Abstract
Currently little is known about the mechanisms regulating the homing and the early engraftment of prenatally transplanted hematopoietic cells due to the lack of a relevant functional assay. In this study, we have defined a reproducible kinetic profile of the homing and the early engraftment events in a murine model of prenatal stem cell transplantation. Light density mononuclear cells (LDMCs) from adult C57Pep3b and SJL/J marrow were transplanted by intraperitoneal (IP) injection into C57BL/6 fetuses (106 LDMCs/fetus) at 14 days of gestation. The fetuses were sacrificed at early time points (1.5 to 96 hours) after transplantation. Recipient fetal liver and cord blood were analyzed for donor cell frequency and donor cell phenotype by dual color flow cytometry. Pertinent findings included the following: (1) a triphasic kinetic profile exists after in utero hematopoietic stem cell (HSC) transplantation (homing of circulating donor cells, rapid reduction of donor cell frequency, and donor cell competitive equilibration); (2) homing to the fetal liver is nonselective and reflects the phenotypic profile of the donor population; and (3) the kinetics after the prenatal transplantation of congenic or fully allogeneic cells are identical. This model will facilitate a systematic analysis of the mechanisms that regulate the homing of prenatally transplanted hematopoietic cells.
THE MECHANISM of homing is most likely a multistep process consisting of adhesion to endothelial cells of the receptive microenvironment, followed by transmigration, and finally tethering within the extravascular microenvironment where proliferation and differentiation occur.1-4 The mechanisms regulating the homing and the early engraftment of hematopoietic stem cells (HSC) after in utero transplantation are poorly understood due to the lack of a relevant functional assay. In vitro adhesion assays do not adequately model the complex network of integrin-ligand-cytokine interactions present during development. Postnatal homing studies in irradiated recipients reflect homing patterns to radiation altered postnatal microenvironments and are not applicable to homing and engraftment events in the nonirradiated fetal microenvironment.5-7Similarly, studies in mutant or compromised fetuses or neonates fail to recreate a setting of engraftment competition, but rather, offer a competitive engraftment advantage to the normal donor cells.8-10 Therefore, in this study, we have developed a novel murine model of in utero HSC transplantation to investigate the homing and the engraftment of transplanted bone marrow (BM) to the fetal liver after in utero HSC transplantation into normal recipients.
Normal hematopoietic ontogeny is characterized by a sequential migration of hematopoiesis from the yolk sac and/or periaortic splanchnopleure, to the fetal liver, and finally to the spleen and BM.11-13 In the mouse, and in the human, the fetal liver is the predominant source of hematopoiesis until just before term. We took advantage of this fact to develop an in vivo assay system for homing and engraftment to the fetal liver. At 14 to 15 days gestation in the mouse, hematopoiesis is derived exclusively from the fetal liver.13 Hematopoietic cells are not observed in the spleen until 15 days gestation and in the marrow until soon thereafter.13 Therefore, analysis of the early homing and engraftment events to the fetal liver can be performed in isolation of other confounding hematopoietic environments at this stage of development.
In many studies of prenatal and postnatal homing of transplanted cells, observations on homing have not been dissociated from observations on engraftment.14-17 If homing is defined as a process by which parenterally administered hematopoietic cells lodge and firmly anchor themselves within the hematopoietic tissues, then the parameters that influence this process need to be studied immediately after transplantation, before cell proliferation ensues. The findings from a number of postnatal studies suggest that the homing process is complete within a few hours after transplantation, and that delayed analysis must account for graft apoptosis and proliferation.18-20
Our goals in this study were to describe the kinetics of cellular trafficking immediately after in utero HSC transplantation. We analyzed multiple time points to assess the early events after transplantation that reflect the homing of transplanted cells, as well as their subsequent engraftment. We have also analyzed the kinetics of circulating donor cells and their clearance from the circulation, which is a necessary corollary for any analysis of homing. In addition, we sought to better characterize the types of cells that home to the fetal liver to determine if homing within the prenatal environment is limited to a particular cell type. Lastly, we examined if there were any differences in the kinetics of homing between congenic and fully allogeneic donor cells.
Our findings included the following: (1) a triphasic kinetic profile exists after in utero HSC transplantation (homing of circulating donor cells, rapid reduction of donor cell frequency, and donor cell competitive equilibration); (2) homing to the fetal liver is nonselective and reflects the phenotypic profile of the donor population; and (3) the kinetics after the prenatal transplantation of congenic or fully allogeneic cells are identical.
MATERIALS AND METHODS
Animals.
Breeding stock for inbred strains of mice, C57BL/6 (H-2b, CD45.2), C57Pep3b (H-2b, CD45.1), and SJL/J (H-2s, CD45.1) were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in our colony. Animals were mated and the females were checked daily for introital plugging. The day of plugging was defined as gestational day 0 for time dating. All animals were housed in the Laboratory Animal Facility of the Abramson Pediatric Research Center at the Children’s Hospital of Philadelphia. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Children’s Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Donor BM harvest.
Donor BM was harvested from 4- to 8-month-old adult mice by flushing the tibias and femurs with phosphate-buffered saline (PBS) (GIBCO-BRL, Gaithersburg, MD) using a 26-gauge needle. A single cell suspension was made by 3 gentle passes through the needle. Cell suspensions were then filtered through a 70-μm nylon mesh and layered over Ficoll (Histopaque 1077; Sigma, St Louis, MO). After centrifugation at 600g for 15 minutes, the light density mononuclear cell (LDMC) layer was removed and washed twice with sterile PBS. The cells were counted and greater than 95% viability was ensured by trypan blue exclusion.
Marrow transplantation.
Recipient mice were injected on day 14 of gestation. Using methoxyfluorane general anesthesia and sterile technique, a midline laparotomy was made and the uterine horns were delivered from the wound. Each fetus was transplanted by intraperitoneal (IP) injection with 1 × 106 cells in 5 μL of PBS under direct visualization through the intact uterine wall using a 100-μm beveled glass micropipette. After return of the uterus to the maternal peritoneal cavity, abdominal closure was achieved using 2 layers of absorbable 5-0 suture.
Kinetics of transplanted cells and assessment of long-term chimerism.
Transplantation kinetics and long-term chimerism levels were assessed in recipients of Pep3b and SJL marrow by harvest of recipient cord blood and fetal liver at time points of 1.5, 4, 24, 48, and 96 hours after transplantation and of recipient peripheral blood and BM at 12 months of age. Cord blood and fetal liver were individually harvested sequentially in petri dishes filled with cold, heparinized PBS. The cord was occluded at harvest and the fetus transferred to the petri dish. The fetus was allowed to exsanguinate into the dish and the media processed for cord blood cells. The fetus was then transferred to another petri dish and the fetal liver harvested by dissection. The fetal livers were then individually passed through a 1-cc syringe to form a single cell suspension. Fetal liver, BM, cord, and peripheral blood suspensions were ficoll separated for enrichment of LDMC. LDMCs were then stained with directly conjugated anti-CD45 phycoerythrin (PE) (specific for either CD45 isoform) and anti-CD45.1 fluorescein isothiocyanate (FITC) (Pep3b and SJL) antibodies (Pharmingen, San Diego, CA) then counted by 2-color flow cytometry (FACScan; Becton Dickinson, Mansfield, MA). Dead cells were excluded by propidium iodide. The percent donor cells was defined as:
This ratio provides an estimate of fractional donor hematopoiesis without being confounded by the overwhelming erythropoiesis of the fetal liver that is predominantly composed of CD45−Ter119+ erythroid precursors.
Analysis of transplanted cell phenotype.
Lineage analysis of fetal liver LDMCs was performed at the same time points. Purified antibodies to CD3, Mac-1, B220, Gr-1, and Ter119 were individually combined with directly conjugated anti-CD45.1 PE in the first step. After cold incubation and repeated washings, the cells were then incubated with an isotype-specific FITC-conjugated secondary antibody. By gating on the donor cell population, the frequency of donor cells expressing various lineage differentiation antigens was made. A total of 5,000 to 10,000 donor cells was counted in each group.
RESULTS
The early engraftment of transplanted marrow is specific and is characterized by three phases: (1) homing, (2) graft reduction and redistribution, and (3) equilibration.
Single-cell suspensions of the LDMCs from the recipient fetal liver and cord blood were analyzed at serial time points from 90 minutes to 96 hours by dual-color flow cytometry using monoclonal antibodies that were specific for donor cell surface antigens. Preliminary experiments at earlier time points showed that deposition of donor cells within the fetal liver occurred as early as 45 minutes after transplantation (data not shown). Later time points showed only small changes in chimerism beyond the 96-hour levels through 12 months (see Fig 2). Therefore, these time points were chosen because they occur during the time of maximal cellular trafficking. These methods provided a dynamic picture of donor cell migration.
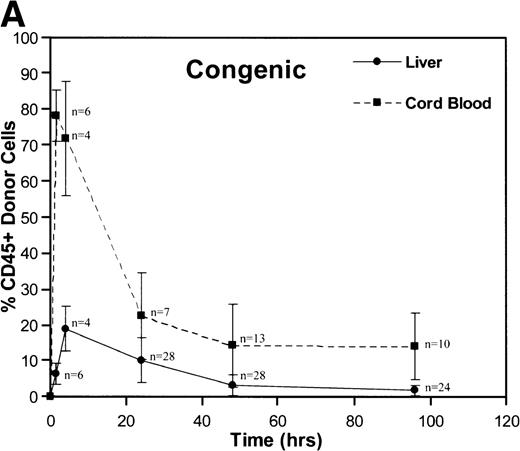
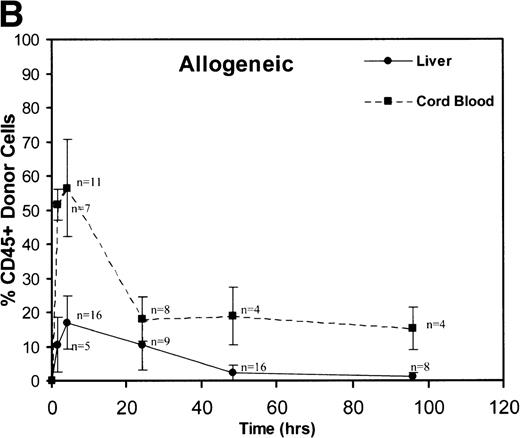
A total of 163 injections were performed and 160 fetuses survived for analysis establishing an overall fetal loss rate of 1.8%. Figure 1 illustrates the frequency of CD45+ donor cells in the recipient fetal liver and cord blood over time. The resulting donor hematopoietic cell fractions that were determined by flow cytometry of the 160 viable recipients are plotted. As shown, a triphasic profile of donor cell frequency within the recipient is evident. After an early peak at 90 minutes (52% to 78%), the donor cell fraction in the fetal cord blood fell dramatically over the subsequent 24 hours (18% to 22%). Thereafter, the level within the cord blood remained essentially stable throughout the remainder of the study (15% to 18% at 96 hours). No differences were noted between the congenic and allogeneic groups. On the other hand, the peak donor cell fraction within the recipient liver occurred at 4 hours (17% to 19%), lagging slightly behind the peak in cord blood levels. The subsequent drop in the levels within the fetal liver was less dramatic and was essentially a linear decline over the ensuing 48 hours (2.2% to 3.2%). During this time, the total number of recipient CD45+ cells expanded nearly 10-fold (Table 1). Donor hematopoiesis in both groups remained stable for the next 48 hours at 2.5% to 3.0%, then exhibited a slow decay over the early weeks of postnatal life, reaching a stable level of 0.5% to 1.3% in the marrow at 12 months as shown in Fig 2. Again, no significant differences were noted between the groups.
These graphs depict the triphasic kinetic profiles for the frequency of donor cells circulating within the recipient fetal peripheral blood (cord blood) or lodged within the fetal liver at various time points (1.5 to 96 hours) after in utero transplantation. The nearly identical profiles for both the congenic (A) and the fully allogeneic (B) strain combinations are shown. The donor cell frequency is calculated as the percentage of cells expressing the donor CD45.1 isoform among all CD45+ cells within the host cord blood or fetal liver.
These graphs depict the triphasic kinetic profiles for the frequency of donor cells circulating within the recipient fetal peripheral blood (cord blood) or lodged within the fetal liver at various time points (1.5 to 96 hours) after in utero transplantation. The nearly identical profiles for both the congenic (A) and the fully allogeneic (B) strain combinations are shown. The donor cell frequency is calculated as the percentage of cells expressing the donor CD45.1 isoform among all CD45+ cells within the host cord blood or fetal liver.
Average Number of Cells Expressing CD45 Within the Murine Fetal Liver According to Gestational Age
| Gestational Age . | No. . | No. of CD45+ Cells per Fetal Liver (×106) . |
|---|---|---|
| 14 | 74 | 0.26 ± 0.08 |
| 15 | 36 | 1.09 ± 0.41 |
| 16 | 16 | 2.61 ± 0.53 |
| Gestational Age . | No. . | No. of CD45+ Cells per Fetal Liver (×106) . |
|---|---|---|
| 14 | 74 | 0.26 ± 0.08 |
| 15 | 36 | 1.09 ± 0.41 |
| 16 | 16 | 2.61 ± 0.53 |
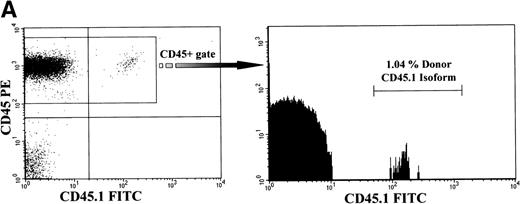
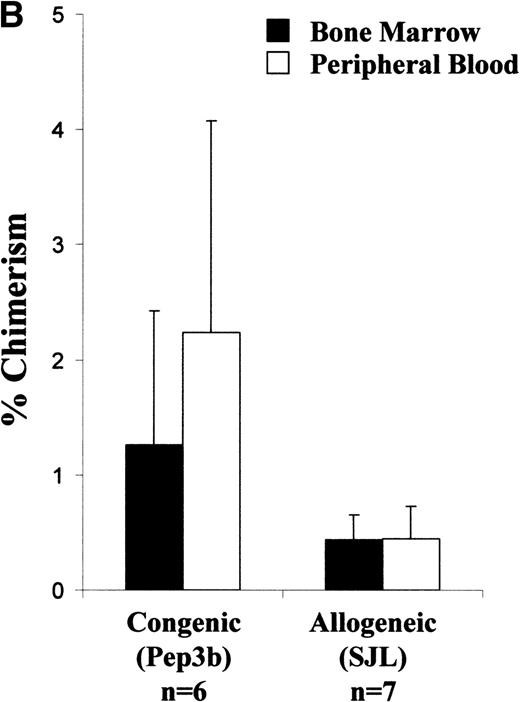
Analysis of long-term chimerism. (A) Sample dot plot and histogram are shown from dual-color analysis of donor (CD45.1 FITC) peripheral blood chimerism among cells expressing either CD45 isoform (CD45 PE) within the recipients at 6 months of age. (B) Peripheral blood and BM chimerism levels by FACS analysis at 12 months of age in recipients of either congenic or allogeneic marrow.
Analysis of long-term chimerism. (A) Sample dot plot and histogram are shown from dual-color analysis of donor (CD45.1 FITC) peripheral blood chimerism among cells expressing either CD45 isoform (CD45 PE) within the recipients at 6 months of age. (B) Peripheral blood and BM chimerism levels by FACS analysis at 12 months of age in recipients of either congenic or allogeneic marrow.
Table 2 displays the relative homing efficiency for the congenic and allogeneic groups. The homing efficiency is defined as the percentage of donor CD45+cells, which were recovered from the fetal liver. As shown, the homing efficiency to the fetal liver at 4 hours for either group was 4.4% to 4.9% without a significant difference.
Efficiency of Transplanted Cell Homing to the Recipient Fetal Liver at 4 Hours
| Donor Cell Type . | % Fetal Liver Chimerism at 4 Hours . | No. of CD45+ Cells per Recipient Fetal Liver . | No. of Homed Donor CD45+ Cells . | No. of CD45+ Cells in Donor Inoculum . | Homing Efficiency (%) . |
|---|---|---|---|---|---|
| Pep3b marrow | 19.0 ± 6.3 | 2.6 ± 0.8 × 105 | 4.8 ± 1.6 × 104 | 9.9 × 105 | 4.9 ± 1.6 |
| SJL marrow | 16.9 ± 7.7 | 2.6 ± 0.8 × 105 | 4.3 ± 1.9 × 104 | 9.9 × 105 | 4.4 ± 2.0 |
| Donor Cell Type . | % Fetal Liver Chimerism at 4 Hours . | No. of CD45+ Cells per Recipient Fetal Liver . | No. of Homed Donor CD45+ Cells . | No. of CD45+ Cells in Donor Inoculum . | Homing Efficiency (%) . |
|---|---|---|---|---|---|
| Pep3b marrow | 19.0 ± 6.3 | 2.6 ± 0.8 × 105 | 4.8 ± 1.6 × 104 | 9.9 × 105 | 4.9 ± 1.6 |
| SJL marrow | 16.9 ± 7.7 | 2.6 ± 0.8 × 105 | 4.3 ± 1.9 × 104 | 9.9 × 105 | 4.4 ± 2.0 |
The homing efficiency is defined as the percentage of donor CD45+ cells from the original transplant inoculum that were recovered from the fetal liver.
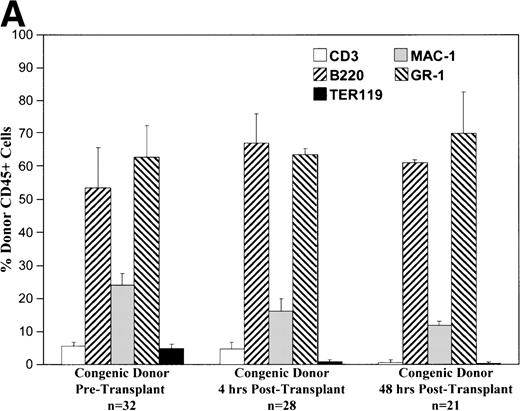
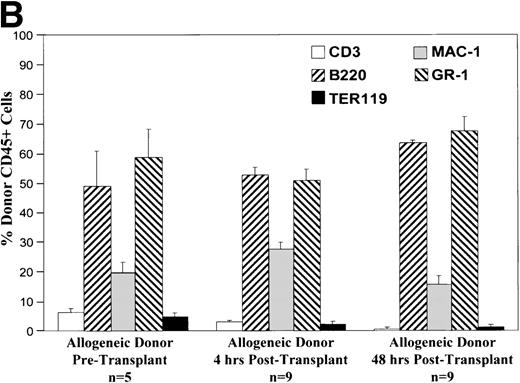
Cells of all lineages are capable of homing to the fetal liver.
Phenotypic analysis of donor cells in the recipient fetal liver at 4 and 48 hours after transplantation shows that various lineages of cells are capable of homing and surviving in the fetal hematopoietic microenvironment. As shown in Fig 3, all lineages of cells from either allogeneic or congenic marrow are capable of homing to the fetal liver. The early lineage analysis shows a pattern similar to that of the donor cell population. After 48 hours, the CD3 expressing cell population is essentially absent. At this point, the pattern of donor hematopoiesis, which includes granulocyte, macrophage, and B-lymphocyte lineages, continues in a manner mirroring host fetal liver hematopoiesis. Analysis of long-term (12 months) chimeras shows a similar pattern of multilineage donor hematopoiesis within recipient BM (data not shown).
These charts summarize the phenotypic profile of the donor cell population at various stages: pretransplant and within the recipient fetal liver at 4 and 48 hours posttransplant. The percentage of donor CD45+ cells expressing each of the differentiation antigens listed is shown for each time point in either the congenic (A) or allogeneic (B) strain combination.
These charts summarize the phenotypic profile of the donor cell population at various stages: pretransplant and within the recipient fetal liver at 4 and 48 hours posttransplant. The percentage of donor CD45+ cells expressing each of the differentiation antigens listed is shown for each time point in either the congenic (A) or allogeneic (B) strain combination.
DISCUSSION
The process of homing and engraftment of hematopoietic cells to the fetal liver has not been previously studied. Our study is unique in several respects. First, we have studied the homing of transplanted cells in a nonirradiated, competitive environment, which should reflect normal events during hematopoietic ontogeny, or after in utero HSC transplantation. Second, because of the developmental timing of these studies, we have been able to examine homing events to the fetal liver (completed 4 hours after transplantation) in isolation from other competitive hematopoietic environments such as the spleen or marrow. This feature is relevant because the hematopoietic environment at this stage of development in the mouse is similar to that in humans when in utero HSC transplantation is optimally performed (12 to 14 weeks gestation).21,22 Finally, our model allows examination of homing and engraftment of allogeneic cells before the development of cell-mediated immunity.13,23 24 Thus, in our model, the influence of histocompatibility on homing and engraftment can be analyzed in the absence of T-cell–mediated immunologic response in an otherwise intact hematopoietic environment.
The kinetic profile found in our study suggests that homing and engraftment to fetal liver has a number of parallels to homing and engraftment to postnatal BM. First, our results demonstrate that the homing events after intraperitoneal transplantation are relatively rapid. We found peak circulating levels of donor cells at 90 minutes after transplantation and peak fetal liver frequencies of donor cells at 4 hours after transplantation. We interpret these events as rapid uptake from the peritoneal cavity with the peak circulating levels of donor cells reflecting a “bolus” effect. Homing and “lodgment” of donor cells in hematopoietic and other tissues then follows resulting in a rapid decrease in the levels of circulating donor cells. The second phase of our engraftment profile between 4 and 48 hours is marked by a rapid decrease in the frequency of donor cells in the fetal liver and steady state circulating levels of donor cells in the cord blood. We interpret this phase as engraftment and dilutional reduction in donor cell frequency due to the exponential increase in host fetal liver cell number at this stage of development as shown in Table 1. In addition, donor cells do not begin cycling for at least 12 to 24 hours after transplantation and the proliferative rate of adult-derived hematopoietic cells is likely to be less than that of endogenous fetal cells.25-27 Further loss of donor cells from the fetal liver may be attributable to the initiation of fetal marrow hematopoiesis on day 15 or 16 of gestation11 13 and as early as 24 hours after transplantation. The third phase of our profile is interpreted as competitive equilibration of donor and host-derived hematopoiesis and extends from 48 hours to the postnatal period. During this time, a slow decrease in donor cell frequency occurs and eventually reaches a steady state, which is presumably ultimately reflective of the fraction of donor HSC to host HSC. Beyond 96 hours, any potential differences between the groups were obscured by the error associated with fluorescence-activated cell sorting (FACS) detection of a target cell frequency below 1%.
Our current phenotypic analysis of the engrafting donor cells supports a nonselective mechanism for lodgment and engraftment. The phenotypic profile of engrafting donor cells is very similar to the phenotype of the injected cells confirming that the vast majority of engrafting cells are committed progenitors of the macrophage, granulocyte, and B-lymphoid lineages. This phenotypic profile remains relatively constant over 48 hours, although there is a trend toward a decrease in donor lymphoid and erythroid cells (CD3+, B220+, and TER119+ cells) with an increase in donor myeloid cells (Mac-1+ and Gr-1+ cells). Loss of T-cell lymphopoiesis might be expected because this is not normally supported by the fetal liver environment and may also reflect redistribution of T cells to a more receptive site such as the thymus.23 Loss of donor erythroid progenitors may reflect the accelerated differentiation of donor erythroblasts in the intensely erythropoietic milieu of the fetal liver.11,13 28
Hematopoiesis during ontogeny is developmentally regulated and presumably involves sequential “activation” and “deactivation” of hematopoietic microenvironments.14Activation requires the development of stromal infrastructure with appropriate expression of chemokines, integrins/ligands, and cytokines to attract and support hematopoiesis. The process of homing and engraftment then becomes critical for the establishment and maintenance of hematopoiesis in a given environment. Deactivation presumably occurs by downregulation of these adhesion and supportive interactions or by the development of a competitive environment with greater affinity for HSC, resulting in the depopulation and quiescence of a once active hematopoietic microenvironment.14 Hence, analysis of homing and engraftment events during development must be taken in the context of the specific stage of hematopoiesis being analyzed and may or may not be applicable to other stages or microenvironments. The molecular mechanisms of this process remain obscure, but their successful completion is required for normal hematopoietic events during development or for successful engraftment after in utero HSC transplantation. In this study, we have developed a model that allows us to focus on a single stage of development and a specific microenvironment, ie, the fetal liver before development of BM hematopoiesis.
Most knowledge about hematopoietic homing and engraftment is based on studies in the irradiated mouse model. Early studies tracked homing and distribution of colony-forming units-spleen (CFU-S) and demonstrated the transient presence of transplanted cells in all organs, as well as the BM and spleen.19,20,29 30 Clearance from the peripheral blood of all CFU-S occurred within 3 to 4 hours and from all, except the hematopoietic organs, within 48 hours. Donor cell cycling and proliferation then began within 24 hours after transplantation. Thus, the initial process of homing, ie, lodgment, does not appear to be specific to hematopoietic organs, but the subsequent survival and engraftment of circulating cells depends on the hematopoietic microenvironment. This supports the concept of a “niche” in the hematopoietic microenvironment that is necessary for survival and proliferation of HSC.
More recently, fluorescently labeled cells have been used to assess the homing and engraftment of syngeneic BM progenitors in nonirradiated, as well as irradiated recipients.17 These studies combined a direct homing assay of enriched marrow with a CFU-S assessment of the recovered cells. Relevant findings included an 8.06% homing efficiency to the marrow of both recipient groups at the measured time points of 17 and 24 hours. In addition, the efficiency was significantly worse in the irradiated recipients when compared with the nonirradiated recipients. These studies once again support the existence of a nonspecific lodgment mechanism. More importantly, however, they confirm that engraftment is an active process requiring communication between the stromal endothelium and the migrating hematopoietic cell and further suggest that radiation preparation may disturb these important mechanisms and impair the success of donor cell engraftment. These findings along with those from other studies challenge the concept that myeloablation is required to create “space” for donor cells.
To our knowledge, only 1 other study has attempted to analyze the kinetics of engraftment after prenatal transplantation of hematopoietic cells.14 In that study, ovine fetuses were transplanted at 40, 50, 60, 70, or 80 days gestation with hematopoietic cells from ovine fetal liver, human fetal liver, and ovine or human adult BM. The recipient fetuses were killed 10, 20, or 30 days later. The liver, marrow, and blood were then analyzed for donor hemoglobin, donor karyotype, and human leukocyte common antigen expression. The investigators found that transplanted cells engrafted in the fetal liver exclusively before formation of the BM. As gestation proceeded, however, the transplanted cells engrafted preferentially to the developing marrow. Over 90% of donor cells were detectable in the fetal liver at 50 days gestation, equal numbers of donor cells were present in fetal liver and BM at 70 days gestation, and by 80 days gestation, nearly 90% of donor cells were detected in the marrow. This shift toward marrow hematopoiesis was in spite of the observation that the vast majority of total hematopoiesis was derived from the fetal liver at all time points studied. These results support a model of increasing microenvironmental affinity for circulating progenitors with the fetal BM having higher affinity for circulating progenitors than the fetal liver. Due to the lack of early time points, however, these studies did not actually address homing, lodgment, or early engraftment of donor cells, but rather, the end result of engraftment, which would include subsequent donor cell redistribution, proliferation, and apoptosis. Although only a semiquantitative analysis of redistribution to the developing fetal BM was possible, we were able to reliably quantify the early events that truly reflect donor cell homing and fetal liver engraftment.
The importance of MHC compatibility in homing and engraftment events has been difficult to evaluate independent of immunologic considerations. The use of genetic or irradiation-induced models of immunodeficiency may or may not reflect events in the immunologically and hematologically intact recipient. In a compelling study, Hashimoto et al31 transplanted donor microenvironment (bone) versus major or minor major histocompatibility complex (MHC)-mismatched microenvironment into irradiated allogeneic recipients and followed this with BM transplantation. Observations included: (1) increased cellularity and number of progenitors in MHC-matched BM versus recipient or third party BM; (2) the ability to engraft HSC when combined with the matched, but not mismatched microenvironment, into previously resistant strain combinations; (3) the dependence of this advantage on MHC with no restriction demonstrated for minor antigen disparity; (4) limitation of engraftment in non–MHC-matched environments was not secondary to T or natural killer (NK)-mediated mechanisms; and (5) MHC restriction was abrogated by pretransplant irradiation of the donor microenvironment. This study suggests that in the nonirradiated microenvironment, MHC-matched cells have an advantage over non–MHC-matched cells, but does not address whether the advantage is due to better homing and engraftment or subsequent ability to maintain and expand hematopoiesis. Our finding that the early homing and engraftment events for congenic or allogeneic donor cells are identical would suggest that any MHC-derived advantage occurs after lodgment and engraftment and is more likely related to ongoing stromal/HSC interaction. It would also support a model of homing and engraftment that is entirely independent of MHC-associated interactions.
A criticism of our current study is that conclusions drawn from transplantation of a heterogeneous BM population may or may not reflect homing and engraftment of long-term repopulating cells. Nevertheless, these findings do represent numerous mechanisms that regulate the trafficking of progenitors and it is likely that there is overlap with the proposed homing mechanisms of HSC. The persistence of detectable chimerism through 12 months within the recipient BM supports the engraftment of donor HSCs. Because the vast majority of engrafting cells do not have long-term repopulating capacity, the donor cell compartment is reduced to a very small fraction of total hematopoiesis.32-34 Based on a phenotypic analysis, Morrison and Weissman33 calculated the frequency of stem cells in the marrow of an adult mouse to be in the range of 0.005% to 0.01%. Slightly lower levels were suggested by the work of Osawa et al,34 who concluded an HSC frequency of 0.004% ± 0.003% based on a more restricted phenotype. From these detailed studies, we conclude that the number of HSCs in the transplanted inoculum was in the range of 50 to 100 cells. Considering the documented homing efficiency of around 5% and assuming an equal homing efficiency for HSCs, transplantation of 1 × 106 BM cells results in the homing of only 2 to 5 HSCs to the fetal liver. This calculation is consistent with the low-level of engraftment that is observed when using this dose of BM cells. The purpose of this study was not to achieve high levels of long-term engraftment, but rather to document the early events after transplantation of a standard dose of adult BM. In separate studies using this model,35 36 we have documented higher levels of multilineage long-term engraftment using fetal liver-derived cells or higher doses of BM-derived cells. This latter finding is consistent with our interpretation of the low-level of long-term engraftment seen in this study.
Although these findings may be applicable to events during normal hematopoietic ontogeny, our primary interest in this model is its potential for the study of in utero HSC transplantation. Despite clinical success with this approach in immunodeficiency disorders in which there is a survival advantage for normal cells (X-linked severe combined immunodeficiency),22,37 broader clinical application of in utero HSC transplantation has been limited by minimal donor cell engraftment in disorders in which there is no competitive advantage. Models of prenatal or neonatal transplantation into mutant or compromised fetuses fail to recreate this setting of engraftment competition, but rather, offer a competitive bias to the normal donor cells.8-10 The receptive environment in the human fetus during the preimmune “window of opportunity” consists of only fetal liver-derived hematopoiesis. Therefore, a developmentally analogous model in which the early homing and engraftment events can be systematically explored and manipulated should prove invaluable in developing clinical strategies to improve donor HSC engraftment. Our ability to reproducibly characterize the kinetics of peripheral blood and fetal liver donor cell expression in this model will pave the way for a systematic analysis to elucidate the adhesion interactions that are critical for fetal liver homing, as well as to examine the homing capability of specific donor cell populations. Further development of the model should allow studies of the competitive capacity of different donor cell populations after engraftment and the ability of fetal liver engraftment to redistribute to other hematopoietic environments.
Supported in part by a National Research Service Award HL09856 from the National Institutes of Health, Public Health Service Grant No. HL53998, and funds from the Ruth and Tristram C. Colket, Jr, Chair of Pediatric Surgery.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Alan W. Flake, MD, The Center for Fetal Diagnosis and Treatment, The Children’s Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal