To the Editor:
The platelet glycoprotein (GP) IIb/IIIa is an important integrin (αIIbβ3) involved in cell-substratum adhesion, which plays a key role in the function of megakaryocytes and platelets. This complex could also play a relevant role in hematopoiesis if expressed in more primitive multilineage hematopoietic progenitor cells. Thus, although an extensive search has been made to identify the expression of this integrin on hematopoietic progenitors, there are conflicting data about the more primitive hematopoietic stem cell (HSC) capable of expressing the platelet GP IIb/IIIa. Antisera against purified GPs IIb and IIIa inhibit colony-forming unit (CFU)-mix in some experiments,1,2 but not in others.3Moreover, the GP IIb/IIIa mRNA has been detected in human CD34+-enriched cell populations.4 Transgenic mice with conditional toxigene suggest that the GP IIb promoter was active in primitive hematopoietic progenitor cells with myeloid, erythroid, and megakaryocytic potential, but decreased progressively as differentiation proceeded toward the erythroid and myeloid lineages.5,6 However, cell surface expression of GP IIb/IIIa in HSC had not been fully demonstrated. Murray et al7 suggest such expression, reporting that the GP IIb/IIIa+ cell population is also positive for the human HSC marker CD34+. The recent report of Ody et al8 is the first evidence of GP IIb/IIIa expression in HSC. By flow cytometry, these authors sorted GP IIb/IIIa+ cells from avian intraaortic clusters and from embryonic and adult bone marrow. Then, by means of clonogenic assays for the multilineage potential of HSC, they showed that the sorted cells were able to differentiate into myeloid, erythroid, and thrombocytic lineages. Thus, they suggest the attractive idea that GP IIb/IIIa is expressed in primitive HSC and, therefore, this integrin should not be considered as a specific marker of the megakaryocytic lineage, but it could be a useful tool for the characterization and localization of hematopoietic progenitors.
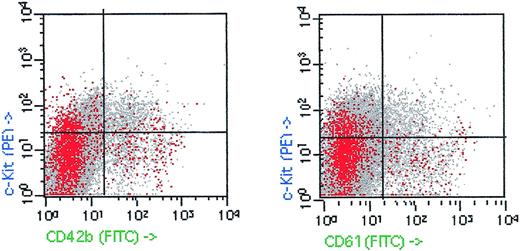
However, some points should be considered with caution when analyzing these results. Firstly, we have observed that a percentage of human primitive multilineage hematopoietic cells (CD34+, c-Kit+) from distinct sources (human cord blood, bone marrow, and recombinant human granulocyte colony-stimulating factor [rhG-CSF] mobilized peripheral blood from healthy subjects) are also positive for platelet markers. Between 10% and 20% of CD34+/c-kit+ cells with light scatter characteristics of true progenitor cells (after convenient exclusion of those CD34+ cells also positive for monocytic antigens) from these sources do present a high expression of GP IIIa (CD61), GP Ibα (CD42b), GP Ia (CD49b), and P-selectin (CD62).
Figure 1 shows a representative example of c-kit, CD34, and platelet-marker (CD42b and CD61) expression on a sample from an rhG-CSF–mobilized donor leukapheresis. Furthermore, we have also observed that the expression of these antigens decreased when samples were washed with EDTA before monoclonal antibody incubation. Therefore, our results cannot be explained by the constitutive expression of these platelet markers in HSC. More likely, these data suggest that the expression of these platelet-associated antigens might be the consequence of platelet adherence to progenitor cell surface. In fact, other authors have reported the in vitro P-selectin–dependent binding of platelet to HSC.9,10Moreover, the recently described expression of the P-selectin glycoprotein-1 (PSGP-1), the putative counterreceptor for P-selectin, on CD34+ progenitor cells does also support platelet adhesion to HSC.11 According with these data, sorting of GP IIb/IIIa+ cells could achieve a positive selection of HSC bound to platelets. Therefore, the culture of these selected cells in different hematopoietic differentiation medium could easily produce a high number of colonies of distinct lineages. The observations suggested by Ody et al could have a fundamental relevance, but we believe it could be necessary to discard the adhesion of platelets to the sorted GP IIb/IIIa+ cells. The use of specific platelet markers, such as GP Ibα, in the sorting assays, and/or the determination of platelet-antigen expression in the sorted GP IIb/IIIa+ population should be performed to clarify this point.
c-Kit, CD42b, (GP Ib), and CD61 (GP IIIa) expression on human leukapheresis product after mobilization with rhG-CSF. CD34+/CD14− low side scatter cells are shown in red. Fifty thousand events were acquired.
c-Kit, CD42b, (GP Ib), and CD61 (GP IIIa) expression on human leukapheresis product after mobilization with rhG-CSF. CD34+/CD14− low side scatter cells are shown in red. Fifty thousand events were acquired.
Response
According to the authors, the immunoreactivity of hematopoietic stem cells could be caused by contaminating adherent platelets. Thus, they question our recently published data.1-1
Platelets do not exist in birds, and thrombocytes are the functional equivalents to mammalian platelets. These are large nucleated cells that express GPIIbIIIa at a high level (Fig 3 in ref 1-1). In the c-kit/GPIIbIIIa double-positive bone marrow population selected by cytofluorimetric cell sorting (Fig 4 in ref 1-1), thrombocytes were excluded by the criteria of their high level of GPIIbIIIa expression. The size of the thromboblasts (thrombocyte precursors) is similar to the size of hematopoietic progenitors in the selected population, and doublets or larger aggregates were excluded in the sorting procedure by the choice of the forward and side scatter window. Therefore, the thromboblasts included in the sorted progenitor population were present as single cells and not bound to hematopoietic progenitors. Furthermore, the potentialities of the double-positive cells were tested in vitro and in vivo and these cells were able to differentiate into all hematopoietic lineages. In conclusion, we showed that c-kit+ multipotential hematopoietic progenitors coexpress GPIIbIIIa.
Nevertheless, in mammals, considering the small size of the platelets, their interaction with hematopoietic cells is more difficult to exclude by flow cytometry. The fluorescence-activated cell sorting (FACS) profiles from Vicente et al using G-CSF–mobilized peripheral blood progenitors show that the c-kit+ population contains cells expressing megakaryocytic markers in the light scatter window characteristic of progenitor cells. These cells could be contaminating platelets adhering to progenitor cells or megakaryocytic precursors expressing lineage markers along with c-kit. By analogy with chicken adult bone marrow staining with c-kit and GPIIbIIIa monoclonal antibodies, the CD61hi cell population may contain adherent platelets because in the chicken, GPIIbIIIahi cells are exclusively composed of thrombocytes (Fig 3 in ref 1-1). In contrast, the CD61lo population could contain megakaryocytic precursors and multipotential hematopoietic progenitors as it is for the chicken (Fig 3 in ref 1-1). From the experiments performed with the chicken, we showed that the multilineage hematopoietic progenitors were exclusively located in the GPIIbIIIalo c-kit+ population.
Considering the in vivo data in mice1-2 1-3 showing that impaired GPIIb expression prevents normal hematopoiesis, we think that GPIIbIIIa expression by multilineage hematopoietic progenitor cells is a general feature and that it plays a functional role in hematopoiesis.
Although it is obviously not the case in the chicken, our data cannot rule out adhesion of platelets to mammalian hematopoietic progenitors. This idea is still intriguing, but the demonstration would require a careful and thorough analysis that is not provided by the current comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal