages. These experiments were repeated 3 times and each demonstrated that the protein expression of p47phox was considerably reduced in C/EBPε−/− phagocytes collected at either 12 or 72 hours (Fig 2C). This effect was most prominent in the sample collected at 12 hours, in which the majority of collected cells were granulocytes: wild-type, 70%; C/EBPε−/−, 56%.
(A, right) Northern blot analysis of gp91 phox expression from C/EBPɛ−/− and wild-type bone marrow cells. RNA from NIH 3T3 fibroblasts was used as negative control. Gp91 phox expression was not reduced in C/EBPɛ−/− bone marrow cells. Hybridization with a beta actin probe was used as a control for equal loading of samples. (A, left) RT-PCR assays to determine expression of the p22 phox, p40 phox, p47 phox, p67 phox and (B) rac-1 and -2 genes. Primers for GAPDH were used to test the integrity of the cDNAs. All genes were expressed in both wild-type and C/EBPɛ−/− bone marrow cells. (C) Western blot comparing the protein expression of gp91 phox and p47 phox in wild-type and C/EBPɛ−/− phagocytes collected at either 12 or 72 hours after intraperitoneal injection of 4% thioglycollate into the peritoneal cavity of wild type and C/EBPɛ−/− mice. The mean percentage of granulocytes and monocytes in the samples were at 12 hours: Wild-type, 70/30; C/EBPɛ−/−, 56/44 and at 72 hours: Wild-type, 75/25; C/EBPɛ−/−, 6/94. GAPDH expression was a control for equal loading. The reduction of p47 phox expression was found in 3 independent experiments. The figure depicts the results of a representative experiment.
(A, right) Northern blot analysis of gp91 phox expression from C/EBPɛ−/− and wild-type bone marrow cells. RNA from NIH 3T3 fibroblasts was used as negative control. Gp91 phox expression was not reduced in C/EBPɛ−/− bone marrow cells. Hybridization with a beta actin probe was used as a control for equal loading of samples. (A, left) RT-PCR assays to determine expression of the p22 phox, p40 phox, p47 phox, p67 phox and (B) rac-1 and -2 genes. Primers for GAPDH were used to test the integrity of the cDNAs. All genes were expressed in both wild-type and C/EBPɛ−/− bone marrow cells. (C) Western blot comparing the protein expression of gp91 phox and p47 phox in wild-type and C/EBPɛ−/− phagocytes collected at either 12 or 72 hours after intraperitoneal injection of 4% thioglycollate into the peritoneal cavity of wild type and C/EBPɛ−/− mice. The mean percentage of granulocytes and monocytes in the samples were at 12 hours: Wild-type, 70/30; C/EBPɛ−/−, 56/44 and at 72 hours: Wild-type, 75/25; C/EBPɛ−/−, 6/94. GAPDH expression was a control for equal loading. The reduction of p47 phox expression was found in 3 independent experiments. The figure depicts the results of a representative experiment.
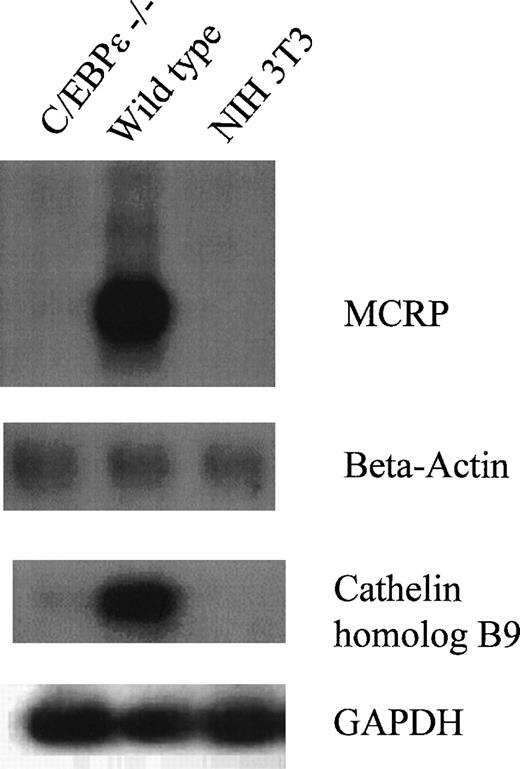
To elucidate further the molecular defects in antimicrobial activity of C/EBPε−/− animals, we tested the cathelin protein family that is important for the generation of antibacterial peptides. The peptides CRAMP1 and CRAMP2, both derived from cathelin-related protein (MCRP), have bactericidal activity, especially against gram-negative bacteria, like Pseudomonas aeroginosa.15,27 Northern blot analysis shows that the 1-kb mRNA of the cathelin-related protein is virtually absent in C/EBPε−/− bone marrow in comparison to a very strong expression in wild-type mice (Fig 3). Also, the expression of a second murine cathelin homolog (B9 protein) was reduced in the C/EBPε−/− bone marrow (Fig 3). This protein is normally expressed in murine promyelocytes.28
Lack of expression of cathelin proteins. First panel shows Northern blot of C/EBPɛ−/− and wild-type bone marrow cells examined for expression of MCRP. Second panel shows that the lanes were balanced for intact RNA by reprobing Northern blot with β-actin. Third panel displays RT-PCR for murine cathelin homolog B9, and the fourth panel assures that cDNA were intact by showing their equal expression of GAPDH. Both genes were markedly diminished in their expression in bone marrow cells from C/EBPɛ−/− mice.
Lack of expression of cathelin proteins. First panel shows Northern blot of C/EBPɛ−/− and wild-type bone marrow cells examined for expression of MCRP. Second panel shows that the lanes were balanced for intact RNA by reprobing Northern blot with β-actin. Third panel displays RT-PCR for murine cathelin homolog B9, and the fourth panel assures that cDNA were intact by showing their equal expression of GAPDH. Both genes were markedly diminished in their expression in bone marrow cells from C/EBPɛ−/− mice.
C/EBPε−/− granulocytic cells were shown to exhibit an impaired migration.1 Expression of receptors for both interleukin-8 and N-formyl-methionyl-leucyl phenylalanine (fMLP) are required for normal granulocytic migration.29-31 Therefore, we examined the expression of these receptors by RT-PCR. Both receptors were expressed in granulocytic cells of C/EBPε−/− mice isolated 4 hours after i.p. thioglycollate injection (data not shown).
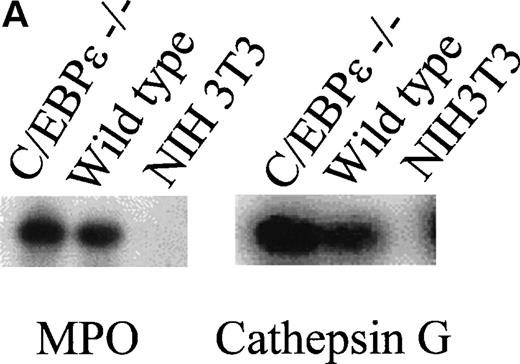
Consistent with the morphological conservation of primary granules in C/EBPε−/− granulocytic cells as observed by electronmicroscopy, RT-PCR showed that the primary granule proteins, myeloperoxidase and cathepsin G, were expressed in the bone marrow cells (Fig 4A). The slightly stronger signal detected in C/EBPε−/− bone marrow most likely reflects myeloid hyperplasia. This notion is reinforced by the increased number of myeloperoxidase (MPO)-positive bone marrow cells seen with MPO immunohistochemistry (data not shown).
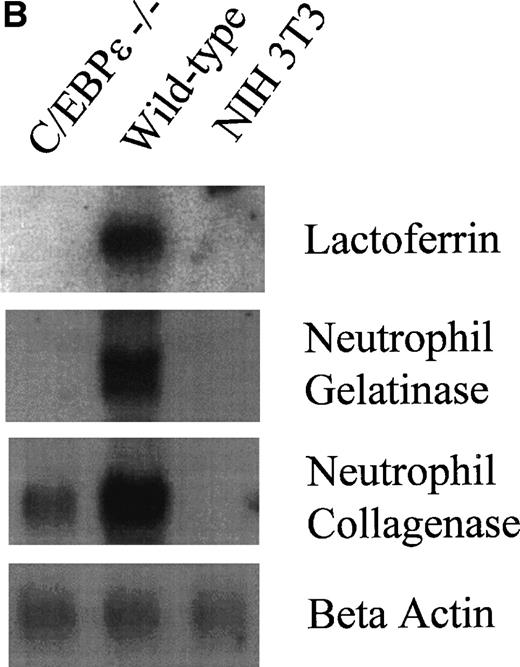
(A) Expression analysis of MPO and cathepsin G mRNAs by RT-PCR. (B) Northern blot analysis for lactoferrin, neutrophil gelatinase, and collagenase mRNA expression.
(A) Expression analysis of MPO and cathepsin G mRNAs by RT-PCR. (B) Northern blot analysis for lactoferrin, neutrophil gelatinase, and collagenase mRNA expression.
A hallmark of late myeloid differentiation is the expression of secondary (specific) granules containing lactoferrin, neutrophil gelatinase, neutrophil collagenase, and transcobalamin I32and tertiary (gelatinase) granules defined by their high concentration of gelatinase. Expression of the secondary granule proteins is at least in part transcriptionally controlled.33 In addition, the downregulation of the binding activity of CCAAT displacement protein (CDP) has been implicated in their coordinate expression.34Northern blot analysis showed the complete absence of lactoferrin and gelatinase mRNA in C/EBPε−/− bone marrow. The mRNA level of neutrophil collagenase was considerably reduced (Fig 4B).
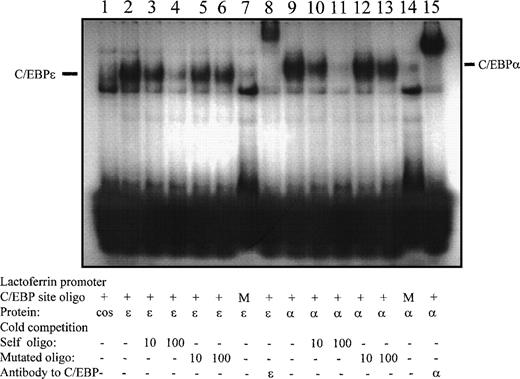
Therefore, we investigated whether the lactoferrin promoter is a direct target for C/EBPε. We first performed electromobility shift assays with 2 large fragments (−750 to −300) and (−300 to +39) of the lactoferrin promoter. Recombinant MBP-C/EBPε bound to the proximal, but not the upstream fragment (data not shown). The proximal fragment contains a predicted C/EBP site at position −55. We designed a double-stranded oligonucleotide representing this putative C/EBP site which is conserved between mouse and humans.35We expressed C/EBPε and C/EBPα protein in cos-1 cells and used 5 μg nuclear extract for the binding assay. Figure 5 shows that C/EBPε and C/EBPα bound specifically to the site. Binding was competed by cold self, but not mutated oligonucleotide. The binding complexes were supershifted by specific antisera against C/EBPε and C/EBPα, respectively. A 32P-γATP-labeled oligonucleotide mutated at the binding site did not bind the C/EBP proteins (Fig 5).
EMSA demonstrating binding of C/EBPɛ and C/EBP to the putative C/EBP site at position −55 of the lactoferrin promoter. Bars point to binding of either C/EBPɛ (lanes 2 through 7), or C/EBP complex (lanes 9 through 14) to double-stranded32P γATP-labeled oligonucleotide– containing C/EBP site (–55) in lactoferrin promoter. Binding is specifically competed by cold oligonucleotides (lanes 3, 4 and 10, 11), but not by mutated oligonucleotides (lanes 5, 6 and 12, 13). The complexes did not bind to labeled mutated oligonucleotides (lanes 7 and 14). Both C/EBPɛ and C/EBP were supershifted by specific antisera (lanes 8 and 15, respectively).
EMSA demonstrating binding of C/EBPɛ and C/EBP to the putative C/EBP site at position −55 of the lactoferrin promoter. Bars point to binding of either C/EBPɛ (lanes 2 through 7), or C/EBP complex (lanes 9 through 14) to double-stranded32P γATP-labeled oligonucleotide– containing C/EBP site (–55) in lactoferrin promoter. Binding is specifically competed by cold oligonucleotides (lanes 3, 4 and 10, 11), but not by mutated oligonucleotides (lanes 5, 6 and 12, 13). The complexes did not bind to labeled mutated oligonucleotides (lanes 7 and 14). Both C/EBPɛ and C/EBP were supershifted by specific antisera (lanes 8 and 15, respectively).
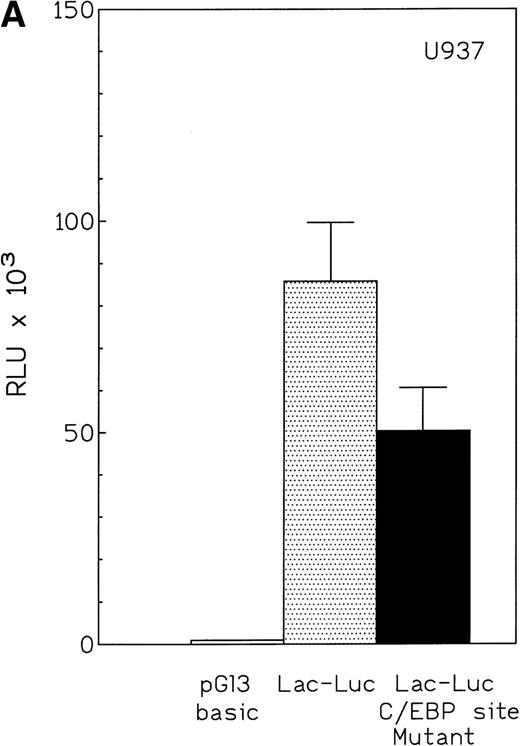
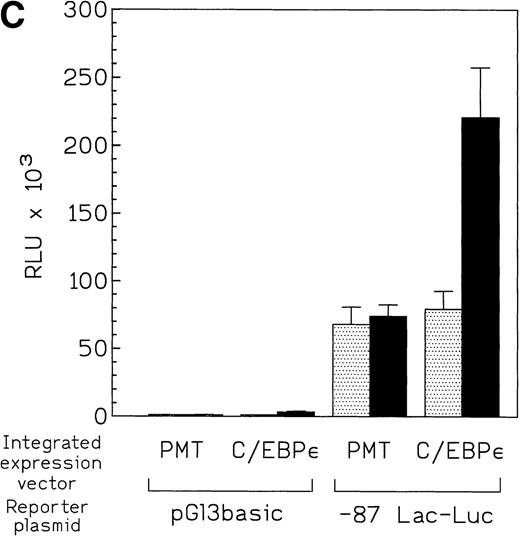
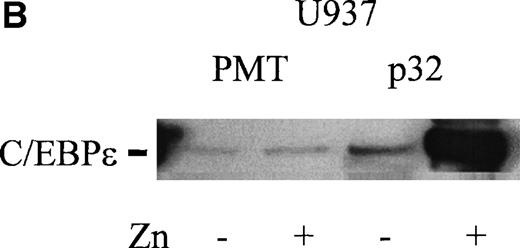
The proximal promoter fragment (−230 to +39) of lactoferrin that contained the putative C/EBP site was fused to a luciferase reporter gene. This construct was 50-fold more active than empty vector when transiently transfected into U937 cells (Fig 6A). Mutation of the putative C/EBP site reduced this activity by 35% (Fig 6A). Furthermore, an 87-bp lactoferrin promoter-reporter construct was transiently transfected into U937 cells that had been engineered so that they could be induced to overexpress C/EBPε because a Zn-inducible C/EBPε expression vector was stably integrated into the cells (Fig 6B). Reporter gene activity was more than 3-fold greater in these U937 cells overexpressing C/EBPε compared with uninduced U937 cells, suggesting indirectly that C/EBPε is a positive regulator of the lactoferrin promoter in myeloid cells (Fig 6C).
(A) Effect of mutation of C/EBP site (−55) on lactoferrin promoter activity in U937 cells. The −230-bp lactoferrin promoter luciferase (Lac-Luc) reporter either with or without a mutation at the C/EBP site as well as β-galactosidase plasmid were transfected into U937 cells. After 16 hours, cells were harvested to determine luciferase activity. Relative light units (RLU) were normalized for β-galactosidase activity. Data shown represent mean ± standard deviation of 3 independent experiments. (B) Western blot for C/EBPɛ expression in U937 cells stably integated with either zinc-inducible C/EBPɛ expression vector or empty expression vector (PMT). Addition of 100 μmol/L zinc-sulfate strongly induces C/EBPɛ protein (labeled p32). (C) U937 cells containing either a stably integrated, zinc-inducible C/EBPɛ expression vector (labeled C/EBPɛ) or empty vector (PMT, control) were transiently transfected with either the −87 lactoferrin promoter reporter plasmid (labeled −87 Lac-Luc) or empty vector pGL 3basic. After transfection, half of the cells were treated with 100 μmol/L zinc (▪) either to induce expression of C/EBPɛ or control for a possible nonspecific zinc effect on promoter activity in the case of U937 with integrated empty vector. Half of the cells were not treated with zinc () and used to determine the basal activity of the −87 Lac-Luc plasmid in U937/C/EBPɛ and U937/PMT. Data represent mean and standard deviation of 3 independent experiments.
(A) Effect of mutation of C/EBP site (−55) on lactoferrin promoter activity in U937 cells. The −230-bp lactoferrin promoter luciferase (Lac-Luc) reporter either with or without a mutation at the C/EBP site as well as β-galactosidase plasmid were transfected into U937 cells. After 16 hours, cells were harvested to determine luciferase activity. Relative light units (RLU) were normalized for β-galactosidase activity. Data shown represent mean ± standard deviation of 3 independent experiments. (B) Western blot for C/EBPɛ expression in U937 cells stably integated with either zinc-inducible C/EBPɛ expression vector or empty expression vector (PMT). Addition of 100 μmol/L zinc-sulfate strongly induces C/EBPɛ protein (labeled p32). (C) U937 cells containing either a stably integrated, zinc-inducible C/EBPɛ expression vector (labeled C/EBPɛ) or empty vector (PMT, control) were transiently transfected with either the −87 lactoferrin promoter reporter plasmid (labeled −87 Lac-Luc) or empty vector pGL 3basic. After transfection, half of the cells were treated with 100 μmol/L zinc (▪) either to induce expression of C/EBPɛ or control for a possible nonspecific zinc effect on promoter activity in the case of U937 with integrated empty vector. Half of the cells were not treated with zinc () and used to determine the basal activity of the −87 Lac-Luc plasmid in U937/C/EBPɛ and U937/PMT. Data represent mean and standard deviation of 3 independent experiments.
DISCUSSION
In this study, we examined the expression of a number of myeloid-specific genes in C/EBPε−/− mice to elucidate further the molecular basis of their phenotype. Although superoxide production is significantly reduced in phagocytes of C/EBPε−/− mice, we found a normal gp91 phox mRNA expression and no apparent lack of p22, p40, p47, and p67 phox, rac-1, and -2 expression, the major components of the NADPH oxidase complex.21-26 Western blot analysis did not show a decrease of gp91phox protein expression. This finding is consistent with our Northern blot data showing the equal expression of gp91phox mRNA in C/EBPε−/− and wild-type bone marrow. p47 phox protein expression, however, was considerably reduced in C/EBPε−/− granulocytes/monocytes. The phosphorylation of p47phox is essential for the assembly of the NADPH oxidase and its translocation to the plasma membrane.36 The reduction of p47phox levels may contribute to the decreased capacity of C/EBPε−/− phagocytes to produce superoxide. The discrepancy beween similar p47phox mRNA expression in wild-type and knock-out as measured by RT-PCR and reduced p47phox protein expression in C/EBPε−/− granulocytes/monocytes is most likely due to the technical limitations of RT-PCR to detect quantitative changes. Recent reports of the expression of C/EBPε in murine monocytes12 are consistent with our finding that their capacity to produce superoxide is also reduced.
The normal expression of the gp91phox gene in C/EBPε−/− mice contrasts to its absence in the granulocytes of PU.1−/− mice.36 The gp91 phox gene is known to be negatively regulated by CDP and was recently shown to be positively regulated by PU.1.37,38 The expression of the gp91phox gene in C/EBPε−/− mice not only implies that C/EBPε is not essential for its expression but, more interestingly, suggests that the mature fraction of C/EBPε−/− granulocytic cells probably lacks the repressive binding activity of CDP. The CDP binding activity is normally downregulated during granulocytic differentiation.37 The gp91 phox promoter has 4 binding sites for CDP and the protein competes with activating factors for binding to the promoter.39 Overexpression of CDP in differentiating myeloid cells silences the gp 91 phox promoter.37 The downregulation of CDP has recently been implicated in the coordinate upregulation of secondary granule proteins.34 40 The absence of lactoferrin and gelatinase mRNA in C/EBPε−/− mice places the maturation arrest of C/EBPε−/− granulocytic cells between gp91phox, and the secondary, respectively tertiary granule proteins. We conclude that in addition to the downregulation of CDP, positive regulators are critical for the expression of secondary granule protein genes. Alternatively, different forms of CDP negatively regulate the gp91phox and the secondary granule protein genes.
Because myeloid leukemia cell lines express CDP binding activity,41 we omitted the CDP binding site at position −880 and potential other CDP sites in the upstream lactoferrin promoter, allowing us to study positive regulators of the lactoferrin promoter in cells which do not express lactoferrin. The reduced activity of the C/EBP site (−55) mutant indicates that C/EBP proteins are involved in the regulation of the lactoferrin promoter. Furthermore, overexpression of C/EBPε in U937 cells increases by 3-fold the already high promoter activity of the proximal −87-bp construct, indicating that C/EBPε can modulate the promoter activity. Likewise, Zn-inducible overexpression of C/EBPε in U937 cells rapidly induces the accumulation of lactoferrin mRNA (data not shown). Our in vitro lactoferrin promoter studies, however, do not explain the complete absence of lactoferrin expression in C/EBPε−/− mice. The proximal 87 bp of the lactoferrin promoter contain at least 2 other binding sites for transcription factors involved in myeloid gene regulation: PU.1 and Sp1.13 Lactoferrin expression is also absent in granulocytes of PU.1−/− mice.36Possibly, 2 or more factors are cooperating in the positive regulation of the lactoferrin promoter in vivo. We hypothesize that the lack of one of these could prevent the assembly of an active complex and thereby render all contributing factors essential.
Our studies using the C/EBPε−/− mouse places C/EBPε downstream of C/EBPα. Because C/EBPε−/− mice express C/EBPα in their bone marrow cells, C/EBPα alone is not sufficient for lactoferrin expression in vivo. Interestingly, the cell line U937 can be driven to express lactoferrin by overexpression of C/EBPα, which, however, also leads to an increase in C/EBPε expression.8 Taken together, C/EBPε most likely directly contributes to the transcriptional activation of the lactoferrin promoter in vivo.
Secondary granule protein deficiency was reported as a rare genetic disorder in humans.42 The patients are susceptible to infections, mainly with gram-positive organisms. Secondary granule protein deficiency probably contributes to the complex phenotype of C/EBPε−/− mice. Nevertheless, the absence of the potent bacteriocidal peptides CRAMP1 and 2 are also probably extremely important for the phenotype of C/EBPε−/− mice. The Northern analysis shows that the mRNA encoding cathelin-related protein is highly abundant in normal murine bone marrow. Because murine granulocytes lack defensins,43 cathelins constitute a major source of bactericidal peptides in murine granulocytes. Their predominant activity against gram-negative bacteria matches the infectious spectrum in C/EBPε−/− mice.15Interestingly, the promoter of the human cathelin homolog Fall 39 has a C/EBP binding site at position −154.44 45 This raises the possibility of a direct involvement of C/EBPε in the transcriptional regulation of the cathelin-related protein. Enhanced expression of C/EBPε might offer a unique approach to augment the ability of an individual to fight potentially serious infections.
ACKNOWLEDGMENT
We thank Dr Robert Lehrer for helpful and very thoughtful discussions and Kai Chien for excellent technical assistance.
Supported in part by the following grants: NIH-C/EBPε, NIH-Genetic Core, Lichtenstein for Leukemia Research, Parker Hughes Grant, and C. and H. Koeffler Grant. W.V. was supported by a Grant of the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H. Phillip Koeffler, MD, Cedars-Sinai Medical Center, Burns and Allen Research-Institute, UCLA School of Medicine, Davis Research Bldg, Room D 5066, 8700 Beverly Blvd, Los Angeles, CA 90048.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal