Abstract
There is evidence from bone marrow transplantation that T cells may be involved in the immunologic control of leukemia. But many patients relapse despite a potent graft-versus-leukemia effect mediated by allogeneic T cells. The expression of the FasL protein has been suggested as a mechanism of tumor immune escape. We, therefore, evaluated the capacity of leukemic cells from patients with acute or chronic myelogenous leukemia to escape the allogeneic or autologous immune response by bearing the FasL molecule. Although almost all leukemic cells express the 37-kD form of FasL, only 54% of acute myeloblastic leukemia and 27% of chronic myeloid leukemia (CML) cells bore a FasL with killing properties, as assessed by the ability of leukemic cells to cause the apoptosis of a Fas-sensitive target cell line or autologous activated T cells in 3 tested leukemic cases. Experiments with a recombinant Fas-Fc molecule confirmed the role of Fas/FasL in leukemic-mediated cell death. Only CML leukemic cells from certain individuals contained the 26-kD truncated form of FasL. Thus, myeloid leukemic cells from some, but not all patients can set up a mechanism of immune escape involving the Fas/FasL pathway. This leukemic escape may have implications for patients eligible for adoptive cellular immunotherapy.
INDUCTION OF COMPLETE remissions with donor lymphocyte injection (DLI) for leukemia relapses after allogeneic bone marrow transplantation (BMT) is a strong indication of an in vivo immune control of hematologic malignancies, at least in the allogeneic setting. This graft-versus-leukemia (GVL) effect is particularly pronounced in cases of chronic myeloid leukemia (CML) and less prominent in acute leukemias.1 However, some relapses occur despite the existence of a potent GVL effect, indicating that the tumor cells escape the immune system.
Cancers can escape immune surveillance by various mechanisms involving antigen processing and presentation, costimulation signals, or immunomodulation with cytokines. Another mechanism relies on the removal of activated tumor-specific T cells or natural killer (NK) cells. The regulation of the immune response may depend on the expression of functional death molecules related to the tumor necrosis factor (TNF) family, by activated T cells, leading to homeostasis of the immune system2 through a paracrine or autocrine pathway.3 Thus, activated T lymphocytes at the tumor site, bearing Fas receptor (Fas), could be killed by contact between Fas and its counterpart FasL abnormally expressed by tumor cells.4Functional membrane-bound FasL was recently found on several types of solid tumors or tumor cell lines (melanoma, astrocytoma, colon cancer, hepatocarcinoma, multiple myeloma), which could result in local immunosuppression by cell-to-cell contacts.5 However, the role of the Fas/FasL interaction in the immune escape of leukemia has not been examined.
We have, therefore, evaluated the role of FasL in the escape of leukemic cells from T lymphocytes. The FasL protein is expressed by acute and chronic myelogenous leukemic cells, but the functional activity of FasL, assessed by its capacity to induce apoptosis of Jurkat T cells, Fas-sensitive targets, was detected only in 27% of CML patients and 54% of acute myeloblastic leukemia (AML) patients. This apoptotic capacity was also observed against autologous activated T cells in 3 studied cases. A 26-kD truncated form of FasL was concomitantly produced by several CML leukemic samples, but not in AML. These results strongly suggest that the Fas/FasL pathway may be implicated in leukemic immune escape.
MATERIALS AND METHODS
Patients.
Peripheral blood mononuclear cells (PBMC) of 27 leukemic patients were collected between November 1995 and March 1998 before the initiation of chemotherapy. All patients gave their informed consent. The PBMC were separated from total blood samples of patients with hyperleukocytosis (WBC>10 109/L) by Ficoll-Hypaque gradient (Pharmacia Biotech AB, Uppsala, Sweden) and frozen in 10% dimethyl sulfoxide, 10% human AB serum, or used fresh. All the PBMC samples contained a majority of tumoral cells with less than 10% residual lymphocytes or normal hemopoietic cells (checked by cytological, cytogenetic, or fluores analysis).
DNA fragmentation and specific killing with the JAM test.
The JAM test was used to study the ability of leukemic cells to kill T cells. DNA fragmentation was assessed by labeling the target Jurkat cells with 3H TdR.6 This test was modified as follows: 5 × 106 Jurkat target cells were labeled with 10 mCi/mL 3H TdR by incubating them in RPMI 10% fetal calf serum in a humidified atmosphere with 5% CO2 at 37°C overnight (15 hours). Excess 3H-Thymidine (Amersham Pharmacia bioTech, Orsay, France) was washed off and 20 × 103 target cells were mixed with varying numbers of effector leukemic cells in 200 μL in 96-well plates (Costar, Cambridge, MA). The plates were incubated for 24 hours at 37°C and 100 μL of the supernatant was removed and added to liquid scintillation fluid. Radioactivity was counted in a β counter (Wallac 1410; EG&G Wallac, Turku, Finland). The radioactivity of the supernatant, reflecting the cell death by apoptosis of labeled Jurkat cells, was normalized to the maximum lysis obtained with the anti-FasR monoclonal antibody (MoAb) (CH-11; Immunotech, Marseille, France) at 100 ng/mL (resulting in 100% apoptosis of Fas-sensitive Jurkat cells). The mean percentage apoptosis induced by effector cells was calculated from triplicate cultures with the formula:
Cpm Total is the radioactivity obtained with 20 × 103 target cells cultured with 100 ng/mL CH-11 antibody, whereas cpm Spontaneous is the spontaneous release of radioactivity from Jurkat cells.
Autologous T cells from patients P22 and P12 were also used as targets in the JAM test. Briefly, adherent cells were removed from PBMC by adherence to plastic culture vessels for 1 hour. Peripheral blood lymphocytes (PBL) were activated with phytohemagglutinin (PHA) (1 μg/mL) for 18 to 20 hours, washed, and cultured in the presence of 25 IU/mL recombinant interleukin-2 (IL-2) (Boehringer Mannheim) for 4 additional days as described by Klas et al.7
Inhibition of apoptosis by blocking Fas/FasL interactions.
Fas:Fc protein (Alexis Biochemicals, Laufelfingen, Switzerland) (7.5 to 30 μg/mL) was used to inhibit FasL-mediated apoptosis and was added to leukemic cells before their coculture with Jurkat cells. The mean radioactivity obtained in the supernatant after overnight incubation was compared with the radioactivity obtained under the same conditions without Fas-Fc. Inhibition was expressed as:
Exp A is the radioactivity in a 10:1 coculture of Jurkat cells with leukemic cells, and Exp B is the radioactivity under the same coculture condition, with Fas-Fc. Spontaneous is the radioactivity released from Jurkat cells alone. The isotype control antibody used in each experiment never inhibited leukemic cell-mediated cell cytotoxicity.
Western blotting.
Cells were incubated in lysis buffer (1% NP-40, 50 mmol/L Tris pH 8.0, 150 mmol/L NaCl, 5 mmol/L ethylenediamine tetraacetate, 6 mmol/L CHAPS, 1 μg/mL leupeptin, aprotinin, pepstatin A, 1 μmol/L phenylmethylsulfonyl fluoride) on ice for 30 minutes and centrifuged at 14,000g. Supernatants were stored at −70°C. Protein concentration was measured with DC Protein Assay (BioRad Laboratories, Hercules, CA). Equal amounts of protein were boiled for 5 minutes in 2X Laemmli sample buffer with 2-βME and run on a 4/20% polyacrylamide gel (BioRad). The separated proteins were then transfered to a nitrocellulose membrane (RPN 2020D; Amersham International, Little Chalfont, UK), immunoblotted with a mouse monoclonal IgG1 specific for FasL (F 37720; Transduction Laboratories, San Diego, CA), and visualized by using horseradish-peroxidase-conjugated sheep anti-mouse IgG F(ab′)2 fragment (NA9310; Amersham International), followed by enhanced chemiluminescence (RPN 2109; Amersham International).
Detection of FasL by flow cytometry analysis.
Cells were incubated for 1 hour at 4°C with purified mouse anti-human FasL (IgG1, Nok1; Pharmingen). The cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG secondary antibody for 30 minutes at 4°C and detected with a flow cytometer (FACScan; Becton Dickinson, Le Pont de Claix, France). Similar experiments were performed with permeabilized cells fixed with formaldehyde (intraPrep permeabilization reagent; Immunotech).
RESULTS
Patients
Leukemic cells from 27 patients with hyperleukocytosis (WBC >10G/L) were selected for this study. The characteristics are shown in Table 1. The PBMC from 11 cases of CML, 13 de novo AML, 2 secondary AML (acute phase of primary myelofibrosis), and 1 acute lymphoblastic leukemia (acute phase of CML) were collected some time after chemotherapy or interferon-γ therapy. The cells were usually taken at the time of diagnosis or relapse of the disease. Three of 13 AML patients who were treated by chemotherapy alone relapsed, 3 died during induction therapy, and 3 never reached complete remission. Five patients were treated with an allogeneic BMT from a sibling donor and 2 relapsed after BMT. Relapses after BMT were treated by donor lymphocyte transfusions to induce a GVL effect. All patients were monitored for 4 months to 3 years after the collection of the cells.
Characteristics of Patients
| Patients . | Diagnosis . | Relapse . | BMT . | Relapse/ Post-BMT . | DLI/ post-BMT . | Evolution . | Jurkat Killing >15% . |
|---|---|---|---|---|---|---|---|
| P2 | CML | — | No | — | — | CP1 | + |
| P3 | CML acc | — | Yes | 4 mo | 7 mo | CR | − |
| P4 | CML | — | No | — | — | CP1 | − |
| P5 | CML | — | Yes | No | — | CR1 | − |
| P6 | CML | — | No | — | — | CP1 | − |
| P7 | CML | — | No | — | — | CP1 | − |
| P8 | CML | — | No | — | — | CP1 | − |
| P9 | CML | — | Yes | No | No | Death in CR | − |
| P10 | CML | — | No | — | — | CP1 | + |
| P12 | CML | — | No | — | — | CP1 | + |
| P13 | CML | — | No | — | — | CP1 | − |
| P25 | AML0 | — | No | — | — | No remission | − |
| P1 | AML1 | — | Yes | 5 mo | 6 mo | Death in relapse | + |
| P16 | AML1 | — | No | — | — | Death induction | − |
| P15 | AML4 | — | No | — | — | CR1 | − |
| P17 | AML4 | — | No | — | — | CR1 24 mo | + |
| P18 | AML4 | 9 mo | No | — | — | Death | − |
| P19 | AML4 | — | No | — | — | Death induction | − |
| P20 | AML4 | — | No | — | — | Death induction | + |
| P11 | AML5 | 12 mo | No | — | — | Death in relapse | + |
| P21 | AML5 | — | No | — | — | CR1 10 months | + |
| P22 | AML5 | 12 mo | No | 6 mo | planned | In relapse | + |
| P23 | AML5 | — | Yes | — | — | CR1 | − |
| P24 | AML5 | — | No | — | — | No remission | + |
| P14 | PM in acut. | — | No | — | — | CR1 | − |
| P26 | PM in acut. | — | No | — | — | No remission | + |
| P27 | ALL | — | No | — | — | CR1 | − |
| Patients . | Diagnosis . | Relapse . | BMT . | Relapse/ Post-BMT . | DLI/ post-BMT . | Evolution . | Jurkat Killing >15% . |
|---|---|---|---|---|---|---|---|
| P2 | CML | — | No | — | — | CP1 | + |
| P3 | CML acc | — | Yes | 4 mo | 7 mo | CR | − |
| P4 | CML | — | No | — | — | CP1 | − |
| P5 | CML | — | Yes | No | — | CR1 | − |
| P6 | CML | — | No | — | — | CP1 | − |
| P7 | CML | — | No | — | — | CP1 | − |
| P8 | CML | — | No | — | — | CP1 | − |
| P9 | CML | — | Yes | No | No | Death in CR | − |
| P10 | CML | — | No | — | — | CP1 | + |
| P12 | CML | — | No | — | — | CP1 | + |
| P13 | CML | — | No | — | — | CP1 | − |
| P25 | AML0 | — | No | — | — | No remission | − |
| P1 | AML1 | — | Yes | 5 mo | 6 mo | Death in relapse | + |
| P16 | AML1 | — | No | — | — | Death induction | − |
| P15 | AML4 | — | No | — | — | CR1 | − |
| P17 | AML4 | — | No | — | — | CR1 24 mo | + |
| P18 | AML4 | 9 mo | No | — | — | Death | − |
| P19 | AML4 | — | No | — | — | Death induction | − |
| P20 | AML4 | — | No | — | — | Death induction | + |
| P11 | AML5 | 12 mo | No | — | — | Death in relapse | + |
| P21 | AML5 | — | No | — | — | CR1 10 months | + |
| P22 | AML5 | 12 mo | No | 6 mo | planned | In relapse | + |
| P23 | AML5 | — | Yes | — | — | CR1 | − |
| P24 | AML5 | — | No | — | — | No remission | + |
| P14 | PM in acut. | — | No | — | — | CR1 | − |
| P26 | PM in acut. | — | No | — | — | No remission | + |
| P27 | ALL | — | No | — | — | CR1 | − |
Abbreviations: CML, chronic myelogenous leukemia; CML acc, CML in acceleration; PM in ac, primary myelofibrosis in blastic phase; AML, acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; CR1, first complete remission; CP1, first chronic phase.
Function of FasL
Apoptosis of Jurkat cells mediated by leukemic cells.
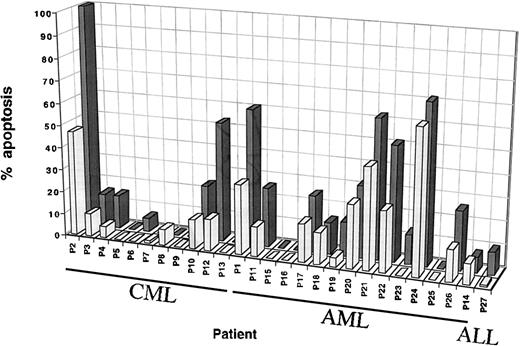
Jurkat cells bearing Fas molecules are sensitive to apoptosis because of Fas ligation by agonistic Fas-MoAb or cells expressing functional FasL. The DNA fragmentation induced by leukemic cells was compared with the effect of 100 ng/mL anti-Fas-antibody, CH-11 MoAb, which induced apoptotic death of 100% Jurkat cells after 24 hours. This MoAb concentration was used as a positive control in the assay. Cell assays were considered to be positive when there was 15% Jurkat cell death at a ratio of 10:1. PBMC from 5 normal donors never caused the lysis of more than 10% of Jurkat cells (data not shown). Experiments with leukemic cells under the same culture conditions showed that PBMC from 3 of the 11 CML patients (P2, P10, P12) and 8 of the 15 AML patients (P1, P11, P17, P20, P21, P22, P24, P26) induced a significant apoptosis of the target cells (Table 1). The mean percent apoptosis was 38% (range, 25% to 71%) at an E/T ratio of 30:1 and 24% (range, 15% to 45%) at an E/T 10:1 (Fig 1). The PBMC from both CML and AML individuals caused more target cell death at an E/T ratio of 30:1 (mean and range), whereas no such effect was noted with PBMC from normal donors (10% at 30:1). The PBMC from the CML patient P2 killed 100% of Jurkat cells at an E/T ratio of 30:1, and the apoptotic effect was still pronounced at an E/T ratio of 3:1 (>40% apoptotic cell death) (data not shown).
Leukemic cell-mediated cytotoxicity. Median percentage apoptosis of Jurkat cells by leukemic samples from 27 patients at E/T ratios of 30:1 (black) and 10:1 (gray). Each sample was tested in duplicate at each time and in at least 2 to 5 separate experiments. Cytotoxicity was assessed by the JAM test, which measured radioactivity released by DNA fragmentation. The target cell Jurkat DNA was labeled with 3H-thymidine, so that the test reflects fragmentation of target cell DNA in response to a signal induced by leukemic cells. The apoptosis induced by PBMC of normal donors, used as a negative control, never exceeded 10% at the highest E/T ratio.
Leukemic cell-mediated cytotoxicity. Median percentage apoptosis of Jurkat cells by leukemic samples from 27 patients at E/T ratios of 30:1 (black) and 10:1 (gray). Each sample was tested in duplicate at each time and in at least 2 to 5 separate experiments. Cytotoxicity was assessed by the JAM test, which measured radioactivity released by DNA fragmentation. The target cell Jurkat DNA was labeled with 3H-thymidine, so that the test reflects fragmentation of target cell DNA in response to a signal induced by leukemic cells. The apoptosis induced by PBMC of normal donors, used as a negative control, never exceeded 10% at the highest E/T ratio.
Four of the 5 AML French-American-British (FAB) type 5 cells were strongly able to induce Jurkat cell lysis (mean, 54%; range, 35% to 71% at an E/T ratio of 30:1).
In some experiments, we used autologous activated T cells (PHA blasts) instead of Jurkat cells in the JAM test. For this test, we selected patients P12, P2 (CML), and P22 (AML) from whom we could obtain PBMC in a period of remission. PHA blasts after 5 days of culture in the presence of PHA and IL-2 are sensitive to Fas-induced apoptosis. This was assessed by their sensitivity to CH11 MoAb mediated lysis. Leukemic cells from patient P2 and P12 were able to induce 70% and 56%, respectively, apoptosis of their autologous T cells at an E/T ratio of 30:1 and 46% and 22% at an E/T ratio of 10:1. Leukemic cells from P22 induced 49% apoptosis of his autologous T cells at an E/T ratio of 30:1 (data not shown). These results are comparable to those obtained with the Jurkat cell line.
Prevention of apoptosis mediated by leukemic cells by an antagonist to the Fas/FasL interaction.
We confirmed that the killing of Jurkat cells cocultured with leukemic cells involved the Fas/FasL interaction. Leukemic cells from 5 patients (3 CML [P2, P10, P12] and 2 AML [P11, P22]) that caused a significant apoptosis of Jurkat cells at an E/T ratio of 30:1 were incubated with recombinant specific MoAb antagonist to Fas/FasL interaction (15 μg/mL Fas-Fc) for 30 minutes, and then with Jurkat cells. Significantly fewer Jurkat cells were killed in all cases. The inhibition of apoptosis reached 64%, 83%, 63%, 75%, and 41% for P2, P10, P11, P12, and P22, respectively (Fig 2A). This dose-dependent effect was up to 100% for the highest concentation of the blocking MoAb (30 μg/mL) at an E/T ratio of 10:1 (Fig 2B). All experiments included an isotype-matched control MoAb (15 μg/mL) that had no protective effect on apoptosis (data not shown).
(A) Fas/FasL interaction mediated cytotoxicity. Jurkat cell apoptosis by leukemic cells from P2, P10, P11, P12, P22 at an E/T ratio of 10:1 was inhibited by the antagonist Fas:Fc. Results are expressed as the percentage inhibition of apoptosis induced by leukemic cells in the presence of the blocking MoAb. Leukemic cells were incubated with 15 μg/mL Fas:Fc for 30 minutes and than used as effector cells in the JAM test. The experiment was repeated 3 times with each patient. (B) Effect of Fas-Fc concentration on apoptosis. Results are expressed as the percentage of Jurkat apoptosis induced by leukemic cells from patient P2, in the presence or absence of the blocking MoAb and shows 1 representative experiment out of 2.
(A) Fas/FasL interaction mediated cytotoxicity. Jurkat cell apoptosis by leukemic cells from P2, P10, P11, P12, P22 at an E/T ratio of 10:1 was inhibited by the antagonist Fas:Fc. Results are expressed as the percentage inhibition of apoptosis induced by leukemic cells in the presence of the blocking MoAb. Leukemic cells were incubated with 15 μg/mL Fas:Fc for 30 minutes and than used as effector cells in the JAM test. The experiment was repeated 3 times with each patient. (B) Effect of Fas-Fc concentration on apoptosis. Results are expressed as the percentage of Jurkat apoptosis induced by leukemic cells from patient P2, in the presence or absence of the blocking MoAb and shows 1 representative experiment out of 2.
FasL Protein Expression by Leukemic Cells
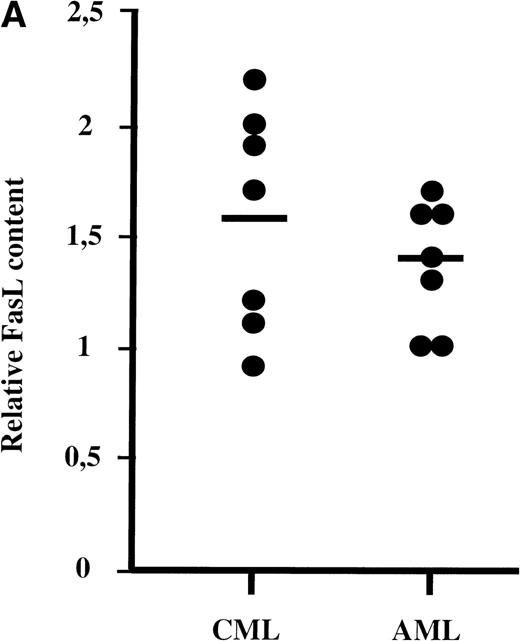
The PBMC from 14 patients (7 AML and 7 CML), which induced a significant apoptosis of Jurkat cells (3 CML: P2, P10, and P12 and 5 AML: P11, P17, P21, P22, and P26) or no apoptosis (4 CML: P4, P5, P6, and P9 and 2 AML: P16 and P23), were assessed for their FasL protein content by Western blot analysis. The predicted 37-kD FasL molecule was detected in all samples of AML and CML cells and in the PBMC of 2 normal donors. However, the amount of FasL protein in leukemic cells was greater in both patient groups than in PBMC from normal donors (Fig 3A).
(A) FasL concentrations in AML and CML patients. FasL was quantified by Western blotting assay by using a National Institutes of Health Image Program 1.6. Data for patients were normalized to the PBMC from 2 normal individuals, for which the data have been included at each time. (B) FasL profile. Western blot showing FasL in cells from leukemic patients. Cells were lysed in lysis buffer, separated on a 4/20% polyacrylamide gel, the protein transferred to a nitrocellulose membrane, and the membrane was probed with an anti-FasL MoAb. Fourteen patients were analyzed for FasL contained by Western blot. All the samples tested contained FasL and several also contained the truncated form of the protein. FasL (37 kD) and short FasL (sFasL, 26 kD) are indicated by arrows. Short FasL was never detected in samples from AML or normal donors.
(A) FasL concentrations in AML and CML patients. FasL was quantified by Western blotting assay by using a National Institutes of Health Image Program 1.6. Data for patients were normalized to the PBMC from 2 normal individuals, for which the data have been included at each time. (B) FasL profile. Western blot showing FasL in cells from leukemic patients. Cells were lysed in lysis buffer, separated on a 4/20% polyacrylamide gel, the protein transferred to a nitrocellulose membrane, and the membrane was probed with an anti-FasL MoAb. Fourteen patients were analyzed for FasL contained by Western blot. All the samples tested contained FasL and several also contained the truncated form of the protein. FasL (37 kD) and short FasL (sFasL, 26 kD) are indicated by arrows. Short FasL was never detected in samples from AML or normal donors.
An additional 26-kD band corresponding to a truncated form of FasL (sFasL) was also found in 4 CML individuals (P2, P5, P6, and P10) but not in any of the samples from AML or normal donors (data not shown) (Fig 3B).
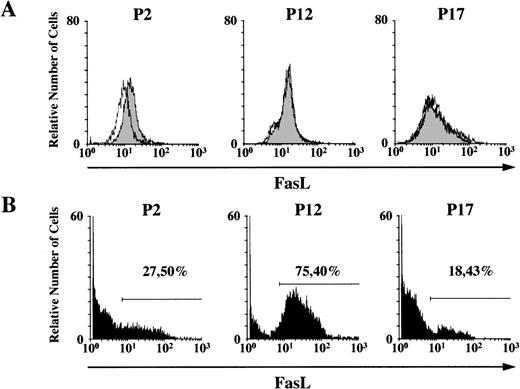
The flow cytometry analysis of FasL expression in the cells showed that some patients (P2) expressed both the intracytoplasmic and membrane bound form of the protein and others exclusively expressed the membrane bound form (P12, P17) (Fig 4). The percentage of leukemic cells expressing the membrane bound form, ranging from 18% to 75% among patients, was not strictly correlated with the capacity to induce apoptosis.
Expression of FasL protein. Flow cytometry analysis of (A) cytoplasmic and (B) membrane bound FasL of leukemic cells from P2 (CML), P12 (CML), and P17 (AML4) individuals. In (A), cytoplasmic FasL (solid peak) is detected in patients (P2) as compared to isotype control (dashed line). In (B), the percentage of leukemic cells expressing membrane bound FasL is shown.
Expression of FasL protein. Flow cytometry analysis of (A) cytoplasmic and (B) membrane bound FasL of leukemic cells from P2 (CML), P12 (CML), and P17 (AML4) individuals. In (A), cytoplasmic FasL (solid peak) is detected in patients (P2) as compared to isotype control (dashed line). In (B), the percentage of leukemic cells expressing membrane bound FasL is shown.
DISCUSSION
Recently, it has been suggested that the abnormal expression of FasL by tumor cells could be responsible for the killing of specific activated T cells in contact with the tumor. This abnormal expression has been reported to be responsible for tumor escape in cases of melanoma, hepatocarcinoma, colon cancer, and multiple myeloma,8 but not yet in human leukemias. The implication of T cells in the immunologic control of leukemias was first suggested in the setting of BMT, where clinical trials have shown that the T-cell depletion of the graft is responsible for a high incidence of relapses.9 The recent practice of giving DLI to treat leukemia relapses after allogeneic transplantation also indicates that T lymphocytes are involved in the eradication of residual leukemic cells from the host.10 This GVL effect seems to be related to an allogeneic recognition of the miHAg on leukemic cells by donor,11 although the expansion of cytotoxic T lymphocytes (CTL) specific for leukemia-associated antigens cannot be ruled out. Our data indicate that FasL, which can kill a Fas-sensitive cell line, is present in 27% of CML and 54% of AML individuals and 70% of patients expressed more FasL as compared with normal individuals. In agreement with our data, a recent report showed that several murine leukemic and lymphoma cell lines expressing FasL can kill specific CTL and Fas-positive activated T cells.4 Thus, the expression of FasL by human leukemic cells could explain, at least partially, the resistance, of some leukemic relapses, to DLI in vivo. A large study showed that about 70% of CML and fewer than 40% of AML patients responded to DLI in vivo,1 which is consistent with our findings. Another indication comes from patients P1 and P22 who quickly suffered a relapse of their AML after an allogeneic transplantation. Patient P1 was also resistant to his DLI, which produced a rapid fatal outcome. PBMC of these 2 patients had a strong FasL activity, pointing to the involvement of these escape mechanisms in vivo. However, a larger number of cases treated by DLI or relapsing after BMT must now be examined to see if there is a correlation between leukemic resistance to DLI and the expression of FasL.
The distribution of the FasL molecule was initially found to be restricted to activated T cells and NK cells in mice and then to sites of immune privilege (testis, anterior ocular chamber).12Its current distribution seems to be wider, because it has also been detected in normal and pathologic hematopoietic human lineages: activated B lymphocytes, T-cell– and NK-cell–derived neoplasms,13 monocytes, activated macrophages, and neutrophil granulocytes.14 Almost all the cases of AML FAB type 5 studied here caused Jurkat cell lysis. The detection of functional FasL in patients with CML or AML FAB type 5 could be due to the presence of circulating mature monocytes belonging to the leukemic clone in CML and to the monoblasts characteristic of AML FAB type 5. However, the high concentration of membrane-bound FasL in another type of AML blasts (P1) without any monocytic compound indicates that this protein can also be produced by immature malignant myeloid cells.
We cannot rule out the involvement of other mechanisms responsible for Jurkat apoptosis in this cell assay. Indeed, signaling along the TNF-α receptor, constitutively expressed by Jurkat cells has been shown to induce programmed cell death via activation of ICE proteases.15 However, the key role of the Fas/FasL pathway for target cell lysis in our system was demonstrated by blocking experiments. Furthermore, we also detected no secretion of TNF-α by any of the leukemic samples, which was assessed by the lack of capacity of the supernatant of leukemic cells to kill a TNF-α sensitive target Wehi cell line (data not shown).
Although the PBMC from 70% of patients bore more FasL than normal individuals, there was no clear relationship between the FasL concentration and the capacity to kill Jurkat cells. In some cases, part of this FasL expression reflected the intracytoplasmic form of FasL. This intracellular form has been described in monocytes, in which FasL is translated toward the membrane on stimulation.14Moreover, there was no strict correlation between the percentage of cells expressing membrane bound FasL and the functional activity. Some CML leukemic samples also contained the 26-kD truncated form of the protein, which may be the soluble form of FasL (sFasL).14This protein can be released into the serum of patients with NK type lymphoma or large granular lymphocyte leukemia.16 This sFasL was recently shown to be capable of inhibiting Fas-mediated cytotoxicity17 and Fas-mediated apoptosis of fresh peripheral blood lymphocytes.18 The serum of our patients containing truncated FasL (P2, P5, P6, P10, and P11) had neither an apoptotic nor an inhibitory effect on the Fas-mediated apoptosis of Jurkat cells (data not shown).
These findings may have clinical implications because various attempts to obtain leukemia-specific effector T cells in vitro are used in autologous or allogeneic systems designed to transfer specific immunity to patients. The expression of FasL by leukemic target cells could impair the results of these strategies. Clearly, a systematic study of the mechanisms used by leukemic cells to escape cellular immunity is necessary in the actual context of development of DLI or cellular immune-based therapies.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, Ligue Nationale Contre le Cancer, Agence Française du Sang, Fondation contre la Leucémie, and Délégation à la Recherche Clinique de l’Assistance Publique-Hôpitaux de Paris.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Agnès Buzyn, MD, INSERM U445, ICGM, Université PARIS V Hôpital COCHIN, 27 Rue du Faubourg Saint-Jacques, 75014 Paris, France; e-mail:buzyn@icgm.cochin.inserm.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal