Abstract
Selective eosinophil accumulation is a hallmark of diseases such as asthma. In a model of chronic eosinophilic inflammation, we have previously shown that the tethering step in eosinophil adhesion is mediated by PSGL-1 binding to P-selectin. The Th2-associated cytokine IL-13 is of potential importance in allergic disease. We have therefore investigated whether IL-13 can mediate eosinophil binding to human umbilical vein endothelial cells (HUVEC) through P-selectin. IL-13 caused dose- and time-dependent increases of P-selectin expression, as assessed by flow and laser scanning cytometry. A similar degree of expression was observed with IL-4. There was no effect on E-selectin or ICAM-1 expression. Tumor necrosis factor- induced the expression of VCAM-1, E-selectin, and ICAM-1 but had no effect on P-selectin expression. IL-13 increased the production of mRNA for surface and soluble variants of P-selectin. Under flow conditions, eosinophils, but not neutrophils, showed enhanced binding to IL-13 and to IL-4–stimulated HUVEC compared to medium-cultured cells. Eosinophil adhesion was completely inhibited by a blocking monoclonal antibody against PSGL-1 and P-selectin. Anti–VLA-4 and anti–VCAM-1 antibodies inhibited binding to a lesser extent. Thus, at physiologic levels of expression induced by Th2 cytokines, P-selectin/PSGL-1 supported eosinophil but not neutrophil adhesion. This mechanism is likely to be a key event leading to the selective accumulation of eosinophils in allergic inflammation.
Eosinophils are important effector cells in a number of allergic conditions, including asthma, allergic rhinitis, and eczema, and in related diseases such as nasal polyposis.1 A feature of these diseases is the selective accumulation of eosinophils, which represent only a small proportion of the peripheral white blood cell pool, without concomitant neutrophil influx. Understanding the mechanisms of preferential eosinophil migration to tissue may provide new targets for more selective therapeutic intervention for diseases such as asthma without the risk of immunosuppression.2Possible mechanisms for the selective eosinophil recruitment that have been investigated are the adhesion pathways used by eosinophils but not by neutrophils. The pathway that has received most attention in this regard is VLA-4 binding to endothelial VCAM-1.3,4 VLA-4 is not expressed by human neutrophils under physiological conditions, and VCAM-1 expression is induced by the Th2 cytokines IL-4 and IL-13, though expression is marginal unless tumor necrosis factor (TNF)-α is included as a costimulant.5,6 Eosinophils have been shown to transmigrate through IL-4– and IL-13–stimulated endothelium in a VLA-4–dependent manner.7 In addition, numerous studies have demonstrated that anti–VLA-4 and anti–VCAM-1 monoclonal antibodies (mAbs) are effective at blocking eosinophil migration to tissue using various animal models of eosinophilic migration.8-10 Using nasal polyps as a model of chronic eosinophilic inflammation, we found that eosinophil and neutrophil adhesion to nasal polyp endothelium was heavily dependent on binding through P-selectin, which was well expressed on nasal polyp endothelium.11 Interestingly, in this model as many as 10 times more eosinophils than neutrophils were able to adhere, suggesting a selective adhesion pathway. One possible explanation for this observation was the ability of eosinophils to bind with greater avidity to low concentrations of P-selectin under flow conditions.4 12
In resting human umbilical vein endothelial cells (HUVEC), P-selectin is localized to intracellular Weibel–Palade bodies and is only transiently expressed on the cell surface after stimulation with secretagogues such as thrombin and histamine.13 This led to the hypothesis that its principal role was to capture neutrophils during the immediate phase of cell recruitment.14 This was supported by the observation that cytokines such as TNF-α, IL-1, interferon-γ and lipopolysaccharide, which induce the expression of other cell adhesion molecules by the up-regulation of rapid-response genes, have no effect on P-selectin expression in humans, though importantly these cytokines do increase P-selectin expression in mice.15,16 However, IL-4 was shown to cause a marked increase in P-selectin mRNA and intracellular protein expression but only a modest increase in surface expression.17 The Th2 cytokine IL-13 shares with IL-4 the IL-4Rα chain as a receptor component,18,19 and its effects are also mediated by the signal-transducing molecule Stat6.20 IL-13 is expressed in eosinophilic inflammation regardless of whether this is related to atopy, whereas IL-4 expression is generally associated only with diseases in which IgE is raised.21
In addition to using flow cytometry to detect P-selectin expression by HUVEC, we used laser-scanning cytometry (LSC) to overcome the problem of having to trypsinize the cells. LSC represents a novel hybrid tool for the cytometric analysis of cells that are found on a microscope slide.22 It uses a 488-nm argon laser beam directed through the optics of an Olympus microscope (Melville, NY) and scans cells that are passed through this laser spotlight by a high-precision stepper motor. Fluorescence-labeled cells are excited within the wavelength of the laser, and the emitted light is transmitted back by the same optical path to photomultiplier tubes. Because this allows for the scanning of even densely packed cells, we postulated that this instrument would be ideally suited for the analysis of adhesion receptor expression by confluent human endothelial monolayers grown in chamber slides.
We therefore investigated using flow cytometry and a novel application of LSC whether IL-13 could induce granular, luminal, and mRNA P-selectin expression and whether the amount of surface expression induced by IL-13 was sufficient to support eosinophil adhesion. We found that IL-13, as well as IL-4, selectively induced P-selectin expression at levels that were able to support eosinophil but not neutrophil adhesion. VLA-4/VCAM-1 played a contributory role in supporting adhesion by these cytokines. Thus, PSGL-1/P–selectin mediates an important pathway of selective eosinophil recruitment in Th2-type inflammatory processes.
Materials and methods
Reagents and antibodies
Mouse IgG1-isotype–matched control antibodies (DAKO, Eli, UK) were used in all immunolabeling experiments. For fluorescence-activated cell sorter (FACS) experiments, we used mAbs RUU-SP1.18 (α-P–selectin) (gift from H. K. Nieuwenhuis, University Hospital Utrecht, Utrecht, The Netherlands). BBA-3 (α-ICAM-1) and BBA-5 (α-VCAM-1) were purchased from R&D Systems (Abingdon, UK), and EN-4 (α-endothelial cell) was obtained from Bradsure Biologicals (Loughborough, UK). Rabbit-α-mouse fluorescein isothiocyanate-conjugated secondary antibodies were from DAKO. For parallel flow assays with blocking antibodies, BBA30 (R&D Systems) or G1 (gift from R. McEver, University of Oklahoma, Oklahoma City, OK) and 4B9 (gift from R. Lobb, Biogen, Cambridge, MA) were used to block P-selectin and VCAM-1, respectively. PL-1 (gift from R. McEver) and HP1.2 (gift from F. Sanchez–Madrid, Servicio de Immunologica, Madrid, Spain) were used to block PSGL-1 and VLA-4, respectively. Recombinant human cytokines IL-4 and IL-13 were obtained from R&D Systems.
Cell culture
HUVEC were extracted and pooled from umbilical cords by using collagenase treatment as described previously.23 Primary cultures were established at 37°C in 5% CO2 in 25-cm2 Falcon tissue culture flasks (Becton Dickinson, Cowley, UK) in endothelial cell basal medium (Clonetics; BioWhittaker, Wokingham, UK) supplemented with 2% fetal calf serum, human endothelial growth factor (10 μg/mL), bovine brain extract (12 μg/mL), and antibiotics (gentamicin 50 μg/mL and amphotericin-B 50 ng/mL (all Clonetics). Cells were passaged using 0.25% trypsin and EDTA 0.02% (Life Technologies, Paisley, UK) into 6-well tissue culture vessels (Becton Dickinson) for FACS and RNA experiments and 0.1% plasma fibronectin (Sigma Aldrich, Poole, UK)-coated Nunc LabTek II chamber slides (Life Technologies, Rockville, MD) for LSC and parallel flow chamber assays. Confluent first-passage cells were used in all experiments.
FACS analysis
Confluent first-passage HUVEC were incubated for variable time periods in the presence of IL-4 (0, 0.2, 2, and 20 ng/mL), IL-13 (0, 0.5, 5, 5, and 50 ng/mL) or medium alone. Cells were harvested using trypsin (0.4%) and EDTA (0.02%; Life Technologies) and transferred to FACS analysis tubes for antibody labeling on ice. Cells were labeled for 10 minutes at optimized concentrations with primary and secondary antibodies, respectively, and washed with phosphate-buffered saline and 0.1% bovine serum albumin. Using a FACScan (Becton Dickinson) flow cytometer, HUVEC were gated using forward- and side-scatter settings that were optimized using EN4 anti-endothelial–specific fluorescence. Only viable cells (typically more than 95%), as determined by propidium iodide exclusion, were analyzed. Specific median fluorescence was determined after the subtraction of fluorescence for isotype-matched control antibody.
Immunostaining for laser scanning cytometry
After incubation for 48 hours in the presence of cytokines or medium alone and after stimulation with histamine 10−5 mol/L (Sigma Aldrich) for 10 minutes, the monolayers were fixed with paraformaldehyde 4% and washed twice. Cells were incubated for 60 minutes with first mAb (α-P–selectin or isotype-matched control mAb). After 2 further washes, cells were incubated for 30 minutes with Oregon green-conjugated rabbit-α-mouse second mAb (Molecular Probes Europe BV, Leiden, The Netherlands), propidium iodide 20 μg/mL (Sigma Aldrich), and pancreatic RNase A (200 ng/mL). To allow propidium iodide to enter the cells, saponin 0.1% (Sigma Aldrich) was included during the second antibody incubation step. For experiments in which granular expression was assessed, saponin 0.1% was also included during the first antibody incubation step. By including immunostaining against von Willebrand factor (vWF) as a control—von Willebrand factor is only expressed within Weibel–Palade bodies of endothelial cells and has no significant surface expression24—it was assured that no inadvertent intracellular staining of P-selectin was detected. Only when saponin 0.1% was included with the first Ab staining buffer was significant vWF staining seen. The microscope slide was finally detached from the culture vessel, and a coverslip was applied with glycerol. Immunostaining was performed on ice, and slides were kept in the dark until analysis by LSC.
Laser scanning procedure
Differentially treated areas on the microscope slide were scanned individually by using the × 20 objective and 5-mW laser output. The LSC captures each cell by software contouring around areas of fluorescence if they are sufficiently distinct from the surrounding background. Using nuclear staining with PI as the threshold parameter, single endothelial cells were identified by their nuclear fluorescence as detected by the photomultiplier tube in the red channel. Green surface fluorescence of each endothelial cell was sampled by defining a perimeter around the nucleus where green fluorescence was quantified. The dimensions of this perimeter were set by adding a defined number of pixels to the threshold contour so that overlap of adjacent cells was avoided. Specific mean fluorescence was determined within this perimeter after the subtraction of fluorescence for the isotype-matched control, and it was corrected for mean perimeter size. Using this correction, the specific mean fluorescence value per pixel (μm2) of cell surface area was established.
Confocal microscopy
Using a Leica (Heidelberg, Germany) confocal microscope, fluorescent images of the same monolayers were obtained.
Duplex reverse transcription–polymerase chain reaction
Semiquantitative analysis of P-selectin mRNA was performed using the primer dropping method as previously described.25 HUVEC total RNA was isolated by TRIzol reagent (Life Technologies). Total RNA (2 mg) was used for first-strand cDNA synthesis. Poly A+RNA was selected using oligo-dT primers (Life Technologies) at 70°C for 10 minutes. Reverse transcription (RT) was performed at 37°C for 1 hour in 20 mL containing 0.2 mmol/L deoxynucleotide triphosphate, 10 mmol/L dithiothreitol, ribonuclease inhibitor RNasin (Promega, Southampton, UK), 1 × RT buffer, and Superscript RT (Life Technologies) overlaid with mineral oil, followed by 5 minutes at 95°C. Polymerase chain reaction (PCR) was performed using primers (Cruachem, Glasgow, UK) specific for P-selectin (5′ primer: TGC TCA GAA CTA CAT GT; 3′ primer: AGG ACT CGG GTC AAA TG) and GAPDH (5′ primer: GGG AAG CTC ACT GGC ATG GCC TTC C; 3′ primer: CAT GTG GGC CAT GAG GTC CAC CAC). The P-selectin primers were designed to coamplify both the soluble (260 bp) and the membrane-bound (380 bp) alternately spliced isoforms. Fifty-milliliter PCR reactions contained 25 pmol each primer, 0.2 mmol/L dNTP, 1 × PCR buffer, 1 U BioTaq DNA polymerase (Bioline, London, UK), template cDNA, and 1.5 mmol/L MgCl added as a “hot start” during the denaturation (94°C, 2 minutes). In duplex reactions, secondary primers were added at the appropriate cycle during the denaturation step as determined for each primer set in preliminary experiments. PCR reactions were resolved on an agarose gel, and scanned images of the bands were quantified using National Institutes of Health Image software for IBM-PC (Scion, Frederick, MD).
Eosinophil and neutrophil purification
Granulocytes were isolated from 100 mL blood from healthy donors by dextran sedimentation, slow centrifugation (200g) to remove platelets, and centrifugation on Histopaque 1083 (Sigma Aldrich) followed by negative immunomagnetic selection using CD16 Microbeads and the MACS system (Miltenyi Biotec, Bisley, UK) to purify eosinophils. Eosinophils and neutrophils had more than 98% purity as assessed by Kimura staining and more than 95% viability on trypan blue exclusion testing.
Parallel flow chamber assay
First-passage HUVEC were grown to confluence in 2-well chamber slides (Nunc) coated with 0.1% plasma fibronectin (Sigma Aldrich) and incubated in the presence of IL-4 (20 ng/mL) or IL-13 (5 ng/mL) for 48 hours. Using a parallel flow chamber (provided by M. Lawrence, University of Virginia), Perthése 0.5-mm silicone gasket (Osteotec, Christchurch, UK) and tubing-purified eosinophils or neutrophils were drawn over the monolayer by a Harvard apparatus 22-syringe pump (Edenbridge, UK). Flow rates of 1.5 mL/min (1.5 dyn/cm2) were used. Using inverted videomicroscopy (Zeiss Axiovert25, Jena, Germany), total cell accumulation was quantified by counting neutrophils and eosinophils in 15 to 20 video frames after a 2-minute interaction for each condition. For blocking experiments, eosinophils or HUVEC were preincubated for 10 minutes with specific mAb. Flow experiments were performed at room temperature unless otherwise stated.
Results
IL-4 and IL-13 cause dose- and time-dependent increases of P-selectin expression on human endothelial cells that persist for at least 48 hours
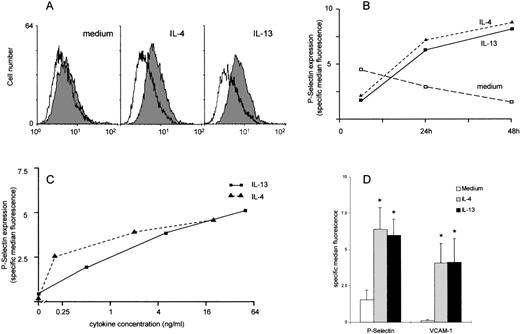
The expression of P-selectin on unstimulated HUVEC was either absent or barely detectable by flow cytometry. In contrast, both IL-4 and IL-13 induced a consistent, if modest, increase in expression of P-selectin on the surface of the endothelial cells (Figures1A and 1D). This increase was readily apparent by 24 hours and was maintained up to at least 48 hours in culture (Figure 1B). The increased P-selectin expression in response to IL-13 was selectively inhibited by anti–IL-13 antibodies (data not shown). The increase was dose dependent (Figure 1C). The increase in P-selectin expression was similar in degree to the increase in VCAM-1 expression. No differences were observed between the effects of IL-4 and those of IL-13 (Figure 1D) on either P-selectin or VCAM-1 expression. Expression of E-selectin was not detectable on IL-4, IL-13, or unstimulated HUVEC. ICAM-1 was constitutively expressed by unstimulated HUVEC, and expression was unaffected by either IL-4 or IL-13. As previously described, TNF-α induced the expression of E-selectin, VCAM-1, and ICAM-1 but did not induce P-selectin expression. TNF-α or IL-1β, in combination with IL-4 or IL-13, had no effect on P-selectin beyond that seen with IL-4 and IL-13 alone (all data not shown).
Analysis of expression of P-selectin on HUVEC by flow cytometry.
HUVEC were cultured with either medium, IL-4, or IL-13 and were stimulated for up to 48 hours. (A) Representative histogram demonstrating P-selectin expression in medium- and cytokine-treated HUVEC (shaded histogram P-selectin, open histogram mouse control immunoglobulin). (B) Time course of P-selectin expression expressed as specific median fluorescence with the mouse control immunoglobulin values subtracted. In this experiment, the low levels of P-selectin in the medium-stimulated cells declined in culture. Expression in cytokine-stimulated cells was near maximal at 24 hours and was maintained for at least 48 hours (data shown from 1 experiment representative of 2). (C) Dose response of P-selectin expression. HUVEC were stimulated for 48 hours in increasing concentrations of IL-4 or IL-13 (data shown from 1 experiment representative of 2). (D) IL-4 and IL-13 induce a significant increase in expression of both P-selectin and VCAM-1 after 48 hours in culture. Data are expressed as specific median fluorescence, n = 7; *P < .05.
Analysis of expression of P-selectin on HUVEC by flow cytometry.
HUVEC were cultured with either medium, IL-4, or IL-13 and were stimulated for up to 48 hours. (A) Representative histogram demonstrating P-selectin expression in medium- and cytokine-treated HUVEC (shaded histogram P-selectin, open histogram mouse control immunoglobulin). (B) Time course of P-selectin expression expressed as specific median fluorescence with the mouse control immunoglobulin values subtracted. In this experiment, the low levels of P-selectin in the medium-stimulated cells declined in culture. Expression in cytokine-stimulated cells was near maximal at 24 hours and was maintained for at least 48 hours (data shown from 1 experiment representative of 2). (C) Dose response of P-selectin expression. HUVEC were stimulated for 48 hours in increasing concentrations of IL-4 or IL-13 (data shown from 1 experiment representative of 2). (D) IL-4 and IL-13 induce a significant increase in expression of both P-selectin and VCAM-1 after 48 hours in culture. Data are expressed as specific median fluorescence, n = 7; *P < .05.
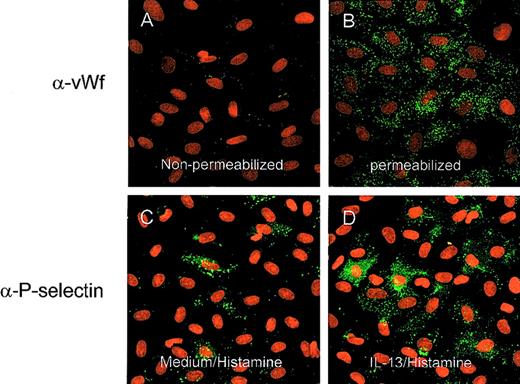
To address the possible effects of trypsinization, which we had to use for flow cytometry analysis, and to measure the transient expression seen after histamine stimulation, we investigated P-selectin expression using LSC. This is a novel technique that allows detailed, quantitative immunofluorescent analysis of cells on microscope slides. Analysis was undertaken on HUVEC cultures grown directly into chamber slides. By using a simple protocol of selective, indirect fluorescent immunostaining with reversible permeabilization and saponin in the staining buffer, we were able to distinguish surface and intracytoplasmic (total) antigen fluorescence, which we confirmed by observing differential patterns of staining with anti-vWF antibody in nonpermeabilized and permeabilized cells as illustrated by confocal microscopy (Figures 2A and 2B). We then investigated luminal staining for P-selectin after stimulation with cytokines and histamine and compared this with levels of intracellular expression. P-selectin expression on unstimulated cells was weak or absent, as seen in the flow cytometry experiments. In our hands, histamine alone had only a small effect that was barely detectable and even absent in some experiments. The addition of histamine to IL-4– and IL-13–stimulated HUVEC caused a marked increase in expression. This was illustrated visually using confocal microscopy in Figures 2C and 2D and in histogram form in Figure 3, and the specific mean fluorescence values are shown in Figure4. There was also marked increase in total P-selectin expression (measured as a combination of surface and intracellular expression in permeabilized cells) induced by IL-4 and IL-13 (Figure 4). Between one third and one half of the total amount of P-selectin in IL-4– and IL13–stimulated HUVEC was expressed on the cell surface after histamine.
Confocal images of HUVEC stained for vWF and P-selectin.
Original magnification, ×40. Staining for vWF without (A) and with (B) saponin 0.1% included with the first antibody staining buffer. Only with permeabilization was significant granular vWF staining seen. Confocal images of cells preincubated for 48 hours with medium (C) or IL-13 (50 ng/mL) after histamine (10−5 mol/L) stimulation for 10 minutes (D). A clear increase in P-selectin expression was observed.
Confocal images of HUVEC stained for vWF and P-selectin.
Original magnification, ×40. Staining for vWF without (A) and with (B) saponin 0.1% included with the first antibody staining buffer. Only with permeabilization was significant granular vWF staining seen. Confocal images of cells preincubated for 48 hours with medium (C) or IL-13 (50 ng/mL) after histamine (10−5 mol/L) stimulation for 10 minutes (D). A clear increase in P-selectin expression was observed.
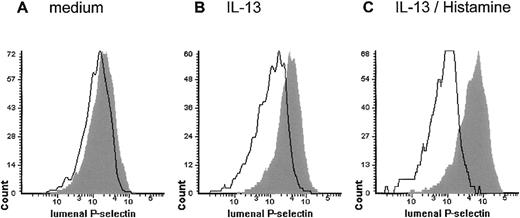
Histograms from measurement of P-selectin expression by laser scanning cytometry
to illustrate the level of P-selectin expression in HUVEC after incubation with medium (A) or IL-13 (50 ng/mL) (B) for 48 hours. Further rapid up-regulation occurs after stimulation with histamine (10−5 mol/L) for 10 minutes (C). Histograms show mean fluorescent values detected within each cell perimeter using anti-P–selectin or isotype control (outline) antibody.
Histograms from measurement of P-selectin expression by laser scanning cytometry
to illustrate the level of P-selectin expression in HUVEC after incubation with medium (A) or IL-13 (50 ng/mL) (B) for 48 hours. Further rapid up-regulation occurs after stimulation with histamine (10−5 mol/L) for 10 minutes (C). Histograms show mean fluorescent values detected within each cell perimeter using anti-P–selectin or isotype control (outline) antibody.
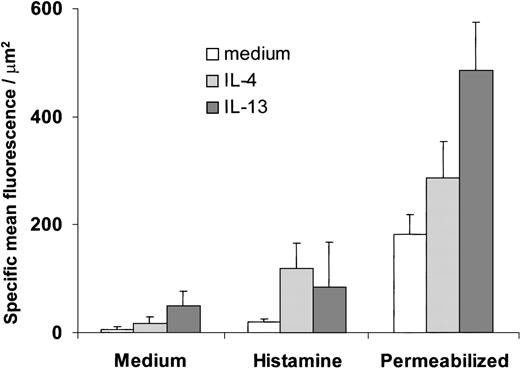
Luminal and total (luminal and intracellular) P-selectin expression measured by LSC.
Luminal P-selectin expression was determined on confluent HUVEC stimulated for 48 hours with IL-4 (20 ng/mL) or IL-13 (50 ng/mL) with or without histamine (10−5 mol/L) added for 10 minutes. Total P-selectin expression was determined for IL-4– and IL-13–stimulated HUVEC using permeabilization with saponin. Specific mean fluorescence was determined as detailed in “Materials and methods.” The data are taken from 3 to 4 independent experiments.
Luminal and total (luminal and intracellular) P-selectin expression measured by LSC.
Luminal P-selectin expression was determined on confluent HUVEC stimulated for 48 hours with IL-4 (20 ng/mL) or IL-13 (50 ng/mL) with or without histamine (10−5 mol/L) added for 10 minutes. Total P-selectin expression was determined for IL-4– and IL-13–stimulated HUVEC using permeabilization with saponin. Specific mean fluorescence was determined as detailed in “Materials and methods.” The data are taken from 3 to 4 independent experiments.
Expression of mRNA for both soluble and surface P-selectin increases in response to allergic cytokine stimulation
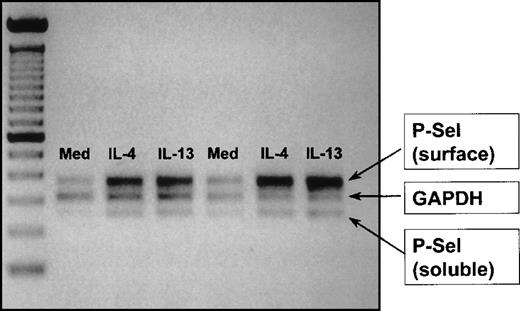
Using a semiquantitative duplex technique, we were able to demonstrate by RT-PCR that IL-13 and IL-4 increased P-selectin mRNA expression for the membrane expressed and the soluble forms of the receptor. Using image analysis for the quantification of the bands (area under the curve), an approximately 2-fold increase in the P-selectin/GAPDH ratio over unstimulated control cells was observed with each cytokine for surface and soluble P-selectin mRNA in 2 independent experiments (Figure 5).
IL-4 and IL-13 induce P-selectin mRNA expression in HUVEC.
Data were obtained using a semiquantitative RT-PCR method as described in “Materials and methods” to determine mRNA expression for soluble and membrane (surface)-bound P-selectin mRNA after 48-hour culture in medium, IL-4, and IL-13. The figure represents a digitally scanned image of the agarose gel. P-selectin mRNA expression was referenced to the housekeeping gene GAPDH. The cytokines induced an approximate doubling in both membrane and soluble P-selectin mRNA expression with no change in GAPDH expression. Data shown are from 2 independent experiments.
IL-4 and IL-13 induce P-selectin mRNA expression in HUVEC.
Data were obtained using a semiquantitative RT-PCR method as described in “Materials and methods” to determine mRNA expression for soluble and membrane (surface)-bound P-selectin mRNA after 48-hour culture in medium, IL-4, and IL-13. The figure represents a digitally scanned image of the agarose gel. P-selectin mRNA expression was referenced to the housekeeping gene GAPDH. The cytokines induced an approximate doubling in both membrane and soluble P-selectin mRNA expression with no change in GAPDH expression. Data shown are from 2 independent experiments.
Eosinophils but not neutrophils adhere to IL-4– and IL-13–stimulated human endothelial cells in flow predominantly through P-selectin/PSGL-1
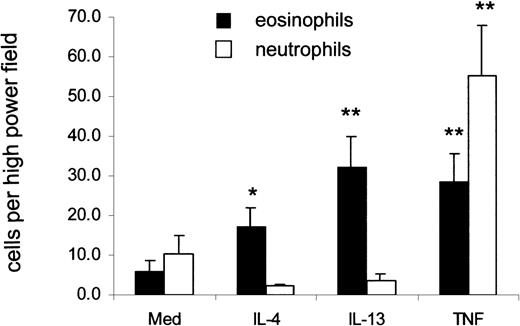
Under flow conditions, eosinophils and neutrophils adhered to untreated HUVEC in low numbers. There was a significant increase in the total number of eosinophils that accumulated after 2 minutes on IL-4– and IL-13–treated HUVEC compared with medium-treated cells (Figure6). Numbers of accumulated eosinophils were equivalent to those accumulating on TNF-α–stimulated HUVEC. There was a trend toward greater accumulation on IL-13–stimulated than on IL-4–stimulated HUVEC, though this did not reach significance. Neutrophils were unable to adhere to IL-4– or IL-13–stimulated HUVEC at shear stresses between 1.0 and 1.5 dynes/cm2. In contrast, they adhered avidly to TNF-α–stimulated HUVEC. Cells (12-20 in number) that tethered to IL-4– and IL-13–stimulated endothelium were observed for their post-tethering behavior. In all experiments, more than 90% of the adherent eosinophils continued to roll, and firm attachment was rarely observed (data not shown).
Eosinophils but not neutrophils adhere to IL-4– and IL-13–stimulated HUVEC under flow.
Eosinophil and neutrophil binding to cytokine-stimulated HUVEC under flow conditions were studied as described in “Materials and methods.” Leukocytes were allowed to bind to the endothelial cells at flow rates of 1.5 dynes/cm2 for 2 minutes, and the average number of cells bound per high-power field from 10 fields taken at random were counted. *P < .05 and **P < .01 compared to medium control; n = 4-10.
Eosinophils but not neutrophils adhere to IL-4– and IL-13–stimulated HUVEC under flow.
Eosinophil and neutrophil binding to cytokine-stimulated HUVEC under flow conditions were studied as described in “Materials and methods.” Leukocytes were allowed to bind to the endothelial cells at flow rates of 1.5 dynes/cm2 for 2 minutes, and the average number of cells bound per high-power field from 10 fields taken at random were counted. *P < .05 and **P < .01 compared to medium control; n = 4-10.
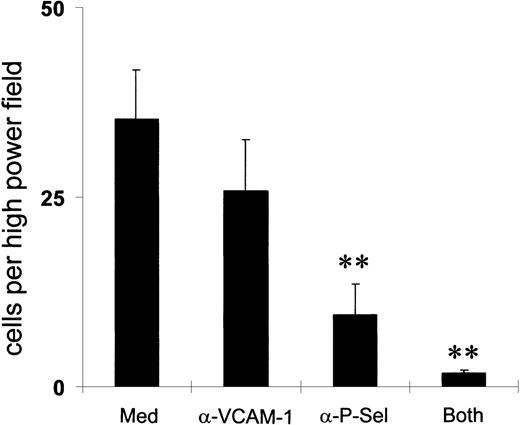
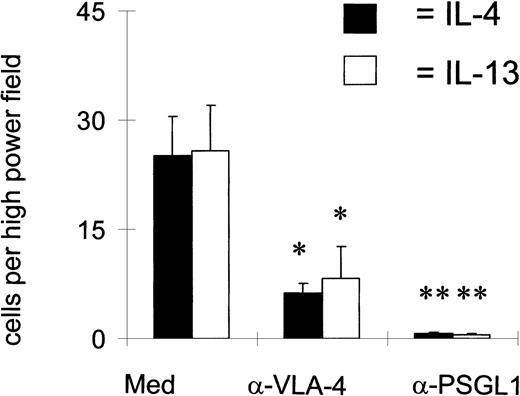
To determine which receptors mediated eosinophil binding to IL-4– and IL-13–stimulated HUVEC, we pretreated the eosinophils with blocking mAbs against PSGL-1 and VLA-4 or the endothelial cells with a blocking mAb against P-selectin and VCAM-1 (Figures 7 and8). Binding with a control IgG antibody was in the region of 25 cells per high-power field. An antibody against PSGL-1 completely ablated binding, whereas an antibody against α4 inhibited binding by approximately 60%. An antibody against P-selectin also reduced binding significantly, in contrast to the blockade of VCAM-1.
P-selectin more than VCAM-1 mediates eosinophil adherence.
Endothelial cells were preincubated with mAbs against either P-selectin or VCAM-1 for 15 minutes before interaction with peripheral blood eosinophils in a parallel flow chamber assay. Total accumulation per high-power field (× 40) after a 2-minute interaction was calculated. **P < .01; n = 5-8.
P-selectin more than VCAM-1 mediates eosinophil adherence.
Endothelial cells were preincubated with mAbs against either P-selectin or VCAM-1 for 15 minutes before interaction with peripheral blood eosinophils in a parallel flow chamber assay. Total accumulation per high-power field (× 40) after a 2-minute interaction was calculated. **P < .01; n = 5-8.
Both 4 and PSGL-1 mediate eosinophil binding.
Eosinophils were preincubated with mAbs against either α4 (HP1.2) or PSGL-1 (PL-1) for 15 minutes before they were allowed to interact with the HUVEC under flow at 1.5 dynes/cm2. After a 2-minute interaction, the number of eosinophils that bound per high-power field was calculated. *P < .05; **P < .01; n = 3.
Both 4 and PSGL-1 mediate eosinophil binding.
Eosinophils were preincubated with mAbs against either α4 (HP1.2) or PSGL-1 (PL-1) for 15 minutes before they were allowed to interact with the HUVEC under flow at 1.5 dynes/cm2. After a 2-minute interaction, the number of eosinophils that bound per high-power field was calculated. *P < .05; **P < .01; n = 3.
Discussion
One of the novel findings of this study is that the Th2 cytokine IL-13 selectively induced chronic expression of P-selectin on cultured endothelial cells, providing further evidence that this receptor is important in mediating Th2-type inflammatory responses and in particular eosinophil trafficking. Until recently, most attention regarding selective pathways of eosinophil adhesion has concentrated on the role of VLA-4 binding to VCAM-1 because this receptor is not expressed by neutrophils. Furthermore, VCAM-1 is selectively induced by IL-426 and IL-13.7 However, in the absence of costimulation with other cytokines such as TNF-α and IL-1, VCAM-1 expression on endothelial cells induced by IL-4 and IL-13 is weak or—in the case of lung microvascular endothelial cells as opposed to neonatal cells—entirely absent.27 This has called into question the importance of VLA-4/VCAM-1 interactions in diseases such as asthma, in which cytokines such as TNF-α, the generation of which is associated with systemic aspects not seen in asthma, are unlikely to feature prominently. Indeed, the expression of VCAM-1 in the upper and the lower airway in asthma, rhinitis, and nasal polyposis is generally weak,28 and we observed only a minor role for VLA-4 in eosinophil adhesion to nasal polyp endothelium.29P-selectin was initially thought only to be transiently expressed on endothelial cells, but evidence for chronic surface expression in a number of inflammatory diseases, including nasal polyposis, was supported by the observation that IL-4 could induce the prolonged expression of P-selectin on HUVEC.17 However, many patients with nasal polyposis and asthma do not have atopy, and they have low levels of IL-4 expression but increased expression of the related cytokine IL-13.30 IL-13, though ineffective in directing Th2-cell differentiation directly,31 characteristically is required for optimal IgE production in the absence of IL-4.32 Considerable and long-lasting levels of IL-13 are also produced by CD8+ cells,33 suggesting a possible role for this cytokine in viral exacerbations of asthma. IL-13 signal transduction, like that of IL-4, is mediated through the Stat6 pathway.20 These cytokines share many similar effects because of their shared signal transducing IL-4Rα chain that is a part of both IL-4 and IL-13 receptor complexes. Our finding that IL-13, like IL-4, can selectively induce P-selectin expression is important because it reinforces the evidence that P-selectin is an adhesion receptor associated with Th2-type inflammatory reactions. In nasal polyps, P-selectin, but not VCAM-1 expression, was inhibited by treatment with glucocorticoids. The reduced expression of P-selectin correlated with a reduction in the expression of IL-13.34In humans, unlike mice, the P-selectin promoter does not, in contrast to the other classical endothelial adhesion receptors, have a traditional NFκB transcription site,35 hence the lack of effect of cytokines such as TNF-α on expression. A Stat6 binding site within the P-selectin promoter region was recently demonstrated.36 The experiment, demonstrating an increase in P-selectin mRNA expression after IL-13 treatment, suggested that like IL-4 the mechanism of action is through inducing de novo transcription, presumably through activation of the Stat6 pathway. The novel observation that the expression of mRNA for the alternatively spliced soluble P-selectin was increased suggests the level of soluble P-selectin may be a marker for Th2-activated endothelial cells and may be increased in serum in diseases such as asthma.
Laser scanning cytometry was originally developed for the analysis of cancerous cells and for DNA ploidy analysis,37-39 and it has also been used for the immunophenotyping of hematologic specimens40 and asthmatic sputum cytospins.41We present here a novel method for the cytometric analysis of confluent monolayers of human endothelial cells without the need for enzymatic or mechanical detachment of these cells from their carrier surfaces and adjacent endothelial cells. Adherent cell cultures are used extensively to investigate the morphology, functional characteristics, and interactions of the diverse cellular constituents within particular tissue architecture. To analyze large numbers of cells quantitatively, cells are commonly detached from their culture vessel to allow the quantification of fluorescent and light-scatter properties of individual cells by conventional cytometry in fluid suspension. However, detachment of naturally adherent cells from their carrier surfaces and immediate cellular ambiance can significantly affect their morphology and functional properties.42-46 It was possible to selectively analyze granular and cell surface P-selectin expression by using LSC in combination with selective permeabilization procedures during indirect immunostaining. Fixation of cells in their intact culture habitat immediately after the rapid functional surface up-regulation of P-selectin with histamine permitted the detection of this evanescent phenomenon by LSC and confocal microscopy. Thus, further up-regulation of P-selectin surface expression after stimulation with histamine is presumably secondary to substantially filled granular stores after allergic cytokine stimulation. Using conventional flow cytometry, it was difficult to detect P-selectin up-regulation to the cell surface on stimulation with histamine, presumably because of the altered functional properties of the delicate granular membrane shuttle mechanism47 that carries this adhesion molecule to the cell surface after trypsinization. The ability to analyze adherent monolayers of endothelial and other cells cytometrically without further disturbance will not only preserve relevant antigens, it will facilitate kinetic studies on such cells that have thus far been fraught with difficulties.
The low levels of P-selectin expression induced by IL-4 and IL-13 made it important to determine the functional relevance of this observation. Another major finding of this study was that eosinophils, but not neutrophils, were able to bind IL-4– and IL-13–stimulated HUVEC through PSGL-1 binding to P-selectin. From studies using blocking monoclonal antibodies, it was apparent that VCAM-1 and P-selectin worked synergistically to support eosinophil adhesion. Patel et al48 recently showed the immediate arrest of eosinophils on HUVEC treated with IL-4 for 24 hours, but they did not compare eosinophil binding to neutrophil binding. We found that eosinophils rolled but did not undergo firm arrest when the experiment was performed either at room temperature or at 37°C (data not shown). The reasons for this difference are unclear but may be related to the earlier time point in that study. In mice, anti–P-selectin has been shown to block eosinophil migration to the lung in models of antigen challenge, and, in a similar model, eosinophil migration was diminished in P-selectin gene-deleted mice.49,50 Moreover, blocking the effects of IL-13 in a murine model of asthma was recently shown independently by 2 groups to reverse features of asthma.51,52 There are 2 possible explanations for the ability of eosinophils but not neutrophils to bind to IL-13–stimulated HUVEC. Either stable binding requires interaction with both P-selectin and VLA-4, or eosinophils can preferentially bind P-selectin at the low level of luminal expression induced by IL-13. Both factors may be responsible. The inhibitory effect of anti–VLA-4/VCAM-1 and anti–PSGL-1/P–selectin in this study demonstrated that the former is at least part of the explanation. We previously observed53that eosinophils bound in greater numbers than neutrophils to purified P-selectin under flow, a difference that was more obvious at the lower dilutions of P-selectin used. In addition, Kitayama et al4found differences in avidity of eosinophil and neutrophil binding to P-selectin. Patel et al54 found no difference in binding, so there remains a degree of uncertainty as to whether eosinophils can preferentially bind P-selectin. Because both IL-4 and IL-13 coordinately induce the expression of P-selectin and VCAM-1, the exact mechanism is to an extent less important than the functional outcome. Because neutrophils have been previously shown to bind to histamine-stimulated HUVEC and the levels of P-selectin expression seen with IL-13 and IL-4 using the LSC were similar to, or even greater than, expression seen after histamine stimulation, it is perhaps surprising that we did not observe neutrophil binding. However, most neutrophil adhesion to histamine-stimulated HUVEC has been undertaken using static assays or more prolonged time points than the 2 minutes we used in our flow assay.
In conclusion, we have shown that IL-13 as well as IL-4 can induce prolonged expression of P-selectin at levels of expression that support eosinophil, but not neutrophil adhesion. This offers further support for an important role for PSGL-1/P-selectin in directing preferential eosinophil adhesion to vascular endothelium in diseases such as asthma characterized by increased expression of Th2 cytokines.
Acknowledgments
We wish to thank R. McEver (University of Oklahoma, Oklahoma City), R. Lobb (Biogen Inc, Cambridge, MA), F. Sanchez-Madrid (Servicio de Immunologica, Madrid, Spain) and H. K. Nieuwenhuis (University Hospital Utrecht, Utrecht, Netherlands) for their generous antibody gifts. We are grateful to D. Ireland for her invaluable assistance with the primer design. We also wish to thank the staff of the Department of Obstetrics and Gynaecology of Leicester Royal Infirmary Hospital for their assistance with the collection of umbilical cords.
Supported by the British Lung Foundation and the National Asthma Campaign UK.
Reprints:Andrew J. Wardlaw, Department of Respiratory Medicine, Glenfield Hospital, Groby Road, Leicester LE3 9QP, UK; e-mail: aw24@le.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal