Abstract
The NUP98 gene is involved in 3 distinct chromosomal rearrangements, t(7;11)(p15;p15), t(2;11)(q31;p15), and inv(11)(p15q22); all of these NUP98 rearrangements have been identified in the malignant cells of patients with therapy-related acute myelogenous leukemia or myelodysplastic syndrome (t-AML/MDS). Here we report the cloning and characterization of a t(11;20)(p15;q11) translocation from patients with t-MDS. The breakpoint on chromosome 11p15 targets the NUP98 gene and results in the separation of the N-terminal FXFG repeats from the RNA-binding domain located in the C-terminus. The breakpoint on chromosome 20q11 occurs within the gene encoding human DNA topoisomerase I (TOP1). As a result, a chimeric mRNA encoding the NUP98 FXFG repeats fused to the body of DNA topoisomerase I is produced. These results indicate that NUP98is a recurrent target in therapy-related malignancies, and thatTOP1 is a previously unrecognized target for chromosomal translocations.
NONRANDOM CHROMOSOMAL aberrations such as translocations, deletions, and inversions are associated with a wide variety of hematological malignancies and are considered causal events in the process of leukemic transformation.1 In some instances, chromosomal translocations fuse sequences encoding a transcription factor or receptor tyrosine kinase to those of a normally unrelated gene, resulting in a chimeric protein with oncogenic properties. In other cases, the translocations result in the coding regions of proto-oncogenes becoming joined to, and regulated by, highly active promoter/enhancer elements such as those of the Ig or T-cell receptor genes.1 Similar chromosomal aberrations associated with therapy-related myelodysplastic syndromes and therapy-related acute myelogenous leukemia (t-MDS/AML) are thought to arise as a direct result of exposure to various forms of chemotherapy and/or radiation therapy.2 Agents that target DNA topoisomerase II (topo II) are associated with a distinct form of t-AML, characterized by a short latency period, monocytic features, and balanced translocations involving the MLL gene on chromosome 11q23.3 Other balanced translocations such as t(8;21),4t(15;17),5 and inv(16)6 have also been reproducibly associated with t-AML after exposure to multiagent chemotherapy.
The NUP98 gene encodes a 98-kD component of the nuclear pore complex (NPC) that is localized to the nucleoplasmic side of the NPC.7 It is thought to function as a docking protein involved in nucleocytoplasmic transport7; this docking function is mediated, at least in part, by multiple FXFG repeats located in the N-terminal portion of the protein.7 We and others have recently reported that the NUP98 gene is found at the breakpoints of 3 distinct chromosomal rearrangements: the t(7;11)(p15;p15),8,9 t(2;11)(q31;p15),10 and inv(11)(p15q22).11 Chimeric mRNAs produced by the t(7;11) and the t(2;11) fuse the FXFG repeats of NUP98 with the homeodomains ofHOXA9 and HOXD13, respectively, while the inv(11)(p15q22) fuses the NUP98 FXFG repeats withDDX10, a putative RNA helicase.8-11 Significantly, all of these translocations have been reported to occur in the leukemic cells of patients with t-MDS/AML.10-12 Based on the above findings, we hypothesized that the NUP98 gene would be disrupted in the t(11;20) translocation, and that a chimeric gene fusing the NUP98 sequences in frame to a gene on chromosome 20q11 would be produced as a result of the translocation.
MATERIALS AND METHODS
Case reports.
Patient no. 923 was a 4-year-old girl who was diagnosed with acute lymphoblastic leukemia; the leukemic cells showed a normal karyotype. She received multiagent chemotherapy that included daunorubicin, vincristine, cortisone, L-asparaginase, doxorubicin, cyclophosphamide, 6-mercaptopurine, methotrexate, etoposide, and cytosine arabinoside. Nine years later, she developed an MDS (French-American-British [FAB] type RAEB-t); the karyotype was 46, XX, t(11;20)(p15;q11.2). This patient has previously been reported in a series of t-AML patients (patient no. 14 in ref 13), and had an MLL gene pseudorearrangement. Patient no. 1138 was a 15-year-old boy who was diagnosed with non-Hodgkin’s lymphoma and received therapy with doxorubicin, vincristine, cyclophosphamide, cortisone, etoposide, ifosphamide, cytosine arabinoside, cisplatin, L-asparaginase, and methotrexate. Approximately 14 months after initiation of treatment, an MDS (FAB type RAEB) was diagnosed. Cytogenetic analysis showed a 46, XY, t(11; 20) (p15;q11.2) [17]/45, idem, −5[3]. This patient has also been previously reported (patient no. 34 in ref 14).
Nucleic acid isolation.
Genomic DNA and total RNA was extracted from cryopreserved leukemic cells as previously described.10 Control DNA and RNA were obtained from peripheral blood mononuclear cells (PBMC) obtained from a normal volunteer. The studies performed were approved by the Institutional Review Board of Roswell Park Cancer Institute, and informed consent for use of patient material in research studies was obtained by the referring institution.
3′-RACE.
3′-RACE was performed using reagents from Life Technologies, Inc (Gaithersburg, MD) and a modified protocol as described previously.10 The polymerase chain reaction (PCR) was performed using an NUP98-specific forward primer (NUP8001, Table 1) and an abridged universal AP (AUAP, Table 1) as the reverse primer. The PCR products were analyzed by hybridization to a terminal deoxynucleotidyl transferase (TdT) end-labeled nested NUP98 oligonucleotide (NUP8002, Table 1) as described previously.10 The PCR products were cloned into the pGEM T-Easy vector using the reagents and protocols supplied by the manufacturer (Promega, Madison, WI). Positive clones were identified by filter lift hybridization to the NUP8002 oligonucleotide.
Oligonucleotide Primer Sequences
| Primer . | Oligonucleotide Sequence (5′-3′) . | Gene . | Nucleotides . |
|---|---|---|---|
| NUP8001 | TGGAGGGCCTCTTGGTACAGG | NUP98 | 1461-1481 |
| AUAP | GGCCACGCGTCGACTAGTAC | ||
| NUP8002 | GCCACTTTGGGCTTTGGAGCCCC | NUP98 | 1516-1538 |
| HA-05 | ATCAGCCTTAGCACTTTTCAGGTC | TOP1 | 2197-2224 |
| NUP8006 | TTTCTGGATATTCAGATGGTGAAGC | NUP98 | 1957-1981 |
| Primer . | Oligonucleotide Sequence (5′-3′) . | Gene . | Nucleotides . |
|---|---|---|---|
| NUP8001 | TGGAGGGCCTCTTGGTACAGG | NUP98 | 1461-1481 |
| AUAP | GGCCACGCGTCGACTAGTAC | ||
| NUP8002 | GCCACTTTGGGCTTTGGAGCCCC | NUP98 | 1516-1538 |
| HA-05 | ATCAGCCTTAGCACTTTTCAGGTC | TOP1 | 2197-2224 |
| NUP8006 | TTTCTGGATATTCAGATGGTGAAGC | NUP98 | 1957-1981 |
Reverse-transcriptase (RT)-PCR analysis.
First-strand cDNA was synthesized as described previously.10 An NUP98 sense primer (NUP8002) and aTOP1 antisense primer (HA-05, Table 1) were used along with Advantage Taq polymerase and reagents (Clontech, Palo Alto, CA). The PCR thermal cycling protocol consisted of 94°C for 1 minute, followed by 35 cycles of 94°C for 30 seconds and 68°C for 6 minutes. The PCR products were subcloned into the pGEM T-Easy (Promega, Madison, WI) vector and sequenced using a Perkin-Elmer ABI PRISM Model 373A Stretch Automated sequencer. (Applied Biosystems, Foster City, CA).
RESULTS AND DISCUSSION
The NUP98 gene was found to be rearranged in the leukemic cells of both patient samples on Southern blot hybridization of genomic DNA to an NUP98 cDNA probe (unpublished observations). To identify a potential fusion transcript involving NUP98, we used 3′-RACE on RNA isolated from patient no. 923. The choice of theNUP98-specific forward primer (NUP8001, Table 1) was based on previously reported NUP98 fusions that occurred at either nucleotide 1552 or 1864 (Genbank accession no. U41815) and on our reasoning that the t(11;20) might produce a transcript with a similar fusion point. The PCR products were subcloned into plasmid vectors, and a clone that hybridized to an internal NUP98 oligonucleotide (NUP8002, Table 1) was selected for further analysis.
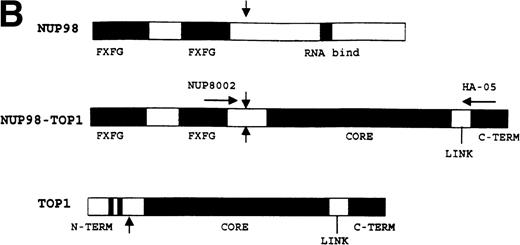
The nucleotide sequence of this 3′-RACE clone diverged from the germline NUP98 sequence at nucleotide 1686 (Genbank accession no. U41815). A BLAST search showed that the sequence immediately 3′ of this divergence was a perfect match for the gene encoding human DNA topoisomerase I (TOP1; GenBank accession no. J03250). The resultant in-frame fusion mRNA joined nucleotide 1686 of NUP98to nucleotide 719 of TOP1 (Fig 1A). The predicted chimeric protein would contain the N-terminus of NUP98, including the FXFG repeats (amino acids 1-514) fused to the majority of DNA topoisomerase I (amino acids 170-765) including the core, linker, and catalytic domains (Fig 1B). We were unable to amplify a potential reciprocal TOP1-NUP98 fusion encoding the DNA topoisomerase I N-terminus and the NUP98 RNA binding domain. These results are consistent with previous reports of NUP98 fusions that have consistently detected N-terminus NUP98 sequences fused to C-terminus partner gene sequences, but not N-terminus partner gene sequences fused to C-terminus NUP98 sequences.8-12
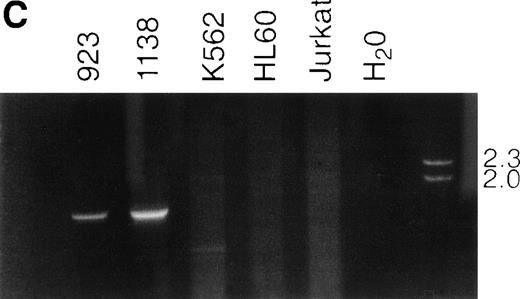
NUP98-TOP1 fusion cDNA. (A) NUP98-TOP1fusion sequence. The germline NUP98 and TOP1 (bold and italic) nucleotide sequences are as shown. The NUP98-TOP1fusion cDNA is as indicated. The amino acids encoded are shown below the nucleotide sequence. (B) Schematic representation of the wild-type and fusion proteins. Functional domains indicated for NUP98 are FXFG, FXFG repeat region; and RNA bind, RNA binding domain. DNA topoisomerase I N-terminus, core, linker, and C-terminus domains, as designated in ref 19, are indicated. NUP98 and TOP1 fusion points are shown with vertical arrows. (C) RT-PCR assay for NUP98-TOP1 fusion mRNA. Primers NUP8002 and HA-05 were used to amplify the 1,677-bp fusion mRNA. Patient samples no. 923 and 1138 are as indicated; no specific PCR product is seen in the negative control lanes (K562, HL60, Jurkat, H2O).
NUP98-TOP1 fusion cDNA. (A) NUP98-TOP1fusion sequence. The germline NUP98 and TOP1 (bold and italic) nucleotide sequences are as shown. The NUP98-TOP1fusion cDNA is as indicated. The amino acids encoded are shown below the nucleotide sequence. (B) Schematic representation of the wild-type and fusion proteins. Functional domains indicated for NUP98 are FXFG, FXFG repeat region; and RNA bind, RNA binding domain. DNA topoisomerase I N-terminus, core, linker, and C-terminus domains, as designated in ref 19, are indicated. NUP98 and TOP1 fusion points are shown with vertical arrows. (C) RT-PCR assay for NUP98-TOP1 fusion mRNA. Primers NUP8002 and HA-05 were used to amplify the 1,677-bp fusion mRNA. Patient samples no. 923 and 1138 are as indicated; no specific PCR product is seen in the negative control lanes (K562, HL60, Jurkat, H2O).
To exclude the possibility of a PCR artifact leading to theNUP98-TOP1 fusion, and to determine if NUP98-TOP1fusion transcripts could be detected in additional patient samples, we designed gene-specific RT-PCR primer pairs (primers NUP8002 and HA-05; Table 1) to amplify the fusion mRNA. As shown in Fig 1C, we were able to amplify identical 1,677-bp PCR products from both patient samples, but not from the control cell lines, showing that identical chimeric transcripts were produced in both patient samples. Sequence analysis confirmed that an identical fusion cDNA was produced in both samples. We were able to successfully amplify the germline NUP98 cDNA sequence from both the patient samples and control cell lines, using primers NUP8001 and NUP8006 (Table 1), demonstrating that both NUP98 alleles were expressed, and confirming the presence of intact RNA in the control samples (data not shown).
The t(11;20)(p15;q11.2) is a rare but recurrent translocation that has been reported in patients with MDS,13 AML,15and polycythemia vera.16 The 2 patients reported here were children who had developed t-MDS/AML after treatment with multi-agent chemotherapy. Of note, the t(2;11) and inv(11)(p15q22) that involveNUP98 were both initially recognized in pediatric patients with t-MDS/AML.10,17 In addition, although the t(7;11) has been most frequently recognized in patients with de novo AML, there is a recent report of t-AML with a t(7;11) after the use of the topo II poison bimolane (ICRF 154) for psoriasis.12 Furthermore, a recent review of children with t-MDS/AML identified 11p15 abnormalities in 7% of patients.18 Taken together, these data suggest that NUP98 may be a more frequent target for therapy-related chromosomal translocations than previously thought, especially in children.
This report provides the first data to suggest that deregulation of DNA topoisomerase I function may be directly related to malignant transformation. DNA topoisomerase I normally catalyzes a cycle of transesterification reactions during which it generates transient single-stranded DNA nicks, resulting in topological transformations of DNA.19,20 DNA topoisomerase I has been shown to be involved in DNA replication, transcription, and recombination, as well as chromosome condensation.19,20 The DNA topoisomerase I protein can be organized into 4 distinct domains: a C-terminus catalytic region that contains the active tyrosine, a nonconserved “linker” domain, a conserved “core” domain, and an N-terminus domain that contains a nuclear localization signal (NLS).20 The core domain has been shown to mediate binding to supercoiled DNA, whereas the N-terminus domain, which is largely lost in the NUP98-TOP1 fusion, is dispensable for DNA topoisomerase I catalytic function in vitro.19-21 However, several recent studies have suggested that the N-terminus domain may have additional, previously unsuspected roles. The N-terminus domain has been shown to interact with nucleolin,22 SV40 large T antigen,23 and the SF2/ASF splicing factor.24 Additionally, DNA topoisomerase I has recently been shown to interact with the C-terminus of p5325; however, the region of DNA topoisomerase I that interacts with p53 is unknown.
The mechanism by which an NUP98-TOP1 fusion might be leukemogenic is unknown. Chromosomal translocations that generate fusion proteins are generally thought to be oncogenic through a gain of function attributed to the fusion protein. Because the predicted NUP98-TOP1 fusion protein lacks the N-terminus of DNA topoisomerase I, loss of regulatory functions dependent on the DNA topoisomerase I N-terminus may lead to a gain of function through the fusion of NUP98 sequences to the catalytic portion of DNA topoisomerase I. Alternatively, it is possible that an NUP98-TOP1 fusion protein binds to and sequesters a protein that normally binds DNA topoisomerase I such as p53. Lastly, it has recently been shown that an NUP98-HOXA9 fusion protein can transform NIH3T3 fibroblasts in vitro.26The mechanism suggested in those experiments is that HOXA9-responsive genes are activated by the NUP98-HOXA9 fusion through a previously unrecognized NUP98 transactivation domain present in the NUP98 FXFG repeat region. However, it is difficult to envision how an NUP98-TOP1 fusion protein could activate transcription in a sequence-specific fashion.
In summary, we have cloned a t(11;20) chromosomal translocation that results in a previously undescribed, in-frame fusion between NUP98 and TOP1. The mechanism by which a NUP98-TOP1 fusion protein might be transforming is unknown and can only be answered by future experiments, although the precedent set by other leukemogenic fusion proteins might suggest that it acts dominantly, through a gain of function. Finally, the finding that this translocation, as well as at least 3 other forms of balanced NUP98 chromosomal rearrangements, occurred in patients with t-MDS/AML suggests that NUP98 is a recurrent target for chromosomal rearrangements caused by genotoxic therapy.
ACKNOWLEDGMENT
We thank Ronald Hancock, Ilan Kirsch, and William Burhans for helpful discussions, Julian Borrows for the NUP98 cDNA probe, and Robert Bash, Naomi Winick, Allan Pyesmany, Thomas Nevill, and Rebecca Eisan for providing patient specimens and clinical information. We also thank Elena Greco for artwork.
P.D.A. is supported by a Leukemia Society of American Scholar Award and National Institutes of Health (NIH) Grants No. CA16056 and CA73773. C.A.F. is supported by a Leukemia Society of America Scholar Award and NIH Grants No. CA66140, CA77683, and CA80175.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Peter D. Aplan, MD, Department of Pediatrics, Roswell Park Cancer Institute, Buffalo, NY 14263; e-mail:paplan@sc3101.med.buffalo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal