Abstract
The generation of immunoregulatory T cells that block the B7(CD86/CD80)-CD28 and/or CD40-CD154 costimulatory pathways has great potential for the induction of long-term transplantation tolerance. In a human polyclonal in vitro model, combined monoclonal antibody (mAb) blocking of the costimulatory ligands CD40 and CD86 lead to allospecific T-cell anergy that cannot be reversed by antigenic rechallenge in the presence of IL-2. Although antigenic restimulation with IL-2 restored the proliferative response, subsequent antigenic restimulation of the restored anergic cells in a tertiary mixed lymphocyte culture still resulted in nonresponsiveness. Importantly, these anergic T cells suppress the response of naive alloreactive T cells in an antigen-specific way via linked recognition. Suppression may partially depend on local IL-10 production, while transforming growth factor–β (TGF-β) did not play a role. Irrespective of the monoclonal antibody combination used, blast formation occurred in a subset of CD4+ cells. These cells were characterized by a sustained CD45RA expression, an increased T-cell receptor density, and a lower level of CD4 expression. A reduced number of CD45RO+/CD8+ T cells was observed whenever anti-CD86 was combined with anti-CD40, which was reflected by an even more attenuated cytotoxic T-cell function. This indicates the importance of CD40-CD154 in the generation of cytotoxic T cells in this transplantation model. We hypothesize that in our model, anergy is induced in the CD4+ T-cell subset, whereby CD8+ cytotoxic effector function is impaired by the lack of both CD40-CD154 signaling and cytokine-mediated help. This costimulatory ligand–directed mAb approach might well be used for the ex vivo generation of antigen-specific immunoregulatory T cells applicable in adoptive immunotherapy.
Several regulatory mechanisms are responsible for controlling homeostasis of immunological responses and maintenance of tolerance.1 In an attempt to prevent allograft rejection in an antigen-specific way, many different studies deliberately evoked these naturally occurring mechanisms to induce and maintain allospecific T-cell tolerance. Although the mechanisms responsible for tolerance induction in mature peripheral T cells are not completely clear, multiple nonmutually exclusive phenomena have been indicated in the context of transplantation.2 These include immunological ignorance,3,4 induction of nonresponsiveness or anergy,5 deletion,6,7 and immunoregulation.8-13 Immunoregulatory T cells have been proposed to act via intercellular interactions 9,13 that are based on competition for antigen-presenting cell (APC) surface antigens and/or locally produced cytokines.9,14 Maintenance of the tolerant state by immunoregulatory T cells might be of clinical importance for long-term graft survival.15,16 With regard to the mitigation of alloresponses in a polyclonal situation, an important aspect of this type of regulation is that alloreactive T-cell clones, which are made tolerant toward a specific alloantigen, can potentially down-regulate the response of another T cell that is directed against a distinct second alloantigen. This is accomplished provided that the antigen is coexpressed on the same APC as the tolerance-inducing antigen. This phenomenon, called linked suppression,17,18 might in fact be one of the first steps in a self-sustaining form of tolerance known as infectious tolerance.12 19
Activation of mature T lymphocytes is a multistep phenomenon20 requiring both antigen-specific triggering of the T-cell receptor (TcR) complex on the T cell and additional signaling via costimulation.21 A key costimulatory signal results from binding the CD28 receptor on T cells with CD86 (B7-2/B70) and CD80 (B7-1/BB1) ligands on APCs.22-26 Inhibition of this pathway in the presence of antigenic stimulation results in T-cell anergy.20 More recently, the CD40-CD154 (CD40L) pathway was shown to attribute to the regulation of T-cell activation, both by independently costimulating T cells and at least in part by up-regulating CD80/CD86 molecules on APCs.27 28
This knowledge has been successfully used in animal models to prevent allograft rejection by blocking CD86 and/or CD80,29,30thereby leading to long-term graft survival.25,31 Others showed the effectiveness of blocking the CD40-CD154 (CD40L) pathway in this respect.32-36 Combined inhibition of both the B7 and CD40 pathways showed a synergistic effect on graft survival in both rodent and primate transplant models.4,32,37,38 Human in vitro studies have shown the efficacy of blocking the costimulatory ligands in the induction of T-cell anergy in both alloresponses24,39,40 and memory T-cell responses.41
In the present study we elaborate on antigen specificity, immunoregulatory features, maintenance of anergy, and the phenotype of human alloreactive T cells, which were made anergic by monoclonal antibody (mAb) blocking of the CD86 and CD40 costimulatory ligands. The anergic T cells were able to suppress the response of polyclonal alloreactive T cells in an antigen-specific way via linked recognition, which was mediated partially via IL-10. Importantly, the anergic state was maintained even after restoration of the hyporesponsiveness, which indicates a profound anergy-inducing protocol. Collectively, these data support the therapeutic potential of anergic T cells generated by mAb blocking of CD86 and CD40 in the polyclonal primary human mixed lymphocyte culture (MLC).
Materials and methods
Cells
For all experiments, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Lymphoprep; Nycomed Pharma, Oslo, Norway) from buffy coats obtained from healthy blood donors. The cells were frozen and stored in liquid nitrogen until use. After thawing, viability of the cells was determined by trypan blue dye exclusion. All donors were human leukocyte antigen–typed (HLA-typed), and we developed MLCs that exploited different degrees of matching.
HLA typing
Serological HLA-A, HLA-B, HLA-DR, and HLA-DQ phenotyping (broad specificities and splits) was performed using the standard microcytoxicity assay. Additional class I and II typing and subtyping was performed by molecular methods. Genomic DNA was prepared using the QIA-Amp Blood Kit (Qiagen, Hilden, Germany). Low- to intermediate-resolution typing of HLA haplotypes A, B, DR, and DQ was performed using a polymerase chain reaction–sequence-specific primers (PCR-SSP) technique (Pel-Freeze Clinical Systems, Deerbrook Trail, WI). HLA-DRB and HLA-DQB subtyping was performed using another PCR-SSP technique (Dynal DRB1*, B3*, B4*, B5*, and DQB subtyping kits; Dynal, Oslo, Norway).
Mixed lymphocyte cultures
Primary one-way MLCs were performed by culturing 1 × 105 30 Gy γ-irradiated stimulator PBMCs with 1 × 105 responder PBMCs in 96-well round-bottom plates (Greiner, Frickenhausen, Germany) in 200 μL Roswell Park Memorial Institute culture medium (RPMI-1640) and glutamax supplemented with 0.02 mmol/L pyruvate, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco, Paisley, England) and 10% heat-inactivated pooled human serum at 37°C, 95% humidity, and 5% carbon dioxide (CO2). Proliferation was analyzed by3H-thymidine incorporation at day 6 of the culture, and 0.037 MBq (1 μCi) 3H-thymidine (ICN Pharmaceuticals, Irvine, CA; specific activity, 7.4 × 1010 Bq/mmol [2.0 Ci/mmol]) was present during the last 18 hours.3H-thymidine incorporation was analyzed by a gas scintillation counter (Matrix 96 Beta counter; Canberra Packard, Meriden, CT). The 3H incorporation is expressed as mean counts per 5 minutes and SD of at least quadruplicate measurements. Counts per 5 minutes by gas scintillation analysis resemble counts per 1 minute as measured by liquid scintillation analysis. For cytokine measurements, culture supernatants were harvested on days 3 and 6.
To study the secondary response of allo-MHC–primed (allo-major histocompatibility complex–primed) T cells, first bulk primary MLCs were performed by culturing 1 × 106γ-irradiated (30 Gy) stimulator PBMCs and 1 × 106responder PBMCs for 7 days in 24-well culture plates (Greiner) in 2 mL culture medium. Cells were harvested, washed, and allowed to recuperate for 2 days. Dead cells were removed by density gradient centrifugation (Lymphoprep, Nycomed Pharma). Subsequently, 2 × 104recovered viable cells were restimulated with 1 × 105 γ-irradiated (30 Gy) stimulator PBMCs in 96-well round-bottom plates. The proliferative response of the secondary MLCs was examined on day 3, which appeared to be the optimal time-point.42 Antigen specificity was examined by using completely HLA-mismatched or partially HLA-matched third-party PBMCs. For cytokine measurements, culture supernatants were harvested after 48-72 hours.
To investigate the tertiary response, first bulk secondary MLCs were performed in 24-well culture plates. Accordingly, 2 × 105 responder cells from a bulk primary MLC (see above) and 1 × 106 γ-irradiated stimulator PBMCs were cultured for 5 days with or without 12.5 U/mL IL-2 (Proleukine, Eurocetus, The Netherlands). Responder cells were washed and allowed to recuperate for 2 days. Subsequently, 2 × 104 responder cells were restimulated with 1 × 105 irradiated stimulator PBMCs in 96-well round-bottom plates. 3H incorporation was examined at day 3.
Induction of allospecific tolerance in a primary MLC
To generate allospecific anergic T cells in a primary MLC, mAb directed against 500 ng/mL CD40 (5D12; gift from Dr M. de Boer, Tanox Pharma, Amsterdam, The Netherlands) and 500 ng/mL CD86 (1G10; gift from Dr K. Lorré) were added, with or without 1000 ng/mL CD80 (M24; gift from Dr K. Lorré, N.V. Innogenetics, Ghent, Belgium) at the start of the bulk primary MLC. Each of the individual mAb dose-response titrations was performed, and the optimal inhibitory concentration was selected. In vitro tolerance was defined as hyporesponsiveness after antigen-specific restimulation and the reduced capacity to perform a specific cytotoxic response.
Cocultures to determine the immunoregulatory potential of anergic T cells
The regulatory capacity of anergic T cells was analyzed in an in vitro coculture MLC, and anergic or control cells from a primary MLC were added to a newly formed MLC. Previously we showed that both concentration and functional state (eg, irradiated vs living) of the added or regulatory cells are critical components in assessing the immunoregulatory capacity.42 Cocultures were performed in 96-well round-bottom plates; 5 × 103 γ-irradiated (30 Gy) anergic or control cells were added to a newly formed MLC consisting of both original responder PBMCs (5 × 104) and γ-irradiated stimulator PBMCs (2.5, 5, or 10 × 104 PBMCs). All tests were performed in quadruplicate. Antigen specificity of the regulatory phenomenon was examined in cocultures performed with third-party stimulator PBMCs that were either completely HLA-mismatched or partially HLA-matched (with an isolated class I or class II mismatch) to investigate the possibility of suppression via linked recognition. Neutralizing mAbs (5 μg/mL) against IL-10 and TGF-β (MAB217 and MAB1835, respectively; R&D Systems, Minneapolis, MN) were added during the coculture to study the role of these cytokines. Irrelevant isotype-matched antibodies, which never abrogated suppression, were used to control for specificity.
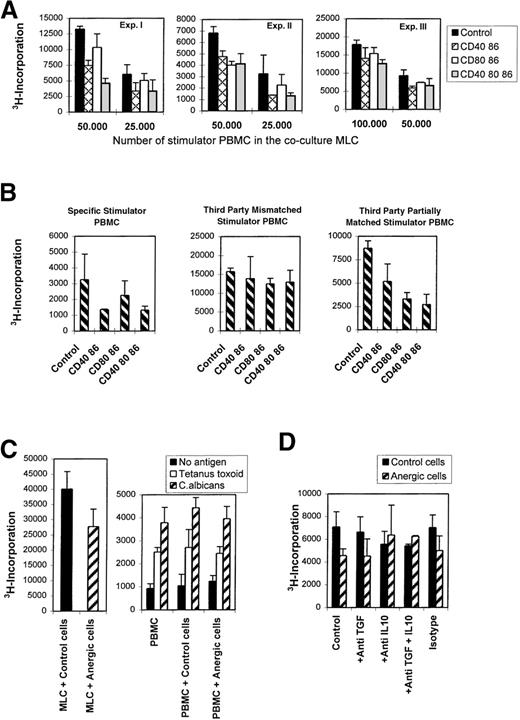
To exclude that a bystander suppression occurred, we examined the effect of anergic T cells on self-restricted recall responses against tetanus toxoid (RIVM, Bilthoven, Lelystad, The Netherlands) and Candida albicans extract (ARTHU Biologicals, Lelystad, The Netherlands). PBMCs (2 × 105) were cultured with 10 μg/mL antigen in the absence or presence of 5000 (30 Gy γ-irradiated) anergic or control cells, and the proliferative response was examined on day 5.
Cytokine assays
Cytokines were measured in culture supernatants. Interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-10 production were analyzed by enzyme-linked immunosorbent assay (ELISA) (Pelikine-compact ELISA kit; CLB, Amsterdam, The Netherlands), and biological active IL-2 was determined by the IL-2 sensitive cell line (CTLL-2) bioassay.43 The production of TGF-β was measured in culture supernatants of cells that were cultured in serum-free medium (Stem Cell Technologies, Vancouver, British Columbia, Canada). Briefly, soluble type II TGF-β receptor (R&D Systems) was used to capture bioactive TGF-β. A standard curve of 10-2500 pg/mL TGF-β (R&D Systems) was used. Detection took place by anti–TGF-β1 antibody combined with biotinylated antichicken immunoglobulin Y (IgY) (Jackson Immunoresearch, West Grove, PA). Color reaction was performed by a standard high resolution horseradish peroxidase (HRPO) method (streptavidin polyHRP mAb, CLB).
Cytotoxicity by chromium 51–release assay
The cytotoxic capacity of primed alloreactive T cells was examined by chromium 51 (51Cr) release of labeled phytohemagglutinin (PHA) blasts. Briefly, to generate PHA blasts, PBMCs were first cultured with PHA-M (Boehringer Mannheim, Mannheim, Germany) and subsequently with 50 units IL-2/mL. Target cells (2 × 106) were labeled with 3.7 MBq (100 μ Ci)51Cr (Amersham) and used as a target at 1000 cells per well. Different effector/target (E/T) ratios were tested in quadruplicate. Culture supernatants were examined for released51Cr on a γ-irradiation counter (Wallac 1470 γ-counter; Wallac, Turku, Finland). Cytotoxic capacity is shown as a percentage of specific lysis calculated according to the following equation, where CPM means counts per minute:
Flow cytometry
Cells were phenotypically analyzed by a 2-step double labeling procedure. Briefly, cells were washed twice with phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). The cells were labeled first with unconjugated specific antibody, followed by conjugate binding with goat antimouse–phycoerythrin (GAM-PE) or GAM–fluorescein isothiocyanate (GAM-FITC) (Dako, Glostrup, Denmark). Thereafter the cells were labeled with either CD4 or CD8 antibodies. All incubations were for 30 minutes on ice, and thereafter the cells were washed twice. The samples were run on a Coulter Epics XL Flowcytometer (Beckman Coulter, Fullerton, CA), and 5000 or 10 000 events were collected based on live lymphocyte cell gating, as indicated by 5 μg/mL propidium iodide staining. Isotype-matched antibodies were used to define marker settings, and isotype-matched controls were usually below background staining. Data were analyzed by Coulter XL-2 software (Coulter Electronics, Miami, FL) and WINMDI software (Scripps Research Institute, La Jolla, CA). CD4+and CD8+ T cells in the live lymphocyte gate were analyzed by the following mAbs: CD3-FITC/PE (Clone UCHT1.7), CD4-PE (MT310), CD8-PE (DK25), CD14-FITC (TUK4), CD19-PE (HD37 [7mAb]), CD25 (ACT-1), CD45RA (4KB5), and CD45RO (OPD41) (Dako, brand names noted in parentheses); WT31 (anti-TcR; Dr W. Tax, Nijmegen, The Netherlands); and L243 (anti-HLA DRα; American Type Culture Collection, Manassas, VA).
Results
Anti-CD86 mAb is a powerful inhibitor of the primary MLC, but additional CD40 blocking attenuates the cytotoxic response
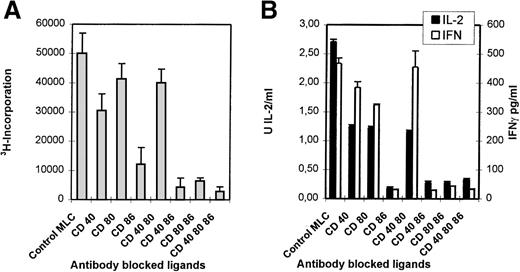
Monoclonal antibodies directed against CD40, CD80, and CD86 ligands were tested in the polyclonal primary MLC to study their applicability to the induction of anergy. In this study 6 distinct responder-stimulator combinations were studied; 5 combinations were mismatched for a single HLA haplotype (ie, 1A,1B,1DR, and 1DQ mismatch), and 1 combination was completely HLA-mismatched. Monoclonal antibody combinations that in particular included the anti-CD86 mAb led to a strong inhibition of proliferation (Figure1A) and a concordant reduction in IL-2 and IFN-γ production (Figure 1B).
Blocking of CD86 inhibits the primary MLC, but additional CD40 blocking results in a declined cytotoxic response.
Indicated mAbs were added at the start of the MLC. (A) The proliferative response was determined by 3H incorporation at day 6 of the cultures. (B) The presence of IL-2 (day 3) and IFN-γ (day 6) was analyzed in culture supernatants. (C) Percentage-specific lysis of allogeneic target cells is shown, in the absence of mAbs, at different E/T ratios. Effector cells were derived from the control of mAb-blocked primary MLCs. SD < 10%. Results are expressed as mean and SE of quadruplicate (A, C) and duplicate (B) measurements. Representative experiments are shown.
Blocking of CD86 inhibits the primary MLC, but additional CD40 blocking results in a declined cytotoxic response.
Indicated mAbs were added at the start of the MLC. (A) The proliferative response was determined by 3H incorporation at day 6 of the cultures. (B) The presence of IL-2 (day 3) and IFN-γ (day 6) was analyzed in culture supernatants. (C) Percentage-specific lysis of allogeneic target cells is shown, in the absence of mAbs, at different E/T ratios. Effector cells were derived from the control of mAb-blocked primary MLCs. SD < 10%. Results are expressed as mean and SE of quadruplicate (A, C) and duplicate (B) measurements. Representative experiments are shown.
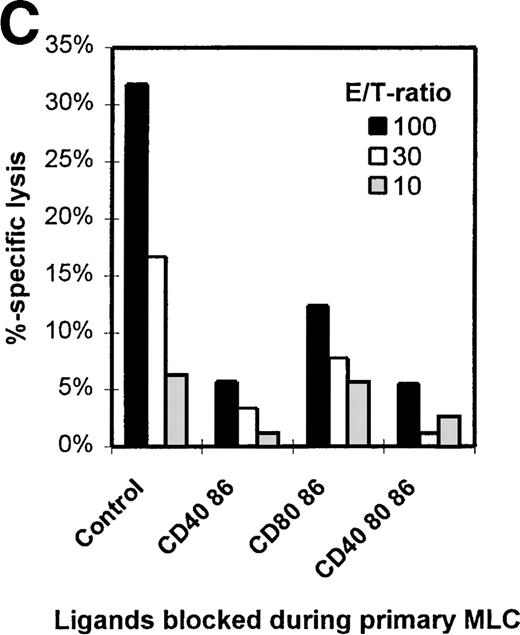
Figure 1C shows the cytotoxic response of T cells primed either in the absence (control) or presence of different mAb combinations. Clearly, mAb blocking of CD86 in the primary MLC reduces the potential to generate a profound antigen-specific cytotoxic effector response. Notably, although the cytotoxic potential was decreased with all mAb combinations, the presence of anti-CD40 led to an additional reduction of the killing capacity.
Thus, alloreactivity in heterogeneous polyclonal T-cell populations depends merely on the interaction with the CD86 costimulatory ligand because blocking of this ligand led to a strong reduction in proliferation, cytokine production, and the induction of cytotoxic effector function. This was found in all HLA combinations tested, irrespective of the degree of HLA mismatch.
Alloantigen priming in the presence of mAbs against CD86, CD40, and/or CD80 induces anergic T cells
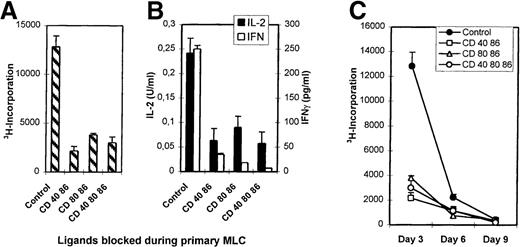
Primary MLCs were performed in the absence (control) or presence of mAb combinations directed against CD86+CD40, CD86+CD80, and CD86+CD40+CD80. Viable cells were harvested and restimulated with the original stimulator cells without mAbs (Figure2A, B). Control T cells responded with secondary proliferative kinetics (ie, maximum response at day 3 and waning in time), while T cells from the mAb-blocked MLCs were hyporesponsive (Figure 2C).The failure to proliferate was accompanied by a seriously impaired IL-2 and IFN-γ production (Figure 2B), while IL-4 and IL-10 concentrations were around detection level for both control and hyporesponsive cells. TGF-β levels were below detection level.
Priming in the presence of mAbs induces hyporesponsiveness.
Primary MLCs were performed for 7 days either in the absence (control) or presence of mAbs. Primed cells (2 × 104) were restimulated with the original allogeneic stimulator PBMCs (1 × 105). (A) Proliferative response at day 3 of culture by 3H incorporation. (B) IFN-γ and IL-2 production were analyzed in the culture supernatants. (C) Proliferation kinetics of secondary response of control and hyporesponsive T cells. Results are expressed as mean and SE of quadruplicate (A, C) and duplicate (B) measurements. Representative experiments are shown.
Priming in the presence of mAbs induces hyporesponsiveness.
Primary MLCs were performed for 7 days either in the absence (control) or presence of mAbs. Primed cells (2 × 104) were restimulated with the original allogeneic stimulator PBMCs (1 × 105). (A) Proliferative response at day 3 of culture by 3H incorporation. (B) IFN-γ and IL-2 production were analyzed in the culture supernatants. (C) Proliferation kinetics of secondary response of control and hyporesponsive T cells. Results are expressed as mean and SE of quadruplicate (A, C) and duplicate (B) measurements. Representative experiments are shown.
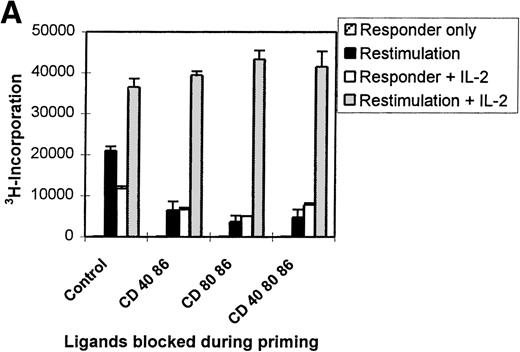
Lack of priming, possibly caused by the presence of mAbs in the primary MLC, might explain the hyporesponsiveness. To exclude this possibility, time response kinetics of the secondary MLC were performed (Figure 2C). If the T cells had been neglected during the primary MLC, restimulation would have led to proliferation with primary kinetics (ie, optimal proliferation at day 6). Restimulation of T cells from an mAb-treated MLC showed neither a secondary nor a primary response. Hyporesponsiveness was not due to deletion; antigenic restimulation in the presence of exogenously added IL-2 led to a comparable response of control and anergic T cells (Figure 3A).
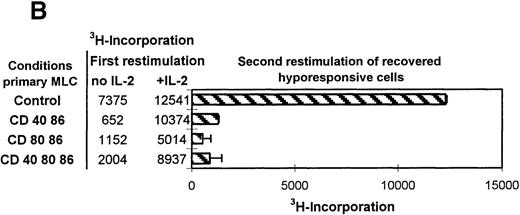
Restored hyporesponsive T cells remain anergic.
(A) Primed control or hyporesponsive T cells (2 × 104), induced by different mAb combinations, were restimulated with the original stimulator PBMCs (1 × 105) in the presence of 12.5 U/mL exogenous IL-2. The proliferative response was examined on day 3. (B) Control and hyporesponsive T cells derived from a primary MLC were recovered with antigen in the presence or absence of exogenously added IL-2 and subsequently restimulated for the second time in a tertiary MLC. The3H incorporation of this first restimulation is shown in Table 1 (SE < 10%). Next, 2 × 104 recovered cells were restimulated for a second time with 1 × 105 stimulator PBMCs in the absence of IL-2. The proliferative response was examined on day 3. Results are expressed as mean and SE of quadruplicate measurements. Representative experiments are shown.
Restored hyporesponsive T cells remain anergic.
(A) Primed control or hyporesponsive T cells (2 × 104), induced by different mAb combinations, were restimulated with the original stimulator PBMCs (1 × 105) in the presence of 12.5 U/mL exogenous IL-2. The proliferative response was examined on day 3. (B) Control and hyporesponsive T cells derived from a primary MLC were recovered with antigen in the presence or absence of exogenously added IL-2 and subsequently restimulated for the second time in a tertiary MLC. The3H incorporation of this first restimulation is shown in Table 1 (SE < 10%). Next, 2 × 104 recovered cells were restimulated for a second time with 1 × 105 stimulator PBMCs in the absence of IL-2. The proliferative response was examined on day 3. Results are expressed as mean and SE of quadruplicate measurements. Representative experiments are shown.
Thus, combined mAb blocking of the costimulatory ligands in the primary polyclonal MLC induces genuine T-cell hyporesponsiveness or anergy, which is not the result of ignorance or cell death. The 3 distinct mAb combinations led to a similar state of hyporesponsiveness.
Reversal of T-cell hyporesponsiveness by exogenous IL-2 and alloantigen does not result in anergy reversal
Various studies have described the potential of exogenously added IL-2 in recovering the proliferative response of anergic T cells in vitro.24 44-47 After tolerance induction we consequently analyzed the antigenic restimulation in the presence of added IL-2. Figure 3A shows the proliferative response of anergic T cells after antigenic restimulation either in the absence or presence of exogenously added IL-2 and the response to IL-2 alone. The presence of both IL-2 and alloantigen resulted in reversal of the hyporesponsive state, while only a residual response was observed with either antigen or IL-2 alone. This indicates that hyporesponsiveness can be restored only if the antigen and IL-2 are present at the same time. Next, we addressed the tertiary proliferative response (second restimulation) of anergic T cells that were first restored with IL-2 and alloantigen in a secondary MLC (Figure 3B). Surprisingly, these restored anergic T cells were still nonresponsive during a subsequent encounter with alloantigen in a tertiary MLC, indicating that anergy reversal did not occur. Collectively, these data show that anergy is maintained even after reversal of hyporesponsiveness by antigenic restimulation in the presence of IL-2.
The anergic state is alloantigen-specific and not dependent on the original APC source
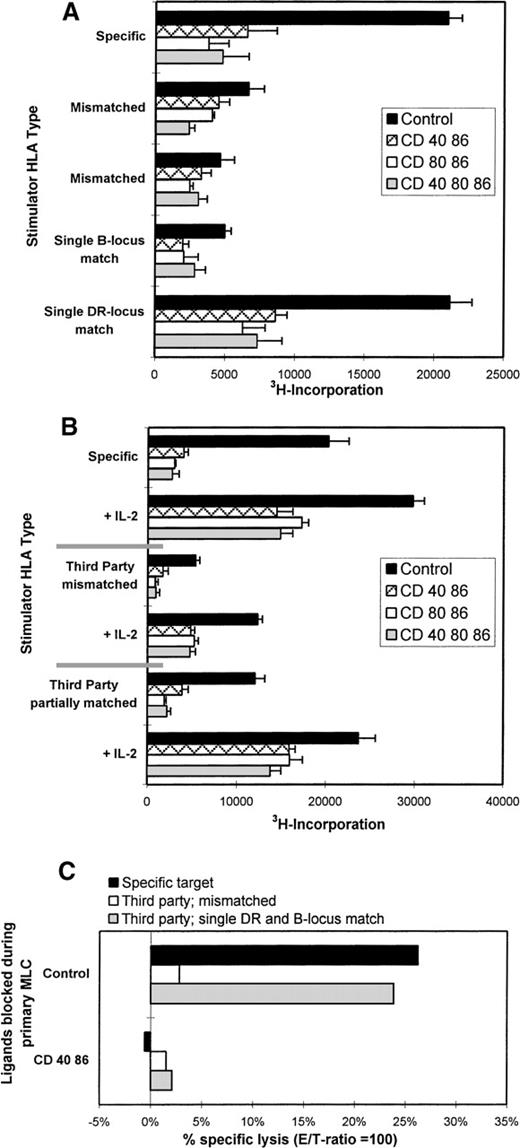
Antigen specificity of the anergic T cells was studied by restimulation experiments using third-party stimulator PBMCs that were either fully HLA-mismatched or partially HLA-matched (match of either HLA class I [B] or class II [DR]), with the stimulator PBMCs originally used for priming. Primed control cells proliferated solely when restimulated with the original PBMCs or third-party PBMCs that shared an HLA class II (DR) antigen with the original stimulators (Figure 4A). No antigen-specific reaction was found against third parties that were either completely HLA-mismatched or shared only a class I (B) locus antigen with the original stimulator cells. As expected, the anergic T cells did not respond irrespective of the third-party HLA type.
Anergic T cells are alloantigen-specific.
(A) Control or hyporesponsive T cells (2 × 104) were restimulated with 1 × 105 specific or third-party stimulator PBMCs that were either completely mismatched or partially matched, ie, in this case, DRB1*0401 (DR4) and B*4901 (B49). (B) IL-2 restored the proliferative response of anergic T cells solely when antigenic restimulation was performed with the specific stimulator PBMCs or the third-party stimulator PBMCs with a shared DR type. Results show the mean 3H incorporation and SE of quadruplicate measurements on day 3 of culture. (C) The cytotoxic response is HLA class I specific. Specific lysis (E/T = 100) is shown against allogeneic target cells, which are either completely mismatched (white bars) or shared MHC antigens (crossed bars) against which the responder cells were generated (here B49 and DR4). Results are expressed as the percentage of specific lysis. Representative experiments are shown.
Anergic T cells are alloantigen-specific.
(A) Control or hyporesponsive T cells (2 × 104) were restimulated with 1 × 105 specific or third-party stimulator PBMCs that were either completely mismatched or partially matched, ie, in this case, DRB1*0401 (DR4) and B*4901 (B49). (B) IL-2 restored the proliferative response of anergic T cells solely when antigenic restimulation was performed with the specific stimulator PBMCs or the third-party stimulator PBMCs with a shared DR type. Results show the mean 3H incorporation and SE of quadruplicate measurements on day 3 of culture. (C) The cytotoxic response is HLA class I specific. Specific lysis (E/T = 100) is shown against allogeneic target cells, which are either completely mismatched (white bars) or shared MHC antigens (crossed bars) against which the responder cells were generated (here B49 and DR4). Results are expressed as the percentage of specific lysis. Representative experiments are shown.
To prove the antigen specificity of the anergic T cells, they were antigenically restimulated in the presence of exogenous IL-2 (Figure4B) as described previously. Hyporesponsiveness was restored solely when antigenic restimulation, in the presence of IL-2, was performed with either the original or third-party stimulator PBMCs with a shared HLA class II type. Thus, anergic T cells were shown to be alloantigen-specific by restimulation with selected third-party stimulator PBMCs expressing the appropriate target HLA in the presence of IL-2. The responsiveness of T cells toward third-party stimulator cells that share antigenic determinants with the original stimulator cell is known as linked recognition.11,12,17,18 The fact that this phenomenon was observed only for the DR locus match and not for the B supports the notion that proliferative and cytokine responses in a MLC are mainly driven by class II.48
Whereas HLA class II mismatches play a major role in proliferation and cytokine production in our experimental setup, the generation of cytotoxic effector T cells appeared to be exclusively induced against HLA class I mismatched antigens, and no cytotoxic response against isolated HLA class II molecules was observed (data not shown). To elucidate the antigen specificity of tolerized cytotoxic T cells, the T cells were tested for their ability to kill third-party target cells that were either completely mismatched or partially matched for HLA class I. Target cells matched by HLA class I were lysed by the alloprimed control T cells, while effector T cells from an mAb-blocked MLC left them untouched. Completely mismatched third-party targets were affected by neither control nor anergic cells (Figure 4C). Together these cytotoxicity data indicate that priming in the presence of mAb blocking results in disabled HLA class I specific cytotoxic T-cell function.
Immunoregulation of anergic T cells via linked recognition
The capacity of anergic T cells to affect a specific alloimmune response was studied in an in vitro coculture MLC; anergic cells were cultured together with a newly performed primary MLC. Previously, we reported on the kinetics of cocultures and showed that this type of coculture has to be performed with low numbers of irradiated anergic cells.42 Furthermore, relative suppression was compared with cocultures of irradiated primed control cells.
Anergic T cells were generated in the primary MLC according to the different mAb tolerizing regimens described above. Figure5A shows the effect of coculturing anergic cells. The relative suppressive effect of the cocultures evoked by the anergic cells was compared with that of cocultures with added irradiated control cells (Table1). Although different levels in suppression were found, sometimes up to 60% of inhibition was found in the following ratio: responder PBMCs:stimulator PBMCs:anergic T cells (5:5:0.5 or 5:2.5:0.5). In general, the suppressive effect became more apparent when the number of stimulator cells was decreased, which suggests the importance of competition between anergic T cells and the responder T cells for antigenic determinants. Antigen-specificity of this suppressive phenomenon was investigated in cocultures using original responder cells and third-party stimulator cells that were either completely HLA-mismatched or shared HLA antigens with the original stimulator PBMCs (anergy-inducing antigens).
Allospecific anergic T cells suppress naive alloreactive T cells via linked recognition mediated partially by IL-10 and do not confer bystander suppression.
(A) Anergic T cells suppress their specific primary MLC, as seen in 3 different representative experiments. T-cell anergy was induced by mAb blocking in the primary MLC (see legends). Next, 5 × 103 irradiated anergic T cells were cocultured with a newly formed MLC using 5 × 104 responder PBMCs and 2.5-5 × 104 (experiments I and II, respectively) or 5-10 × 104 (experiment III) stimulator PBMCs. Control cocultures were performed with control cells that were primed and processed in a way similar to that of the anergic cells but in the absence of mAbs. (B) Anergic T cells mediate suppression via linked recognition. We cocultured 5 × 103 γ-irradiated anergic T cells with a newly incubated MLC consisting of 5 × 104 responder PBMCs and 2 and 5 × 104 γ-irradiated stimulator PBMCs. The stimulator PBMCs were either allospecific (left) or third-party PBMCs being completely HLA-mismatched (middle) or partially HLA class II matched (right) with the original stimulator cells. (C) Anergic cells do not confer bystander suppression. Cocultures were performed by adding 5 × 103 anergic or control cells to a culture of 2 × 105 responder PBMCs in the presence of 10 μg/mL tetanus toxoid or C albicans (right). As a control, these anergic and control cells were cocultured with a newly formed MLC consisting of 5 × 104 responder PBMCs and 5 × 104 γ-irradiated stimulator PBMCs (left). (D) Suppression partially depends on IL-10. Cocultures consisting of 5 × 103 anergic or control cells, 1 × 105 responder PBMCs, and 5 × 104 γ-irradiated stimulator PBMCs were performed in the presence of 5 μg/mL TGF-β and/or IL-10 neutralizing antibodies or an isotype-matched antibody. Anergic T cells used in the experiments shown in (C, D) were generated by blocking CD40 and CD86. In all figures the proliferative response is shown as the mean 3H incorporation and SE of quadruplicate measurements on day 6 (except for recall responses, which were analyzed on day 5) of the cocultures.
Allospecific anergic T cells suppress naive alloreactive T cells via linked recognition mediated partially by IL-10 and do not confer bystander suppression.
(A) Anergic T cells suppress their specific primary MLC, as seen in 3 different representative experiments. T-cell anergy was induced by mAb blocking in the primary MLC (see legends). Next, 5 × 103 irradiated anergic T cells were cocultured with a newly formed MLC using 5 × 104 responder PBMCs and 2.5-5 × 104 (experiments I and II, respectively) or 5-10 × 104 (experiment III) stimulator PBMCs. Control cocultures were performed with control cells that were primed and processed in a way similar to that of the anergic cells but in the absence of mAbs. (B) Anergic T cells mediate suppression via linked recognition. We cocultured 5 × 103 γ-irradiated anergic T cells with a newly incubated MLC consisting of 5 × 104 responder PBMCs and 2 and 5 × 104 γ-irradiated stimulator PBMCs. The stimulator PBMCs were either allospecific (left) or third-party PBMCs being completely HLA-mismatched (middle) or partially HLA class II matched (right) with the original stimulator cells. (C) Anergic cells do not confer bystander suppression. Cocultures were performed by adding 5 × 103 anergic or control cells to a culture of 2 × 105 responder PBMCs in the presence of 10 μg/mL tetanus toxoid or C albicans (right). As a control, these anergic and control cells were cocultured with a newly formed MLC consisting of 5 × 104 responder PBMCs and 5 × 104 γ-irradiated stimulator PBMCs (left). (D) Suppression partially depends on IL-10. Cocultures consisting of 5 × 103 anergic or control cells, 1 × 105 responder PBMCs, and 5 × 104 γ-irradiated stimulator PBMCs were performed in the presence of 5 μg/mL TGF-β and/or IL-10 neutralizing antibodies or an isotype-matched antibody. Anergic T cells used in the experiments shown in (C, D) were generated by blocking CD40 and CD86. In all figures the proliferative response is shown as the mean 3H incorporation and SE of quadruplicate measurements on day 6 (except for recall responses, which were analyzed on day 5) of the cocultures.
Relative suppression of alloreactive T cells by anergic T cells
| Exp . | Stimulator PBMCs no., ×104 . | Anergic cells derived from MLCs blocked for ligands . | ||
|---|---|---|---|---|
| CD40 + CD86, % . | CD80 + CD86, % . | CD40 + CD80 + CD86, % . | ||
| I | 5 | 30 | 41 | 40 |
| 2.5 | 58 | 30 | 59 | |
| II | 5 | 44 | 22 | 65 |
| 2.5 | 44 | 16 | 45 | |
| III | 10 | 21 | 14 | 29 |
| 5 | 36 | 20 | 29 | |
| Exp . | Stimulator PBMCs no., ×104 . | Anergic cells derived from MLCs blocked for ligands . | ||
|---|---|---|---|---|
| CD40 + CD86, % . | CD80 + CD86, % . | CD40 + CD80 + CD86, % . | ||
| I | 5 | 30 | 41 | 40 |
| 2.5 | 58 | 30 | 59 | |
| II | 5 | 44 | 22 | 65 |
| 2.5 | 44 | 16 | 45 | |
| III | 10 | 21 | 14 | 29 |
| 5 | 36 | 20 | 29 | |
Figure 5B shows that only the partially matched third-party MLCs were suppressed to a similar level as the original specific MLC, whereas the completely mismatched third-party MLCs were hardly affected. In the described polyclonal system, this implies that recognition of the specific target antigen on the surface of a third-party APC by these anergic T cells enables them to suppress the primary reaction of neighboring T cells. Thereby distinct alloantigens are recognized, provided that these are present on the same APCs as the anergy-inducing antigens. This mechanism of immunoregulation has been referred to as linked suppression.11,12,17 18 To exclude the fact that bystander suppression occurred, recall responses against tetanus toxoid and C albicans were studied in the presence of either anergic or control T cells. The proliferative response against these antigens was left unaffected by the anergic T cells (Figure 5C). This indicates that anergic allospecific T cells do not interfere in a nonspecific manner in self-MHC–restricted T-cell responses.
IL-10 and TGF-β have been identified important immunosuppressive cytokines.9,10 14 To elucidate the role of these cytokines, neutralizing antibodies against IL-10 and/or TGF-β were added to the cocultures of naive alloreactive cells, stimulator PBMCs, and anergic or control T cells. In contrast to anti-TGFβ, anti–IL-10 antibodies partially prevented suppression by the anergic T cells (Figure 5D). The addition of anti–TGF-β together with anti–IL-10 antibodies did not affect the level of suppression caused by IL-10 alone (Figure 5D). This indicates that IL-10, but not TGF-β, might play a role in the suppression by the anergic T cells. In some control experiments, anti–IL-10, anti–TGF-β+IL-10, or isotype-matched control antibodies themselves led to a small reduction of the proliferative response.
Collectively, these coculture data show that anergic T cells generated in the primary polyclonal MLCs by mAb blocking of costimulatory ligands are able to suppress alloreactive T-cell responses directed to multiple alloantigens, provided these antigens are coexpressed on the same APCs as the anergy-inducing antigens. Suppression was at least partially mediated by IL-10, and nonspecific bystander suppression was not observed. The distinct mAb-tolerizing regimens yielded T cells with comparable suppressive capacity, as measured in our system.
Phenotypical analysis of tolerized T cells
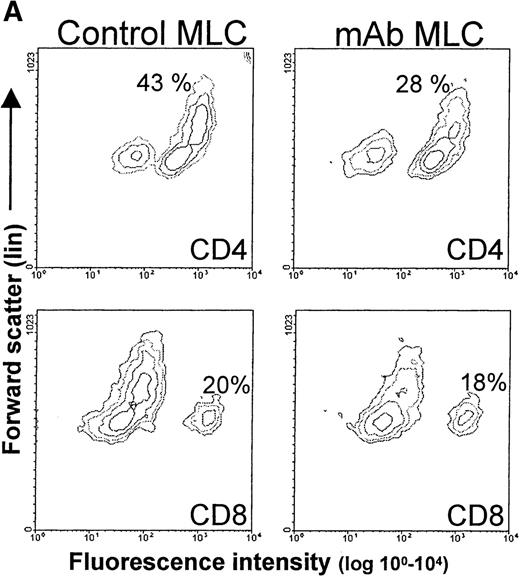
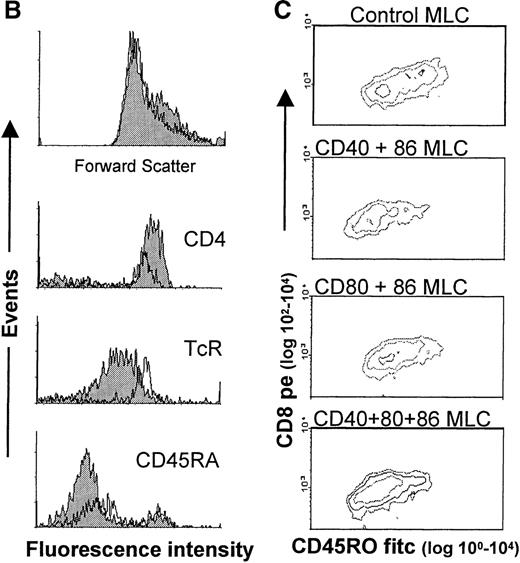
T cells derived from either control or mAb-blocked MLCs were phenotypically analyzed by flow cytometry. Cells were harvested after 7 days of culture, allowed to recuperate for 2 days, and analyzed. From a primary control MLC, 2 clear CD4+/CD3+ T-cell populations emerged with distinct forward scatter characteristics (Figure 6A, B). A similar distribution pattern was found after mAb blocking, and no difference was observed between the different mAb-blocking combinations. However, the blast-like large-sized CD4+ T-cell population had markedly reduced cell numbers (Figure 6A). Notably, the large-sized T cells from the mAb-blocked MLC showed an increase in TcR expression and a decrease in CD4 expression (Figure 6B) as compared with large-sized T cells from a control MLC. Irrespective of the presence or absence of mAbs in the primary MLC, the majority of the blast-like CD4+ T-cell population was CD45RO+, but in the case of the mAb-blocked MLC, a clear relative increase in CD45RA+ large-sized CD4+ cells was observed (Figure 6B). Large-sized CD4+ T cells from both the control and mAb-blocked MLCs were HLA class II positive, and they generally expressed CD25 to similar levels (not shown).
Phenotypical analysis of tolerized T-cell populations.
After a 7-day primary MLC in the absence or presence of mAbs, the responder lymphocytes were harvested, allowed to rest, and analyzed by flow cytometry. (A) Contour plots showing forward scatter (linear scale) and either CD4 (upper 2 panels) or CD8 (lower 2 panels) expression (fluorescence log scale) in the live lymphocyte gate. The left panel shows T cells from a control MLC, and the right panel shows T cells from an mAb-blocked MLC. (There were no observed differences between mAb combinations). The percentages in the upper 2 panels indicate the relative number of CD4+ T cells with a large blast-like appearance, while in the lower 2 panels, the total percentage of CD8+ T cells is indicated. (B) The upper panel shows the size difference in a forward scatter histogram. The 3 lower panels show the expression of CD4, TcR (WT31), and CD45RA on the large-sized backgated CD4+ T-cell population, which was derived from either a control (shaded histogram) or an mAb-blocked MLC (open histogram). The histograms show the number of events on the vertical axis (lin scale) and fluorescence intensity on the horizontal axis (log scale). (C) CD8+ cells from an MLC with simultaneous blocking of CD86 and CD40 showed a decrease in the number of CD45RO expressing cells and CD45RO intensity. Additional CD80 blocking had no effect. Cells were derived from either a control MLC or an mAb-blocked MLC, where the indicated mAbs were present. The contour plots show the fluorescence intensity of CD45RO on CD8-expressing T cells. Note that the Y axis shows a log scale from 102 to 104, which indicates that all cells shown are CD8+.
Phenotypical analysis of tolerized T-cell populations.
After a 7-day primary MLC in the absence or presence of mAbs, the responder lymphocytes were harvested, allowed to rest, and analyzed by flow cytometry. (A) Contour plots showing forward scatter (linear scale) and either CD4 (upper 2 panels) or CD8 (lower 2 panels) expression (fluorescence log scale) in the live lymphocyte gate. The left panel shows T cells from a control MLC, and the right panel shows T cells from an mAb-blocked MLC. (There were no observed differences between mAb combinations). The percentages in the upper 2 panels indicate the relative number of CD4+ T cells with a large blast-like appearance, while in the lower 2 panels, the total percentage of CD8+ T cells is indicated. (B) The upper panel shows the size difference in a forward scatter histogram. The 3 lower panels show the expression of CD4, TcR (WT31), and CD45RA on the large-sized backgated CD4+ T-cell population, which was derived from either a control (shaded histogram) or an mAb-blocked MLC (open histogram). The histograms show the number of events on the vertical axis (lin scale) and fluorescence intensity on the horizontal axis (log scale). (C) CD8+ cells from an MLC with simultaneous blocking of CD86 and CD40 showed a decrease in the number of CD45RO expressing cells and CD45RO intensity. Additional CD80 blocking had no effect. Cells were derived from either a control MLC or an mAb-blocked MLC, where the indicated mAbs were present. The contour plots show the fluorescence intensity of CD45RO on CD8-expressing T cells. Note that the Y axis shows a log scale from 102 to 104, which indicates that all cells shown are CD8+.
The CD8+ population from both the control and mAb-blocked MLCs comprised 1 uniformly sized population, with similar CD8+ cell numbers and CD8 expression levels (Figure 6A). CD8+ cells derived from an MLC blocked with anti–CD86+CD40 or anti–CD86+CD40+CD80 showed a reduced number (and expression) of CD45RO+/CD8+ T cells, which was not observed in the CD8+ T cells from the CD80+CD86 blocked MLC (Figure 1C). This indicates the importance of the CD154-CD40 interaction in the activation of CD8+ T cells and might explain the relatively higher cytotoxic response of T cells derived from a CD80+CD86 MLC as compared with T cells from an mAb-blocked MLC where CD40 was also blocked (Figure 1C). These data support an important role for the CD154-CD40 pathway in the generation of cytotoxic effector function in the human polyclonal MLC, as was recently demonstrated in murine models.49-51
In addition, phenotypical analysis excluded the presence of putatively tolerizing APCs (ie, the presence of B cells or monocytes with cell-surface bound anti-CD80, anti-CD86, and/or anti-CD40 mAbs) that might interfere during restimulation and would inadvertently lead to the observed hyporesponsiveness (data not shown).
Discussion
Alloantigen-specific immunosuppression is one of the main goals in preventing graft rejection. Here we demonstrate the ex vivo generation of anergic allospecific T cells from a primary polyclonal MLC by mAb blocking of CD86, CD40, and/or CD80. These anergic T cells have an antigen-specific immunoregulatory function because they are able to suppress the response of naive alloreactive T cells via linked recognition. Importantly, although hyporesponsiveness of these T cells was recovered by antigenic restimulation with exogenous IL-2, this did not extend to anergy reversal; nonresponsiveness was still observed in a tertiary MLC, implying that anergy was maintained. Anergic immunoregulatory T cells generated by this anti–CD86+CD40–based tolerizing protocol might be a putative tool for antigen-specific adoptive immunotherapy in transplant medicine.15
During the anergy induction phase, there was a strong inhibition of the primary MLC, especially by anti-CD86. This dominant effect, sorted on proliferation and cytokine production, might be explained by the constitutive expression of CD86 on the majority of APCs. Activation through costimulatory ligands also appeared to be essential for the induction of allospecific cytotoxic effector T-cell function because combined mAb blocking of both CD86 and CD40 in the primary MLC resulted in a strongly affected cytotoxic response. This inability was not the result of differences in CD8+ T-cell numbers, but rather it reflects an intrinsic defect caused either directly or indirectly by the lack of help. Moreover, although minimal cytolytic activity was found after anti-CD86 blocking of the primary MLC, the cytotoxic response was even more attenuated after additional blocking with anti-CD40 mAb. This fits the notion that the CD40-CD154 pathway actively contributes to the induction of cytotoxic effector function.49-51 Of interest here is the change observed in CD45RO expression in the CD8+ population; CD8+cells from a CD40+CD86–blocked MLC clearly showed a decreased number of CD45RO+ cells, which suggests that CD40-CD154 ligation delivers an important signal for differentiation into cytotoxic effector cells.
The large-sized, blast-like CD4+ T cells derived from the mAb-blocked MLCs all expressed the CD45RO marker, indicating that activation occurred. In contrast to the CD4+ blastoid cells from the control MLC, blast-like CD4+ T cells from the tolerized MLCs had higher CD45RA numbers, which might be characteristic of anergized cells in our model. These large-sized tolerized CD4+ T cells are indulged to spot antigen, as judged by their increased TcR expression. At the same time, however, the decrease in CD4 coreceptor density might result in the loss of proliferation. This is reminiscent of the data described by Madrenas et al52 showing that partial activation is the result of inefficient CD4 recruitment to the TcR.
Recovery of the proliferative response of anergic T cells by exogenously added IL-2 was previously demonstrated in distinct experimental settings, albeit using 2 distinct approaches. Either anergic T cells were restimulated in the presence of IL-2,24,44 as in our experiments, or alternatively, anergic T cells were first left in culture medium with exogenously added IL-2 only and subsequently restimulated.45,46 We show that hyporesponsive T cells were recovered by antigenic restimulation in the presence of IL-2. This, however, did not lead to reversal of the anergic state as such because a subsequent antigenic restimulation of these recovered cells in a tertiary MLC still left these cells nonresponsive, and an anergy persisted. This particular characteristic was also found in a T cell to T cell (T-T) presentation model,53 revealing the importance of the absence of costimulation in this type of anergy.
The implications of this recovery-sensitive persistent anergy for the in vivo situation are as yet speculative, but it might serve the purpose of specific tolerance induction after transplantation. After the initial period of trauma, the transplanted organ itself could serve as a source to maintain tolerance, and there would be little risk of reversing the anergic state of circulating anergic allospecific T cells upon encounter with IL-2 far from the site of transplantation (in the absence of antigen). The proliferative capacity of the anergic cells could be reestablished only if local inflammation occurs and IL-2 is produced at the transplant site. However, because these cells remain dependent on IL-2, suppression of this particular cytokine would restore the tolerant state.
It is difficult to demonstrate antigen specificity of anergic T cells in a polyclonal MLC with stimulator cells carrying many potential target antigens. To circumvent this problem we used the characteristics of anergic cells to recover their response upon antigenic restimulation in the presence of exogenously added IL-2. We reasoned that if the anergy is antigen-specific, this state can be recovered only by third-party stimulator PBMCs that share a specific HLA with the stimulator cells which were used for anergy induction. In our experimental system it appeared that the proliferative response was directed against class II and not class I antigens, and consequently that anergy was induced against the former. This is not entirely surprising because we and others48 have obtained evidence that proliferation and cytokine production in a primary MLC are mainly driven by the class II antigens. An isolated class I mismatch appears to be an insufficient trigger for a substantial response to take place during a standard 7-day MLC.
Recently, it was shown that in vivo anergized T cells displayed a phenotype of regulatory cells which were not able to proliferate. Nevertheless, the cells produced high levels of IL-10 after in vitro stimulation.54 Others also showed that IL-10 and TGF-β were generated in anergic or regulatory T-cell subsets.10,14,39 55 In our model we did not observe elevated IL-10 or TGF-β production after restimulation of the anergic T cells. This is probably the consequence of the limited number of anergic cells per well due to the heterogeneous cellular composition in our polyclonal system. In fact only a small percentage of the T cells present will have specificity for the stimulator cells used, and therefore the bulk will be left untouched. However, IL-10 did play a role in suppression; neutralizing IL-10 antibody counteracted the suppression caused by the anergic T cells. Apparently very small amounts of IL-10, which confer a suppressive function in the microenvironment comprising anergic T cells, naive alloreactive T cells, and stimulator PBMCs, are produced.
Nonspecific suppression in a system like this can occur in several ways. Previously we have reported on the kinetics of this in vitro coculture model. It appeared that the addition of more than 1 × 104 control cells (live or irradiated) prohibited the response of the responding cells.42Irradiation proved necessary because primed T cells respond with secondary kinetics upon subsequent encounter with the antigen and thus prohibit a newly cultured primary MLC simply by consuming culture nutrients and overcrowding. Consequently, to detect an immunoregulatory phenomenon in a primary MLC, small numbers of irradiated anergic or control cells were cocultured with freshly isolated responder and irradiated stimulator cells. Others40 demonstrated suppression of primary MLCs with large numbers of nonirradiated anergic T cells, and their anergic T cells:responder PBMCs:stimulator PBMCs ratio had a setting of 3:1:1 × 105 compared with our setting of 5 × 103:5 × 104:2.5-5 × 104. This raises questions about the mechanism and specificity of the suppression described by this group.40 In addition, even when using small numbers, we and others56 observed that control T cells added to a culture can affect the response of naive T cells in a nonspecific manner, therefore the antigen-specific immunoregulatory effect can only be deduced after comparison with appropriate control cultures. Consequently, we compared cocultures of anergic cells with antigen-primed control T cells and showed that HLA-specific suppression occurs. The anergic T cells suppressed naive responder T cells directed toward third-party HLAs only when third-party stimulators shared an HLA with the stimulator PBMCs used for anergy induction. This antigen-specific manner of suppression has previously been shown to occur locally via linked recognition,11,12,17,18 and it implies that suppression will occur when direct contact is obtained between T cells of different specificities.9,13 Lombardi et al9 showed that local competition for the antigen binding site might be one of the suppressive mechanisms; indeed reduction of stimulator APC numbers in our study led to an increase in suppression in most cocultures. Furthermore, specificity of the response in this kind of system is not always easy to confirm because linked recognition might also lead to third-party tolerance via minor antigens.17
Finally, from our model we hypothesize that blocking the CD86-CD28 pathway induces anergy in the CD4+ T-cell subset, which as a consequence provides insufficient cytokine mediated help for complete activation of CD8+ cytotoxic effector T cells. The CD40-CD154 interaction predominantly controls the activation of CD8+ cytotoxic T cells in a direct way because the cytolytic response was attenuated by mAb blocking of CD40.
Acknowledgment
We are indebted to Alwin Scharstuhl for analyzing TGF-β.
Reprints:Irma Joosten, Department for Blood Transfusion and Transplantation Immunology/OV603, University Medical Center, St Radboud, 6500 HB Nijmegen, The Netherlands; e-mail:i.joosten@utdts.azn.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal