Abstract
The impact of cell dose (number of nucleated donor cells per kilogram recipient weight) on transplantation outcome is controversial and may differ for allogeneic and identical twin (syngeneic) bone marrow transplants. We studied the association between cell dose and outcome in 100 unmanipulated identical twin bone marrow transplantations for leukemia, reported to the International Bone Marrow Transplant Registry between 1985 and 1994, using Cox proportional hazards regression for multivariate analyses. Cell doses ranged from 0.3 to 7.4 × 108 nucleated cells/kg (median, 3.0 × 108cells/kg). Median follow-up was 75 months. Five-year cumulative incidences of transplant-related mortality with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses were 2% (95% confidence interval [CI], 0% to 8%) versus 10% (95% CI, 4% to 20%), respectively. Five-year probabilities of leukemia-free survival were 53% (95% CI, 39% to 67%) and 37% (95% CI, 23% to 52%), respectively. In multivariate analysis, among patients surviving in remission at least 9 months after transplantation, those receiving high cell doses were at significantly lower risk for treatment failure (relapse or death) than those receiving low cell doses (RR, 0.27; 95% CI, 0.12 to 0.6;P = .001). Lower treatment failure resulted from fewer relapses in the high cell dose group (RR for relapse, 0.28; 95% CI, 1.2 to 0.66; P = .003). These findings suggest that outcomes after syngeneic bone marrow transplantation could be improved by transplanting more than 3 × 108 nucleated cells per kilogram. The benefit of high cell dose on relapse may represent a delayed graft-versus-leukemia effect.
Bone marrow transplantations for leukemia using identical twin donors have low transplant-related mortality rates and negligible graft-versus-host disease (GVHD) but higher risks for leukemia relapse than allografts.1,2 Factors determining outcome after identical twin bone marrow transplantation may differ significantly from those determining allograft outcome because there are no allogeneic effects. They may also differ from autotransplants because the graft was not previously exposed to cytotoxic agents and is not at risk for leukemia contamination. In related3-5 and unrelated6 donor allogeneic bone marrow transplantation, low nucleated graft cell doses are associated with higher transplant-related mortality rates, with variable impacts on incidence and severity of acute GVHD. Because proportions of cell types in grafts vary, it is not possible to determine whether lymphocytes, stem cells, or both are responsible for these cell dose effects.7,8However, in a study of T-cell–depleted allografts, low CD34+ cell doses (less than 106/kg) resulted in higher early transplant-related deaths.3
In syngeneic bone marrow transplantation, cell dose is one of the few variables (other than the preparative regimen) that can be controlled. It is therefore important to determine its impact on transplantation outcome. We studied this effect in 100 syngeneic transplantations reported to the International Bone Marrow Transplant Registry (IBMTR).
Patients and methods
Patient selection
Patients receiving an identical twin bone marrow transplant for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), or chronic myelogenous leukemia (CML) between 1985 and 1994 were identified in the IBMTR research database. From 150 potentially eligible identical twin bone marrow transplants, we studied 100 patients who received a conditioning regimen of cyclophosphamide and total body irradiation (CyTBI) or busulfan and cyclophosphamide (BuCy), with no GVHD prophylaxis, and with complete data on nucleated cell dose. Twenty-seven patients were excluded because a different conditioning regimen was used, 19 because GVHD prophylaxis was given, and 4 because data on cell dose were unavailable.
Outcomes
The primary outcome was time to treatment failure, defined as time to death or relapse, the inverse of leukemia-free survival (LFS). Other outcomes analyzed were treatment-related mortality (time to death in continuous complete remission), leukemia relapse (time to onset of clinical/hematologic leukemia recurrence), survival, and cause of death.
International Bone Marrow Transplant Registry
The IBMTR is a voluntary working group of more than 350 transplant teams worldwide who contribute detailed data on their allogeneic and syngeneic blood and bone marrow transplantations to the Statistical Center at the Medical College of Wisconsin. Participants are required to report all consecutive transplantations; compliance is monitored by on-site audits. Approximately 60% of all active transplant centers report their data to the IBMTR. The IBMTR database includes 40% to 45% of all recipients of allogeneic and syngeneic transplants since 1970. Patients are followed up longitudinally. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality.
Statistical methods
Using treatment failure as the outcome, we first examined cut points of cell dose ranging from 2.5 to 3.5 × 108 nucleated cells/kg to determine the value that best discriminated between high and low cell doses. Cell dose was subsequently treated as a dichotomous variable in all analyses—greater than versus equal to or less than the cut point. Characteristics of patients receiving high versus low nucleated cell doses were compared using the chi-square statistic and the Wilcoxon rank sum test for categorical and continuous variables, respectively. Cumulative incidence rates of transplant-related mortality, leukemia relapse, and Kaplan–Meier estimates of LFS were compared using the log-rank test.9 Cox proportional hazards regression was used to examine the effect of cell dose on transplant-related mortality rate, relapse rate, and treatment failure in multivariate analyses adjusting for other significant covariates.10 Because cell dose was the main variable of interest, all models contained the dichotomous cell dose covariate (high versus low). Other factors considered in multivariate model building were disease type (AML versus ALL versus CML); disease stage (advanced [refractory or relapsed acute leukemia, blastic-phase CML] versus intermediate [acute leukemia in second or higher remission, accelerated-phase CML] versus early [acute leukemia in first remission, chronic-phase CML]); recipient and donor sex (female versus male); year of transplantation (1989-1994 versus 1985-1988); conditioning regimen (CyTBI versus BuCy); white blood cell (WBC) count at diagnosis (greater than or equal to 25 versus less than 25 × 109/L); Karnofsky score (greater than or equal to 90% versus less than or equal to 80%) and age at transplant (older than 25 versus 25 years or younger). The assumption of proportional hazards over time was tested for all explanatory covariates using a time-dependent covariate. The tests indicated that high versus low cell dose (more than 3.0 versus less than or equal to 3.0 × 108 nucleated cells/kg) had a time-varying effect on relapse and treatment failure. Various cut points were tested for the early versus late effect; the cut point giving the greatest discrimination between early and late effects was selected for regression analysis. At the optimum cut points, proportionality assumptions were again tested to validate proportionality between the high and low cell dose groups. A forward stepwise model was used to identify the factors significantly associated with outcomes. Within each model one additional significant factor was added at each step, and the remaining factors were reexamined. When all covariates significant at P = .05 were entered, model building was stopped. First-order interactions between each significant covariate and the main effect of cell dose were also examined. Adjusted probabilities of LFS were generated from the final Cox model stratified on cell dose and weighted averages of covariates using the sample proportion as the weight function. Adjusted probabilities represent predicted outcomes for similar groups of patients receiving each treatment.
Results
Patient characteristics and overall outcome
Patient characteristics are shown in Table1. In the higher cell dose group, there was a significantly greater number of transplants between female twins (P = .01) and a trend toward more advanced disease (P = .07). Body weights were somewhat lower in the high cell dose group (59 vs 67 kg; P = .05). Otherwise the 2 groups were similar.
Patient-, disease-, and transplant-related characteristics
| Variable . | Cell dose . | P . | |||
|---|---|---|---|---|---|
| N . | ≤3.0 × 108 cells/kg . | N . | >3.0 × 108 cells/kg . | ||
| Number of patients | 50 | 50 | |||
| Disease type | 50 | 50 | .48 | ||
| AML | 21 (42) | 27 (54) | |||
| ALL | 14 (28) | 11 (22) | |||
| CML | 15 (30) | 12 (24) | |||
| Disease stage before transplantation | 50 | 50 | .07 | ||
| Early | 38 (76) | 27 (54) | |||
| Intermediate | 8 (16) | 16 (32) | |||
| Advanced | 4 (8) | 7 (14) | |||
| Male sex | 50 | 36 (72) | 50 | 22 (44) | .01 |
| Age at transplantation, years | 50 | 27 (5-59) | 50 | 26 (5-59) | .81 |
| Year of transplantation | 50 | 50 | .32 | ||
| 1985-1988 | 25 (50) | 20 (40) | |||
| 1989-1994 | 25 (50) | 30 (60) | |||
| Conditioning regimen | 50 | 50 | .83 | ||
| BuCY ± other | 18 (36) | 17 (34) | |||
| CyTBI ± other | 32 (64) | 33 (66) | |||
| Patient CMV-positive | 45 | 22 (49) | 44 | 20 (45) | .75 |
| Donor CMV-positive | 44 | 18 (41) | 42 | 16 (38) | .79 |
| Karnofsky score, ≤80% | 50 | 8 (16) | 50 | 10 (20) | .60 |
| WBC at diagnosis | 46 | 18 (1-371) | 45 | 24 (1-490) | .24 |
| Neutrophil >0.5 × 109/L, median, days | 49 | 17 (9-34) | 46 | 14 (8-52) | .32* |
| Platelets >25 × 109/L, median, days | 50 | 23 (0-85) | 43 | 18 (5-248) | .15* |
| Acute GVHD | 50 | 49 | .51 | ||
| None | 46 (92) | 43 (88) | |||
| Grade I | 3 (8) | 2 (4) | |||
| Grade II | 1 (2) | 3 (6) | |||
| Grade III | 0 (0) | 1 (2) | |||
| Chronic GVHD | 48 | 48 | .21 | ||
| None | 48 (100) | 45 (94) | |||
| Limited | 0 (0) | 3 (6) | |||
| Median follow-up, months | 77.4 | 74.6 | .22* | ||
| Variable . | Cell dose . | P . | |||
|---|---|---|---|---|---|
| N . | ≤3.0 × 108 cells/kg . | N . | >3.0 × 108 cells/kg . | ||
| Number of patients | 50 | 50 | |||
| Disease type | 50 | 50 | .48 | ||
| AML | 21 (42) | 27 (54) | |||
| ALL | 14 (28) | 11 (22) | |||
| CML | 15 (30) | 12 (24) | |||
| Disease stage before transplantation | 50 | 50 | .07 | ||
| Early | 38 (76) | 27 (54) | |||
| Intermediate | 8 (16) | 16 (32) | |||
| Advanced | 4 (8) | 7 (14) | |||
| Male sex | 50 | 36 (72) | 50 | 22 (44) | .01 |
| Age at transplantation, years | 50 | 27 (5-59) | 50 | 26 (5-59) | .81 |
| Year of transplantation | 50 | 50 | .32 | ||
| 1985-1988 | 25 (50) | 20 (40) | |||
| 1989-1994 | 25 (50) | 30 (60) | |||
| Conditioning regimen | 50 | 50 | .83 | ||
| BuCY ± other | 18 (36) | 17 (34) | |||
| CyTBI ± other | 32 (64) | 33 (66) | |||
| Patient CMV-positive | 45 | 22 (49) | 44 | 20 (45) | .75 |
| Donor CMV-positive | 44 | 18 (41) | 42 | 16 (38) | .79 |
| Karnofsky score, ≤80% | 50 | 8 (16) | 50 | 10 (20) | .60 |
| WBC at diagnosis | 46 | 18 (1-371) | 45 | 24 (1-490) | .24 |
| Neutrophil >0.5 × 109/L, median, days | 49 | 17 (9-34) | 46 | 14 (8-52) | .32* |
| Platelets >25 × 109/L, median, days | 50 | 23 (0-85) | 43 | 18 (5-248) | .15* |
| Acute GVHD | 50 | 49 | .51 | ||
| None | 46 (92) | 43 (88) | |||
| Grade I | 3 (8) | 2 (4) | |||
| Grade II | 1 (2) | 3 (6) | |||
| Grade III | 0 (0) | 1 (2) | |||
| Chronic GVHD | 48 | 48 | .21 | ||
| None | 48 (100) | 45 (94) | |||
| Limited | 0 (0) | 3 (6) | |||
| Median follow-up, months | 77.4 | 74.6 | .22* | ||
Log-rank test.
AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; TX, transplant; Bu, busulfan; Cy, cyclophosphamide; TBI, total body irradiation; CMV, cytomegalovirus; WBC, white blood cells; DX, diagnosis; GVHD, graft-versus-host disease.
Cell doses ranged from 0.3 to 7.9 × 108/kg recipient weight. Between cut points of 2.5-3.5 × 108, we found that a cut point at approximately the median of 3.0 × 108 nucleated cells/kg gave the greatest discrimination for treatment failure risk between high (n = 50; median dose, 3.9 × 108/kg) and low (n = 50; median dose, 2.3 × 108/kg) doses. Fifty-nine of 100 patients were alive at last contact, with a median follow-up of 75 months (range, 4 to 146 months). Five-year probabilities (95% CI) of overall survival and LFS were 58% (48% to 68%) and 45% (35% to 55%), respectively. Five-year cumulative incidence rates of transplant-related mortality and relapse were 6% (2% to 12%) and 49% (38% to 59%), respectively. Thirty-four recipients died of recurrent leukemia and 6 of transplant-related causes.
Comparison of high and low cell doses
Hematologic recovery.
There was a nonsignificant trend toward slower recovery of neutrophils (P = .32) and platelets (P = .15) in the low cell dose group (Table 1).
GVHD.
A clinical syndrome consistent with acute GVHD developed in 10 patients. The median onset was 16 days (range, 7-31 days) after transplantation. In 7 patients (biopsy taken in 5), GVHD affected only skin, and its severity was stage I or II. All patients received topical or systemic steroids, and GVHD resolved in a median of 16 days (range, 10 to 45 days). Three patients had clinical features (no biopsies) of more extensive GVHD that affected skin, gut, and liver (overall grades II, II, and III). After treatment with systemic steroids with or without other agents, GVHD resolved in 1 patient after 25 days; in 2 patients it progressed to limited chronic GVHD. Median nucleated cell dose in these 10 patients was 3.3 (range, 1.1 to 4.7) × 108/kg; 4 patients received fewer than 3 × 108 nucleated cells/kg. De novo chronic GVHD did not develop in any patient.
Transplant-related mortality.
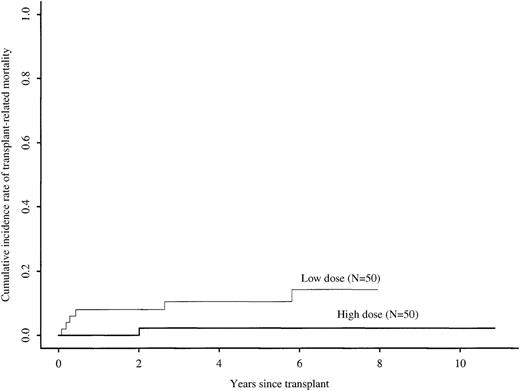
Five-year cumulative incidence rates of transplant-related mortality with high versus low cell doses were 2% (0% to 8%) versus 10% (3% to 20%), respectively (univariate log rank test, P = .04) (Figure 1). In multivariate analysis, the relative risk of transplant-related mortality with high versus low cell doses was 0.16 (95% CI, 0.02 to 1.29; P = .08) (Table2). The only other factor significantly associated with transplant-related mortality in multivariate analysis was advanced disease versus early or intermediate stage disease (RR, 5.97; 95% CI, 1.14 to 31.31; P = .03). Table3 compares treatment-related mortality rates in high and low cell dose groups according to disease and treatment characteristics. Treatment-related mortality rates were higher in the low cell dose group in all categories.
Cumulative incidence rates of transplant-related mortality after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Cumulative incidence rates of transplant-related mortality after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Relative risk of transplant-related mortality, relapse, and treatment failure with high versus low cell doses
| Event . | Variable . | RR (95% CI) . | P . |
|---|---|---|---|
| Transplant-related mortality | High vs low cell dose Advanced vs early/int disease | 0.16 (0.02-1.29) 5.97 (1.14-31.31) | .08 .03 |
| Relapse | High vs low cell dose: | ||
| First 9 mos after transplantation | 1.65 (0.69-3.95) | .26 | |
| >9 mos after transplantation | 0.28 (0.12-0.66) | .003 | |
| Advanced vs early/int disease | 6.21 (2.66-14.47) | <.001 | |
| WBC at diagnosis | |||
| >25 vs ≤25 × 109/L | 2.56 (1.39-4.69) | .002 | |
| CyTBI vs BuCY for conditioning | 0.51 (0.28-0.92) | .02 | |
| Treatment failure | High vs low cell dose: | ||
| First 9 mos after transplantation | 1.16 (0.53-2.53) | .71 | |
| >9 mos after transplantation | 0.27 (0.12-0.60) | .001 | |
| Advanced vs early/int disease | 6.14 (2.87-13.12) | <.001 | |
| WBC at diagnosis | |||
| >25 vs ≤25 × 109/L | 2.42 (1.39-4.21) | .002 | |
| CyTBI vs BuCY for conditioning | 0.57 (0.33-0.99) | .05 | |
| Karnofsky score ≥90% vs <90% | 0.49 (0.24-0.99) | .05 |
| Event . | Variable . | RR (95% CI) . | P . |
|---|---|---|---|
| Transplant-related mortality | High vs low cell dose Advanced vs early/int disease | 0.16 (0.02-1.29) 5.97 (1.14-31.31) | .08 .03 |
| Relapse | High vs low cell dose: | ||
| First 9 mos after transplantation | 1.65 (0.69-3.95) | .26 | |
| >9 mos after transplantation | 0.28 (0.12-0.66) | .003 | |
| Advanced vs early/int disease | 6.21 (2.66-14.47) | <.001 | |
| WBC at diagnosis | |||
| >25 vs ≤25 × 109/L | 2.56 (1.39-4.69) | .002 | |
| CyTBI vs BuCY for conditioning | 0.51 (0.28-0.92) | .02 | |
| Treatment failure | High vs low cell dose: | ||
| First 9 mos after transplantation | 1.16 (0.53-2.53) | .71 | |
| >9 mos after transplantation | 0.27 (0.12-0.60) | .001 | |
| Advanced vs early/int disease | 6.14 (2.87-13.12) | <.001 | |
| WBC at diagnosis | |||
| >25 vs ≤25 × 109/L | 2.42 (1.39-4.21) | .002 | |
| CyTBI vs BuCY for conditioning | 0.57 (0.33-0.99) | .05 | |
| Karnofsky score ≥90% vs <90% | 0.49 (0.24-0.99) | .05 |
RR, relative risk; CI, confidence interval; int, intermediate; Cy, cyclophosphamide; TBI, total body irradiation; Bu, busulfan.
Frequencies of relapse and treatment-related mortality according to disease and treatment characteristics
| . | Relapse . | Treatment-related mortality rates . | ||
|---|---|---|---|---|
| Low cell dose (%) . | High cell dose (%) . | Low cell dose (%) . | High cell dose (%) . | |
| Disease | ||||
| Acute leukemia | 16/35 (46) | 16/38 (42) | 5/35 (14) | 1/38 (3) |
| CML | 12/15 (80) | 6/12 (55) | 1/15 (7) | 0/12 (0) |
| Stage before transplantation | ||||
| Early | 22/38 (58) | 9/27 (33) | 4/38 (11) | 1/27 (4) |
| Intermediate | 5/8 (62) | 6/16 (38) | 0/8 (0) | 0/16 (0) |
| Advanced | 1/4 (25) | 7/7 (100) | 2/4 (50) | 0/7 (0) |
| WBC at diagnosis | ||||
| ≤25 × 109/L | 11/29 (38) | 11/28 (39) | 3/29 (10) | 1/28 (4) |
| >25 × 109/L | 17/21 (81) | 11/22 (50) | 3/21 (14) | 0/22 (0) |
| Conditioning | ||||
| BuCY | 12/18 (67) | 8/17 (47) | 2/18 (11) | 0/17 (0) |
| CyTBI | 16/32 (50) | 14/33 (42) | 4/32 (13) | 1/33 (3) |
| . | Relapse . | Treatment-related mortality rates . | ||
|---|---|---|---|---|
| Low cell dose (%) . | High cell dose (%) . | Low cell dose (%) . | High cell dose (%) . | |
| Disease | ||||
| Acute leukemia | 16/35 (46) | 16/38 (42) | 5/35 (14) | 1/38 (3) |
| CML | 12/15 (80) | 6/12 (55) | 1/15 (7) | 0/12 (0) |
| Stage before transplantation | ||||
| Early | 22/38 (58) | 9/27 (33) | 4/38 (11) | 1/27 (4) |
| Intermediate | 5/8 (62) | 6/16 (38) | 0/8 (0) | 0/16 (0) |
| Advanced | 1/4 (25) | 7/7 (100) | 2/4 (50) | 0/7 (0) |
| WBC at diagnosis | ||||
| ≤25 × 109/L | 11/29 (38) | 11/28 (39) | 3/29 (10) | 1/28 (4) |
| >25 × 109/L | 17/21 (81) | 11/22 (50) | 3/21 (14) | 0/22 (0) |
| Conditioning | ||||
| BuCY | 12/18 (67) | 8/17 (47) | 2/18 (11) | 0/17 (0) |
| CyTBI | 16/32 (50) | 14/33 (42) | 4/32 (13) | 1/33 (3) |
Relapse.
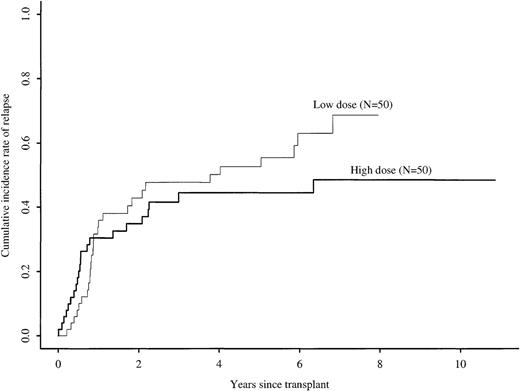
Five-year cumulative incidence rates of relapse with high versus low cell doses were 45% (31% to 59%) versus 53% (38% to 67%), respectively (P = .19) (Figure2). In multivariate analysis, cell dose had a time-varying effect on relapse (Table 2). The maximum likelihood method indicated that the relative hazard for relapse changed at approximately 9 months after transplantation. In the first 9 months after transplantation, the risk for relapse was similar in the high and low cell dose groups. Among patients surviving in remission 9 months after transplantation, the relative risk for subsequent relapse in the high versus low cell dose groups was 0.28 (95% CI, 0.12 to 0.66;P = .003). Other factors associated with increased relapse risk were advanced disease, high WBC count at diagnosis, and use of BuCY rather than CyTBI for pretransplant conditioning. Table 3 compares relapse in high and low cell dose groups according to disease and treatment characteristics. For all subcategories, patients receiving low cell doses had higher relapse frequencies than those receiving high cell doses, except for patients with advanced disease. However, only 4 patients with advanced disease received low cell doses, and 2 of them died of treatment-related causes.
Cumulative incidence rates of relapse after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Cumulative incidence rates of relapse after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Treatment failure.
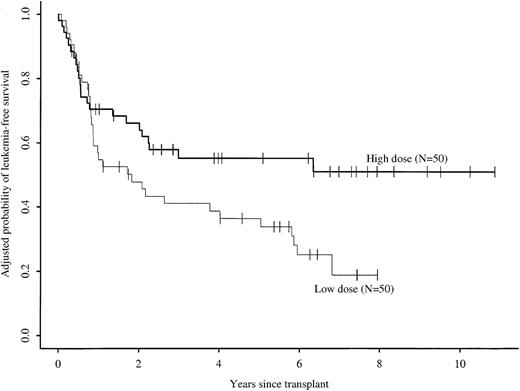
Cell dose also had a time-varying effect on treatment failure (relapse or death) in multivariate analysis (Table 2). In the first 9 months after transplantation, the risk for treatment failure was similar in the high and low cell dose groups. Among patients surviving in remission 9 months after transplantation, the relative risk for subsequent treatment failure in the high versus low cell dose groups was 0.27 (95% CI, 0.12 to 0.6; P < .01). Other factors associated with higher risks for treatment failure were advanced disease, high WBC count at diagnosis, BuCY rather than CyTBI for pretransplant conditioning, and Karnofsky score less than 90% before transplantation. These significant factors were considered in calculating adjusted probabilities of LFS. Such adjusted probabilities represent predicted probabilities of LFS for similar groups of patients receiving high or low cell doses. After adjusting for these prognostic factors, 5-year probabilities of LFS were 55% (43% to 67%) and 36% (24% to 49%) for the high versus low cell dose groups, respectively (P = .05) (Figure 3). There were no significant interactions between other prognostic variables and the cell dose effect. The lower risk for treatment failure with higher cell doses was significant for the following subgroups: early and intermediate or advanced leukemia; age older than 25 years; male or female sex; presenting WBC count more than or less than 25 × 109/L; and BuCY or CyTBI conditioning regimens.
Adjusted probabilities of leukemia-free survival rates after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Adjusted probabilities of leukemia-free survival rates after identical twin bone marrow transplantations with high (more than 3 × 108 cells/kg) versus low (less than or equal to 3 × 108 cells/kg) cell doses.
Causes of death are shown in Table 4. Fifteen of 50 patients in the high-dose group died, 14 from leukemia relapse and 1 from transplant-related causes; 26 of 50 patients in the low-dose group died, 20 from relapse and 5 from transplant-related causes.
Causes of death by cell dose
| Cause of death . | Cell dose . | |
|---|---|---|
| ≤3.0 × 108 cells/kg . | >3.0 × 108 cells/kg . | |
| Primary leukemia | 20 | 14 |
| Interstitial pneumonitis | 1 | 0 |
| Viral infection | 1 | 0 |
| Organ failure | 2 | 0 |
| Hemorrhage | 1 | 0 |
| Other or unknown | 1 | 1 |
| Total number of deaths | 26 | 15 |
| Cause of death . | Cell dose . | |
|---|---|---|
| ≤3.0 × 108 cells/kg . | >3.0 × 108 cells/kg . | |
| Primary leukemia | 20 | 14 |
| Interstitial pneumonitis | 1 | 0 |
| Viral infection | 1 | 0 |
| Organ failure | 2 | 0 |
| Hemorrhage | 1 | 0 |
| Other or unknown | 1 | 1 |
| Total number of deaths | 26 | 15 |
Discussion
In this series of patients receiving identical twin bone marrow transplants for leukemia, we found almost a log variation in nucleated bone marrow cell doses given. High-nucleated cell doses were associated with significantly lower late relapse rates and superior LFS. A statistically nonsignificant reduction in transplant-related mortality might also have contributed to the higher LFS.
We sought confounding variables that could explain these findings. In multivariate analysis, other factors independently associated with the risk for relapse, LFS, or both were advanced disease, presenting WBC, pretransplant conditioning regimen, and pretransplant Karnofsky score. A favorable effect of high cell dose on LFS was apparent in all these categories. Although we were unable to identify a confounding variable, it remains possible that factors not analyzed here could be responsible for the findings.
How then may these results be interpreted? An effect of high stem cell dose improving survival might be robust hematologic and immunologic recovery, reducing early complications of infection.5 11There was a small and not statistically significant difference in neutrophil and platelet recovery between recipients of high and low nucleated cell doses. However, any improvement in hematologic recovery from higher transplant cell doses did not affect early transplant-related mortality rates, which were very low (only 2 patients in each group died before day 100). It would be necessary to analyze larger numbers of recipients of syngeneic transplants to determine whether the small decrease in probability of transplant-related mortality rates (10% to 2%) with a higher cell dose is real.
Bone marrow transplantations from identical twin donors are characterized by low transplant-related mortality rates but relatively high risks for leukemia relapse.1,2 Transplant-related mortality rates are low because immune recovery is favored by the absence of GVHD prophylaxis and is only occasionally compromised by the development of GVHD. Relapse is presumed to be higher because residual leukemia does not present alloantigens to donor cells.12Attempts to reduce relapse after syngeneic transplantation have involved intensifying the preparative regimen or vaccination of the donor with irradiated leukemia cells of the recipient.13-15Neither approach is clearly beneficial. Because the dose of marrow cells is, within limits, under the control of the clinician and because in syngeneic transplantation there are no obvious contraindications to increasing lymphocyte dose, the finding that cell dose may influence relapse is important. Insight into potential mechanisms for this effect would have been helped by analysis of CD34+ cell and T-cell doses, which were unavailable for most patients. High nucleated marrow cell doses are a surrogate for higher stem cell doses and higher lymphocyte doses, but the relationship between these 2 components is imprecise.7,8 Whatever the mechanism, a favorable effect of higher total nucleated cell dose on mortality rates is described in an animal model13 and in several transplant series.4-6
An effect of nucleated cell dose on relapse in identical twin bone marrow transplantations may be explained by a syngeneic graft-versus-leukemia (GVL) effect linked to syngeneic GVHD. Well-documented reports of syngeneic GVHD indicate that some form of immune response against a genetically identical recipient may occur; this could theoretically include a GVL effect.16-20 In this series, clinical features of GVHD developed in 10% of patients. However the incidence of GVHD was similar in recipients of high and low cell doses, ruling out GVHD per se as the cause of lower relapse rates in recipients of high cell doses. A syngeneic GVL effect might be conferred in 2 alternative ways.1 High nucleated cell dose transplants include more T lymphocytes,8 which might induce syngeneic GVL in the absence of clinical GVHD. Full T-cell–mediated immune recovery, though faster after syngeneic transplantations than after allogeneic transplantations, still requires approximately 6 months.20The delayed benefit of high cell dose on relapse, apparent at 9 months after transplant, is therefore consistent with a lymphocyte-mediated GVL mechanism.2 Higher cell doses might also provide more CD34 cells. High CD34 cell doses reduced relapse in 1 animal model.21
These findings support transplanting more than 3 × 108 nucleated marrow cells/kg to optimize identical twin bone marrow transplant outcomes. They also indicate the importance of measuring individual cell components to better characterize the relationship between graft composition and outcome.
Other authors, members of the GVHD/GVL Working Committee of the International Bone Marrow Transplant Registry, are as follows:
Jennifer Bird, Southmead Hospital, University of Bristol, Bristol, UK; Gerald J. Elfenbein, University of South Florida, Tampa, FL; Joseph W. Fay, Baylor University Medical Center, Dallas, TX; P. Jean Henslee-Downey, Richland Memorial Hospital, University of South Carolina, Columbia, SC; Mary M. Horowitz, International Bone Marrow Transplant Registry, Health Policy Institute, Medical College of Wisconsin, Milwaukee, WI; Mark R. Litzow, Mayo Clinic, Rochester, MN; Philip L. McCarthy, Roswell Park Cancer Institute, Buffalo, NY; Hakumei Oh, INOUE Memorial Hospital, Chiba, Japan; Anibal J. Robinson, Navy Hospital Pedro Mallo, Buenos Aires, Argentina; James A. Russell, Tom Baker Cancer Centre, Calgary, Alberta, Canada; and Gérard Socié, Hôpital Saint Louis, Paris, France.
Supported by Public Health Service grants P01-CA40053 and U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of the U.S. Department of Health and Human Services; and by grants from Alpha Therapeutic Corp; Amgen, Inc; Anonymous; Baxter Fenwal; Berlex Laboratories; BioWhittaker, Inc; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol–Myers Squibb Co; Cell Therapeutics, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies, Inc; Chiron Therapeutics; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Human Genome Sciences; Immunex Corporation; The Kettering Family Foundation; Kirin Brewery Co; Robert J. Kleberg, Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals, Inc; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; RGK Foundation; Roche Laboratories; Rockwell Automation Allen Bradley Co; SangStat Medical Corp; Schering AG; Schering-Plough Oncology; Searle; SEQUUS Pharmaceuticals; SmithKline Beecham Pharmaceutical; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; SyStemix; United Resource Networks; and Wyeth-Ayerst Laboratories.
Reprints:Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal