Abstract
Clinically advanced and rapidly progressive AIDS-associated Kaposi sarcoma (AIDS-KS) tumors require an aggressive tumor-directed therapy. We have observed that AIDS-KS cells express high levels of receptors for immune regulatory cytokine, interleukin-13 (IL-13). Two tumorigenic AIDS-KS cell lines, KS Y-1 and KS-imm, expressed 4560 and 9480 IL-13 binding sites per cell with an affinity (kd) of ∼0.9 and 3.7 nmol/L, respectively. IL-13 cytotoxin IL13-PE38QQR, consisting of human IL-13 and a derivative of Pseudomonas exotoxin, is specifically cytotoxic to KS tumor cells. Systemic and loco regional administration of IL13-PE38QQR in immunodeficient mice with established human KS tumors produced remarkable antitumor activity. Three intratumoral (IT) injections of IL-13 toxin (250 μg/kg per dose) on alternate days (qod) or 5 daily (qd) IT injections with lower doses (50 or 100 μg/kg per dose) resulted in a complete regression of established subcutaneous tumors in most animals. Daily IT treatment with 250 μg/kg of IL-13 toxin in another KS-derived cell line also produced complete responses. Twice daily intraperitoneal injections of IL13-PE38QQR (25 or 50 μg/kg per dose) for 10 days (total injections = 20) also completely eradicated KS Y-1 tumors. Intravenous administration of IL13-PE38QQR also suppressed tumor growth; however, complete responses were not observed. All animals tolerated the therapeutic doses of IL-13 toxin without any visible signs of toxicity. The efficacy of receptor-directed IL13-PE38QQR therapy in mice warrants further exploration of this drug for AIDS-KS treatment.
Highly vascularized Kaposi's sarcoma (KS) is the most prevalent tumor occurring in individuals infected with HIV. KS is an angioproliferative tumor often characterized by 3 components: a proliferative component made up of endothelial and spindle cells; an inflammatory component that includes B cells, T cells, and monocytes; and a neovascular component.1 At least in the early stage, KS behaves as a hyperplastic-proliferative lesion sustained by the actions of inflammatory/angiogenic cytokines and growth factors. The detection of novel DNA fragments for KS-associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8) in virtually all KS lesional tissues2-4 has further complicated the complex characteristics of KS. The association of herpesvirus to KS may have important pathobiologic functions. It has recently been shown that the presence of inflammatory cytokine in KS lesions may reactivate KSHV infection. High HHV-8 load associated with KS development suggests that infected cells, particularly monocytes, may carry the virus to tissues and differentiate into KS-like spindle cells. Viral IL-6 encoded by KSHV is also implicated to play an important role in pathogenesis of certain KSHV-associated disorders.5-7
KS-derived spindle cells have been shown to elaborate a number of autocrine and paracrine growth factors. Of particular interest are basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), oncostatin M, tumor necrosis factor (TNF), platelet-derived growth factor (PDGF), transforming growth factor (TGF), granulocyte macrophage colony-stimulating growth factor (GM-CSF), hepatocyte growth factor (HGF), interleukin (IL)-1β, IL-6, and IL-8.8-14 These cytokines and factors exert their effect through their cognate receptors. KS cells have been shown to express receptors for a variety of cytokines, including IL-4, IL-6, VEGF, and TNF.15-18 In addition, we have previously reported that KS cell lines express a large number of high-affinity IL-13 receptors.19
IL-13, predominantly produced by activated Th2 cells and mast cells,20 inhibits the production of inflammatory cytokines in monocytes.21 IL-13 shares a number of biologic functions associated with the pleiotropic immunoregulatory cytokine, IL-4, because of the similarities between their receptors.22IL-13, like IL-4, has also been shown to inhibit HIV-1 replication in primary blood-derived human macrophages.23 We have reported that IL-13 modestly inhibits the growth of AIDS-KS cell lines in vitro.19 Because AIDS-KS cells express receptors for IL-13, we produced a fusion protein comprised of IL-13 and a mutated form ofPseudomonas exotoxin (PE).24 This recombinant agent, IL13-PE38QQR (also termed IL-13 toxin) is highly cytotoxic to various IL-13R positive solid tumor cells, including AIDS-KS primary cell cultures.19 24-26 We have further examined the structure of IL-13R on KS cells and investigated the in vivo antitumor activity of IL13-PE38QQR against 2 types of AIDS-KS tumors. We have also investigated pharmacokinetics and distribution of IL-13 toxin after intratumor administration.
Material and methods
Cell culture and reagents
The AIDS-KS cell line, KS Y-1 was isolated from pleural effusion of an AIDS patient.27 KS-imm cells were derived from a KS biopsy sample.28 Recombinant human IL-13 was expressed inEscherichia coli and purified in our laboratory (Joshi et al, Oshima et al, unpublished results). Human serum albumin was purchased from Sigma (St. Louis, MO).
Bacterial expression and purification of IL-13 toxin
The recombinant chimeric toxin, IL13-PE38QQR, was expressed in E coli and purified under denaturing conditions24 in our laboratory as described by Joshi et al (unpublished results).
Quantitation of IL-13 receptors by binding assay
The labeling of recombinant IL-13 radiolabeled with 125I (Amersham Corp, Piscataway, NJ) has been described elsewhere.19 This radiolabeling procedure consistently produced radiolabeled IL-13 with a high specific activity (0.555-0.666 MBq[15-18 μCi]/μg). The binding of125I-IL-13 to IL-13R receptors and Scatchard analysis using the LIGAND program29 was performed on KS cell lines as described previously.22
RNA isolation and reverse transcriptase-polymerase chain reaction analysis
The AIDS-KS cells were trypsinized and washed in ice-cold phosphate-buffered saline (PBS). Total RNA was extracted with TRIzol (Life Technologies, Gaithersburg, MD) as described earlier.15 Reverse transcription, 2.5 μg of total RNA was transcribed to complementary DNA (cDNA) in 50 μL reaction buffer.15 The synthesized cDNA (10 μL) was amplified by polymerase chain reaction (PCR) in a 50 μL volume by using the GeneAmp RNA PCR kit (Perkin Elmer, Branchburg, NJ) with 0.1 μg of IL-13Rα (+52) 5′-AATGGCTTTCGTTTGCTTTGG-3′/(+489) 5′-ACGCAATCCATATCCT GAAC or IL-13Rα′ (+957) 5′-GGAGAATACATCTTGTTTCATGG/(+1104) 5′-GCGCTTCTTACCTA TACTCATTTCTTGG primers.30 The PCR products were separated on 2% Nusieve 3:1 (FMC Bioproduct, Rockland, ME) gel.
Protein synthesis inhibition assay
Protein synthesis was determined by the incorporation of [3H]leucine into AIDS-KS tumor cells as previously described.15 KS Y-1 or KS-imm cells (104 per well) were preincubated with an excess of IL-13 (2 μg/mL) for 45 minutes before addition of IL13-PE38QQR. Cells were then incubated for 20 to 24 hours at 37°C and 0.037 MBq (1 μCi) of [3H]leucine (NEN, Boston, MA) was added to each well and allowed to incubate for an additional 4 hours. The cells were harvested on a fiberglass filtermat to count the cell-associated radioactivity using a Beta Plate Counter (Wallac, Gaithersburg, MD). The concentration of IL13-PE38QQR at which 50% inhibition of protein synthesis (IC50) occurred was calculated.
AIDS-Kaposi's sarcoma tumor transplant in nude mice
The 4- to 6-week-old immunodeficient athymic female nude mice of Balb/C origin were purchased from the Frederick Cancer Center Animal Facilities, National Cancer Institute, Frederick, MD. The animals were housed in filter-top cages in laminar flow hoods under pathogen-free conditions with 12 hours light/12 hours dark cycles. All studies described herein have been approved by the CBER Animal Care Use committee and conform to the guidelines set forth by the NIH Animal Advisory Committee.
KS Y-1 or KS-imm tumors were implanted into the flank of nude mice as described earlier.31 Briefly, KS Y-1 tumor pieces (30-40 mg) or KS-imm cells, 2 to 3 × 106 in 50 μL, were mixed with 100 μL of 10 mg/mL Matrigel (Collaborative Biomedical Products, Bedford, MA) and SC implanted in the flank of the immunodeficient mice. Both KS tumors were established consistently within 3 to 5 days of implantation. Tumor were measured by Vernier calipers. In general, 5 to 7 animals were used for each dose group unless specified otherwise. The treatment procedure, involving different routes of administration, was comparable to one previously described by Husain et al.31 32 The control mice in all experiments received 0.2% human serum albumin (HSA) in PBS as excipient only. The statistical significance of tumor regression was calculated by Student ttest.
Western blot analysis
To measure the concentration of IL-13 toxin in the tumor bed after a single intratumoral injection of 250 μg/kg, tumors were excised at 2, 6, or 24 hours after injection of cytotoxin. Tumor pieces (100 mg) were homogenized in 100 μL of Tris-HCl (10 mmol/L, pH 7.5) buffer containing 1.0 mmol/L EDTA, 0.1 mmol/L phenylmethyl sulfonyl fluoride, and 10 μL protease inhibitor cocktail (Sigma, St. Louis, MO). The supernatants were collected and frozen at −70°C until analyzed. The supernatants were run on 10% SDS-PAGE gel and transferred to PVDF membrane (Novex, San Diego, CA). The membrane was probed with an antibody against PE, washed 3 times, and incubated with biotinylated antirabbit IgG (Vector Laboratories, Burlingame, CA). The blot was later incubated with Avidin:Biotinylated enzyme Complex (ABC) reagents and bands were visualized by submersing the blot in peroxidase substrate as per the manufacturer's instructions (Vector Laboratories). A standard curve was generated by running different amounts of IL-13 toxin in the same gel. The quantity of IL-13 toxin contained in tumor tissue homogenates was calculated by comparing band intensities.
Serum level of IL-13 toxin and its antibody
To determine the half-life of IL-13 toxin, sera were collected at 2, 5, 10, 15, 30, 45, 60, 90, 120, 240, and 360 minutes after a single intravenous injection of 100 μg/kg of IL-13 toxin and stored at −70°C. Two mice were used for each time point of serum collection. The serum cytotoxicity was measured by a protein synthesis inhibition assay run in quadruplicate for each mouse (n = 2) as described before. Varying dilutions (1:100 and 1:500) of sera were added to human pm-renal cell carcinoma (PM-RCC) cells known for their sensitivity to IL-13 toxin.26 Toxin levels were calculated from standard curves run, using IL-13 toxin admixed with control mouse serum.
To detect the presence of PE antibody in sera collected on day 47 after repeated intraperitoneal injections of IL-13 toxin, diluted serum samples (1:200) mixed with IL-13 toxin (0.2, 1.0, or 5 ng/mL) were subjected to a protein synthesis inhibition assay usingpm-RCC as indicator cells. Similar concentrations of IL-13 toxin mixed with untreated mouse serum were used to generate a standard curve of protein synthesis inhibition. The difference in IC50 of control and treated sera were compared and the neutralization of IL-13 toxin by PE antibodies was estimated. For a positive control, serum from a patient who developed neutralizing antibody to PE was used in the assay.
Results
Abundant expression of high affinity IL-13 receptors on AIDS Kaposi's sarcoma cells
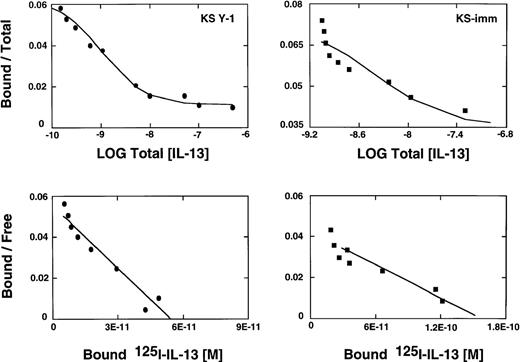
We have reported previously that several primary cell cultures of Kaposi's sarcoma constitutively express large numbers of high affinity IL-13R.19 Scatchard analysis of 2 additional tumorigenic AIDS-KS cell lines, KS Y-1 and KS-imm, revealed the presence of approximately 4560 and 9480 sites per cell, respectively. The affinity constants (Kd) for ligand binding for KS Y-1 and KS-imm were 0.9 and 3.7 nmol/L, respectively (Figure1). In contrast, normal human umbilical vein-derived endothelial cells (HUVEC) shown to respond to IL-1319 and a possible precursor of AIDS-KS expressed low numbers of IL-13R (less than 200 sites per cell). Unlabeled IL-13 displaced 125I-IL-13 binding on both KS cells lines, indicating the specificity of binding.
IL-13 receptor expression on AIDS-KS cells.
AIDS-KS (1.0 × 106) were incubated with125I-IL-13 (500 pmol/L) with increasing concentrations (0 to 200 nmol/L) of unlabeled IL-13 for 2 hours at 4°C. The displacement curves (upper panels) and scatchard analyses (bottom panels) were generated from the binding data using LIGAND program.29
IL-13 receptor expression on AIDS-KS cells.
AIDS-KS (1.0 × 106) were incubated with125I-IL-13 (500 pmol/L) with increasing concentrations (0 to 200 nmol/L) of unlabeled IL-13 for 2 hours at 4°C. The displacement curves (upper panels) and scatchard analyses (bottom panels) were generated from the binding data using LIGAND program.29
Subunit structure of IL-13 receptor expressed on AIDS-Kaposi's sarcoma cells
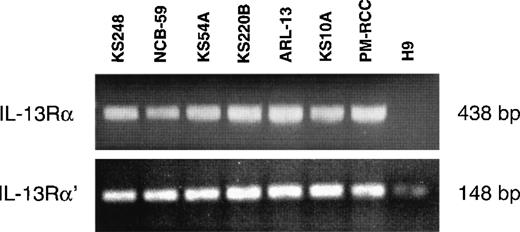
Previous studies have demonstrated that the IL-13R may be composed of IL-13Rα, IL-13Rα′, and IL-4Rβ (also known as IL-4Rα) chains in various cell types, including malignant cells and normal human skin fibroblasts.30 Because fibroblasts are involved in the pathogenesis of KS, it was of interest to investigate which chains of the IL-13R are expressed on AIDS-KS cells. The mRNA of 6 AIDS-KS–derived cells was subjected to reverse transcriptase-PCR (RT-PCR) analysis. Figure 2 demonstrates that IL-13Rα and IL-13Rα′ chains were expressed in all cells lines tested. pm-RCC served as positive control for both chains. The H9 human T-cell line does not express the IL-13Rα chain30 and thus it was used as a negative control. A weak band for IL-13Rα′ was detected in H9 cells. In addition, we previously showed that these AIDS-KS cells also express the IL-4Rβ chain, but not the common γ chain (γc), a component of the IL-4R in immune cells.15 These results confirm that IL-13R expressed on AIDS-KS cells is comprised of IL-13Rα and α′ and IL-4Rβ chains.
RT-PCR analysis of IL-13 receptor transcripts.
Total RNA (2.5 μg) of different AIDS-KS cells were reverse transcribed to cDNA and then amplified by PCR using specific IL-13Rα and IL-13Rα′ primers. The PCR products were run on 2% Nusieve 3:1 gel for ultraviolet analysis. pm-RCC and H9 T cells were used as positive and negative controls, respectively.
RT-PCR analysis of IL-13 receptor transcripts.
Total RNA (2.5 μg) of different AIDS-KS cells were reverse transcribed to cDNA and then amplified by PCR using specific IL-13Rα and IL-13Rα′ primers. The PCR products were run on 2% Nusieve 3:1 gel for ultraviolet analysis. pm-RCC and H9 T cells were used as positive and negative controls, respectively.
Receptor targeted cytotoxicity of IL-13 toxin to AIDS-KS cell lines
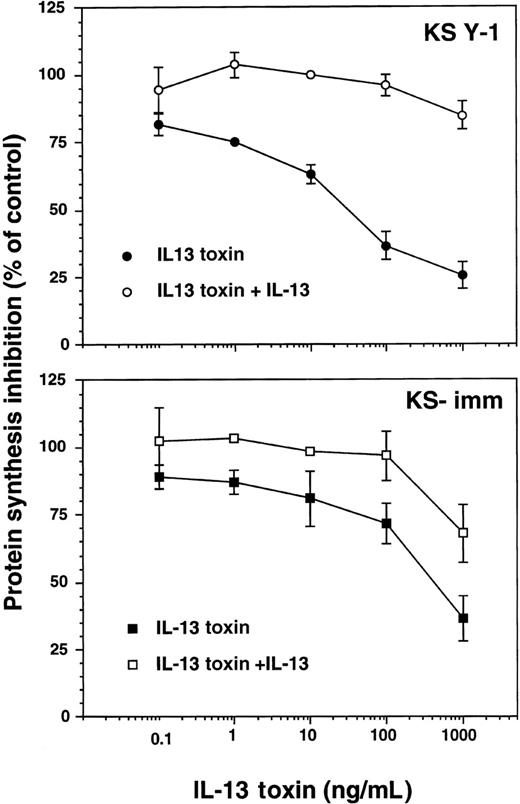
We have previously demonstrated that several types of tumor cells that express surface IL-13R can be effectively and specifically targeted with IL-13 toxin.24-26,33 Because KS Y-1 and KS-imm are tumorigenic cell lines that express IL-13R, it was obviously important that their sensitivity toward IL-13 toxin be assessed. The recombinant IL-13 toxin exhibited potent and specific cytotoxic effects against these 2 cell lines, inhibiting protein synthesis by 50% (IC50) at concentrations of 27 ± 6 and 380 ± 99 ng/mL, respectively (Figure 3). The IL-13 toxin cytotoxic effects were abrogated by addition of excess recombinant IL-13 (2 μg/mL), indicating that cytotoxicity by IL-13 toxin is specifically mediated through IL-13R. Normal endothelial cells that expressed low levels of IL-13R (less than 200 sites per cell) were not sensitive to the cytotoxic effects of IL-13 toxin even at concentrations more than 1000 ng/mL.19
Cytotoxicity of IL-13 toxin against AIDS-KS cells.
Protein synthesis inhibition in KS Y-1 or KS-imm cells was assayed with IL-13 toxin alone (filled symbol) or IL-13 toxin plus 2 μg/mL IL-13 (open symbol). The assay was run in quadruplicate and repeated several times.
Cytotoxicity of IL-13 toxin against AIDS-KS cells.
Protein synthesis inhibition in KS Y-1 or KS-imm cells was assayed with IL-13 toxin alone (filled symbol) or IL-13 toxin plus 2 μg/mL IL-13 (open symbol). The assay was run in quadruplicate and repeated several times.
Intraperitoneal administration of IL-13 toxin results in complete tumor regression
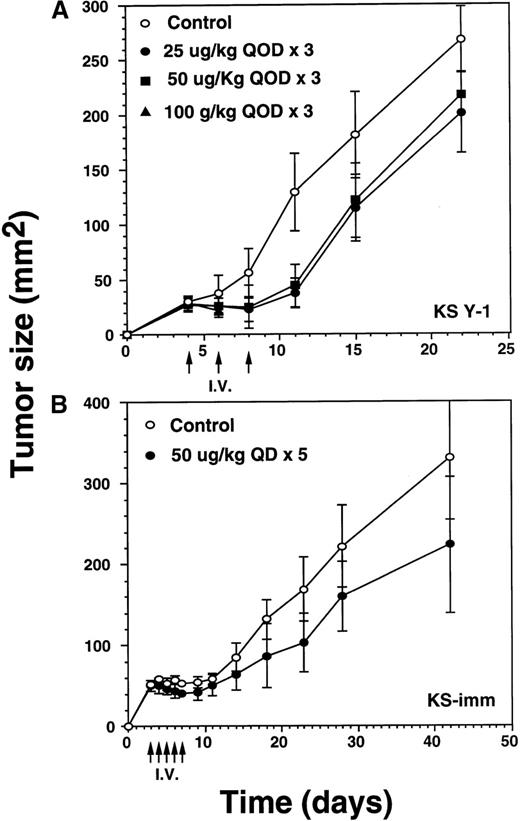
To explore IL-13 toxin antitumor efficacy, we attempted intraperitoneal administration of IL-13 toxin at different doses and schedules into nude mice xenografts. A preliminary experiment using various concentrations of IL-13 toxin (5 to 100 μg/kg per dose) on alternate days (qod) for a total of 3 doses demonstrated tumor regression of KS Y-1 xenografts. Interestingly, a dose-dependent correlation was not established (data not shown). Additional attempts were made to increase the number of injections as well as to alter the IL-13 toxin dosing schedule. Daily injection (qd) of 50 μg/kg per dose for 5 days reduced tumor size by more than 30% when measured on day 15 after tumor implantation. Twice daily (bid) injections at half the dose, 25 μg/kg of toxin for 5 days (total injections = 10), caused a reduction in tumor size comparable to that observed for the 50 μg/kg, qd × 5 schedule (data not shown). These results suggest that continuous availability of toxin at the tumor site is critical to achieve significant tumor regression.
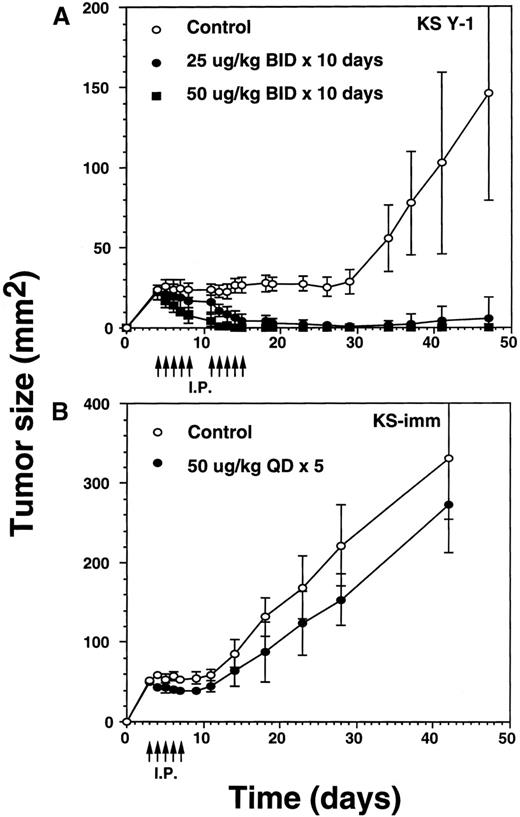
The incomplete regression of tumors in some animals suggested the likelihood that IL-13 toxin was not uniformly available to the tumor bed. To overcome this problem, we reasoned that accumulation of higher concentrations of the drug at the tumor site is required to kill all the tumor cells. Because of dose-limiting toxicity, augmenting the number of injections rather than increasing the dose of IL-13 toxin was considered to be a more suitable option. To execute our hypothesis, we selected identical concentrations of IL-13 toxin as described above. Tumor xenografts were injected bid with IL-13 toxin at doses of 25 or 50 μg/kg for a total of 10 days (total injections = 20). Treatments began on day 4 when the average KS Y-1 tumor size had reached to 23 mm2 (Figure 4A). By day 11, treatment with the 50 μg/kg dose regressed tumors by 79% with 2 of 5 animals being complete responders (CRs). The residual tumors disappeared completely by day 14 and all 5 mice remained tumor free for a total of 47 days, when the experiment was terminated. By day 14, the lower dose of 25 μg/kg also resulted in tumor regression of 76% compared with the control group, with 1 of 6 mice displaying a CR through day 47. By day 34, 5 of 6 mice displayed a CR, with 1 animal bearing a small (9 mm2) tumor that subsequently increased in size to 36 mm2 by the last of day of the experiment (day 47). As was the case previously, complete responders remained tumor-free and healthy until the day they were killed. In control mice, injected with vehicle (0.2% HSA in PBS) only, displayed tumors which grew exponentially, attaining a size of 146 mm2 by day 47. The mice in all groups were killed humanely to collect blood for the determination of IL-13 toxin antibody.
Regression of AIDS-KS tumors by intraperitoneal injections of IL-13 toxin.
(A) Nude mice were implanted subcutaneously with approximately 30 mg of KS Y-1 tumor mass on day 0. The animals then received injections twice a day with IL-13 toxin on day 4 through 8. The control mice received excipient only. The second cycle of injections was given on day 11 through 15. Each group had 6 animals. (B) KS-imm cells (2 × 106/50 μL) mixed with 100 μL of Matrigel were implanted on day 0 and 5 daily injections began on day 3. The control and treatment groups had 7 and 6 mice, respectively. The arrows indicate the day of injections.
Regression of AIDS-KS tumors by intraperitoneal injections of IL-13 toxin.
(A) Nude mice were implanted subcutaneously with approximately 30 mg of KS Y-1 tumor mass on day 0. The animals then received injections twice a day with IL-13 toxin on day 4 through 8. The control mice received excipient only. The second cycle of injections was given on day 11 through 15. Each group had 6 animals. (B) KS-imm cells (2 × 106/50 μL) mixed with 100 μL of Matrigel were implanted on day 0 and 5 daily injections began on day 3. The control and treatment groups had 7 and 6 mice, respectively. The arrows indicate the day of injections.
To investigate whether a second KS xenograft model is also sensitive to IL-13 toxin, we administered IL-13 toxin via intraperitoneal injections into KS-imm tumor-bearing mice. A daily dose of 50 μg/kg per dose, for 5 consecutive days, resulted in 28% reduction in tumor size in 100% (n = 6) of the mice by day 8, compared with matched controls (Figure 4B). When the injections were concluded, treated tumors began to regrow and reached to an average size of 273 ± 60 mm2 compared with 331 ± 77 mm2 for the tumors in control animals measured on the last day of experiment. Because of heavy tumor burden, animals were killed on day 42. The smaller decrease in the size of tumors in treated animals may be due to the fact that KS-imm cells express IL-13 receptors of lower affinity (3.7 nmol/L) and show lesser sensitivity toward IL-13 toxin in vitro. No complete responders were observed in this treated group.
Significant decrease of tumors by intravenous injection of IL-13 toxin
Because late stage AIDS-KS may involve major organs, eg, lung, liver, spleen, pancreas, and gastrointestinal tract,34 it was of interest to investigate the antitumor activity of IL-13 toxin when administered via the intravenous route. Three intravenous injections of IL-13 toxin (25, 50, or 100 μg/kg) were administered on alternate days (qod) to animals bearing 29 mm2 KS Y-1 tumors on day 4 after the implantation (Figure5A). Tumor regression of 65% to 71% in the mice dosed at 25 or 50 μg/kg per dose were evident by day 11, compared with exponentially growing tumors in control mice. The tumors then began to grow slowly but remained significantly (P < .010) smaller in size compared with control tumors by day 22. Although tumor growth was delayed by 40% at an IL-13 toxin dose of 100 μg/kg, this dose was toxic to animals and 5 of 6 mice died after the second injection. The last 100 μg/kg animal died on day 11. No complete response was observed in any of the treated groups. Tumors in the control mice began to ulcerate resulting in termination of the experiment on day 22. These results suggest that intravenous injection of an optimal dose of IL-13 toxin can diminish tumor growth rate significantly.
Regression of AIDS-KS tumor growth by intravenous injection of IL-13 toxin.
(A) After implanting KS Y-1 tumors (approximately 20 mg) at day 0, the xenografts received 3 injections of IL-13 toxin in 200 μL in the tail vein on day 4, 6, and 8. In the 100 μg/kg dose group, all 6 mice died by day 11. (B) The KS-imm xenografts were given 5 daily injections of 50 μg/kg of IL-13 toxin on day 3 through 7. The arrows indicate the days of injections.
Regression of AIDS-KS tumor growth by intravenous injection of IL-13 toxin.
(A) After implanting KS Y-1 tumors (approximately 20 mg) at day 0, the xenografts received 3 injections of IL-13 toxin in 200 μL in the tail vein on day 4, 6, and 8. In the 100 μg/kg dose group, all 6 mice died by day 11. (B) The KS-imm xenografts were given 5 daily injections of 50 μg/kg of IL-13 toxin on day 3 through 7. The arrows indicate the days of injections.
In the KS-imm tumor model, IL-13 toxin (50 μg/kg per dose) was intravenously injected daily for 5 days, beginning on day 3, when the average tumor size reached approximately 50 mm2 (Figure5B). By the day of the last injection (day 8), tumors had regressed by 24% compared with control tumors. Thereafter, the treated tumors continued to grow, but at a rate significantly (P < .050) lower than observed in control tumors on day 42. The study suggests that KS-imm tumors also respond to IL-13 toxin, though no complete responses were observed.
Total eradication of AIDS-Kaposi's sarcoma tumors by intratumoral IL-13 toxin
Intratumoral treatment eradicates KS Y-1 tumors.
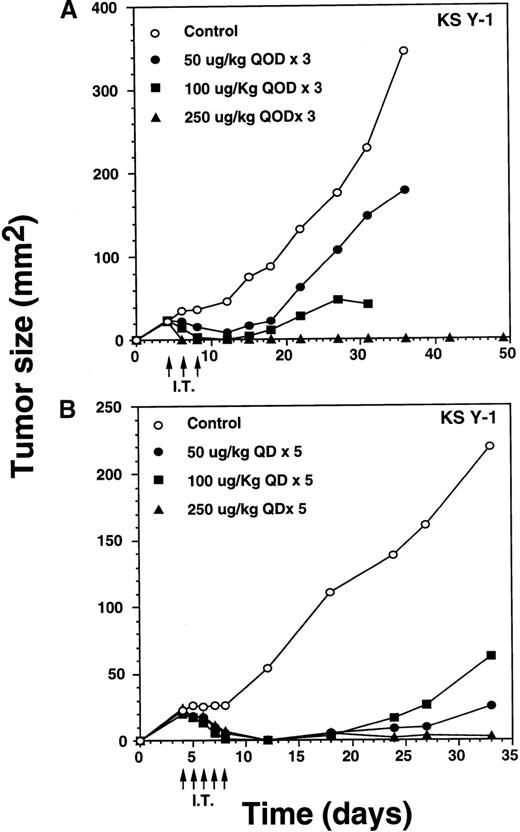
IT injection of IL-13 toxin into the KS Y-1 and KS-imm tumors was performed in an attempt to enhance the availability of the toxin at the tumor site. Doses of 50, 100, or 250 μg/kg per dose of toxin were administered directly into KS Y-1 tumors, qod × 3, beginning on day 4 after implantation (ie, day 4, 6, 8) when tumors measured 24 ± 1 mm2 (Figure 6A). By day 6, 100% of the 250 μg/kg dose (n = 4) showed complete regression. By day 12, doses of 50 or 100 μg/kg per dose also caused complete tumor regression in 2 of 4 and all 4 mice, respectively. Tumor growth in 2 noncomplete responder mice in the 50 μg/kg dose group was inhibited by 73% compared with the animals in the control group. Later, tumors began to reappear in all the mice from the 50 and 100 μg/kg dose, averaging 179 ± 72 mm2 (50 μg/kg dose) and 25 mm2 (100 μg/kg dose) compared with a mean control tumor size of 345 ± 86 mm2 by day 36. Although tumors reappeared in all mice in both groups, tumor growth at the 100 μg/kg dose was completely arrested never expanding beyond the original size of tumors on the day of the first injection. By day 36, 2 of 4 mice dosed at 100 μg/kg died (cause of death unknown). All surviving mice in the 0, 50, and 100 μg/kg groups were killed on day 36 because of substantial tumor burden. All CRs in the 250 μg/kg dose group remained tumor free through at least day 124. These results clearly suggest that antitumor activity of IL-13 toxin is dose dependent.
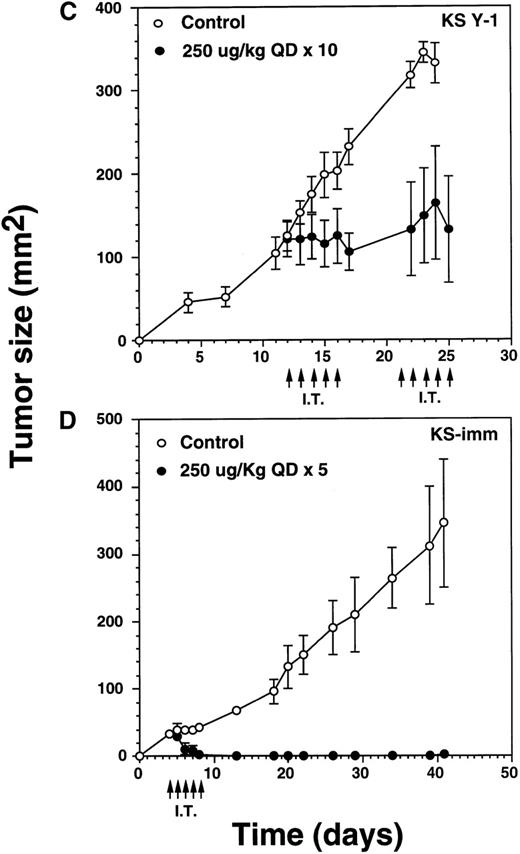
Complete regression of AIDS-KS tumors by intratumoral administration of IL-13 toxin.
The nude mice (n = 4 per group) with established KS Y-1 tumors received 30 μL of IL-13 toxin (A) slowly into all quadrants of tumor (7.5 μL per quadrant) by 26-guage syringe on day 4, 6, and 8. The control animals received excipient only. One animal died on day 6 in the 250 μg/kg dose group. (B) The similar doses as of Figure 6a, but with a schedule of daily injections were administered on day 4 through 8. Because some mice in the treatment groups obtained CR, the error bars in mean tumor size were large and are not shown. The mean values for 0, 50, and 100 μg/kg doses were 345 ± 91, 179 ± 72, and 25 ± 5, respectively, on day 36. The arrows point to the days of treatments. (C) Large KS Y-1 tumors were injected with 2 cycles of 5 injections of IL-13 toxin as shown by arrows on the x-axis. Each group had 7 mice. (D) Established KS-imm xenografts received 5 daily injections of IL-13 toxin beginning on day 4.
Complete regression of AIDS-KS tumors by intratumoral administration of IL-13 toxin.
The nude mice (n = 4 per group) with established KS Y-1 tumors received 30 μL of IL-13 toxin (A) slowly into all quadrants of tumor (7.5 μL per quadrant) by 26-guage syringe on day 4, 6, and 8. The control animals received excipient only. One animal died on day 6 in the 250 μg/kg dose group. (B) The similar doses as of Figure 6a, but with a schedule of daily injections were administered on day 4 through 8. Because some mice in the treatment groups obtained CR, the error bars in mean tumor size were large and are not shown. The mean values for 0, 50, and 100 μg/kg doses were 345 ± 91, 179 ± 72, and 25 ± 5, respectively, on day 36. The arrows point to the days of treatments. (C) Large KS Y-1 tumors were injected with 2 cycles of 5 injections of IL-13 toxin as shown by arrows on the x-axis. Each group had 7 mice. (D) Established KS-imm xenografts received 5 daily injections of IL-13 toxin beginning on day 4.
To determine whether an alteration to the IL-13 toxin dosing schedule would result in permanent, complete regression of KS Y-1 tumors, dose levels of 50, 100, or 250 μg/kg per day were IT injected daily for 5 consecutive days (Figure 6B). Treatment began on day 4 when the tumor size reached approximately 23 mm2. By the final injection (day 8), doses of 50 and 100 μg/kg resulted in complete responses in 3 of 6 and 5 of 6 mice, respectively. By day 12, all mice (n = 6) in both groups exhibited complete regression. Although the tumor began to recur in some of the animals in both IL-13 toxin groups, by the end of the experiment (day 33), 3 of 6 (50 μg/kg) and 4 of 6 (100 μg/kg) mice remained tumor free. Recurrence of tumor growth was significantly delayed (50 μg/kg, P < .008; 100 μg/kg,P < .009), reaching an average size of 63 or 25 mm2at 50 or 100 μg/kg, respectively by day 33.
The daily dosing schedule (qd × 5) of IL-13 toxin at 50 and 100 μg/kg per day resulted in tumor regression, which persisted for a longer duration, compared with a dosing schedule of qod × 3. All mice (n = 6) dosed at 250 μg/kg, qd × 5 displayed complete responses by day 12, and 5 of 6 mice remained tumor free until they were killed on day 33. The presence of a small tumor (16 mm2) in 1 of the animals did not grow beyond its regressed size. The data show that 250 μg/kg, qd × 5 injection was similar to the qod × 3 schedule (Figure 6A) in regressing tumors completely. Even 3 qod injections at the highest dose produced CRs in all animals.
To determine the antitumor activity of IL-13 toxin against relatively larger tumors, we grew KS Y-1 tumors to a size about 5 times larger than in previous experiments. The tumors (124 mm2) received 5 intratumoral injections of 250 μg/kg on days 12 through 16, followed by another cycle of injections from day 21 through 25 (Figure6C). Tumor growth was completely arrested in all treated (n = 7) mice and tumors did not grow beyond their initial size. Additional injections on day 21 through 25 did not allow tumors to grow further; however, no additional regression in size was observed. The control tumors continued to grow unabated and attained a size of 332 ± 24 mm2 on day 25, which was at least 2.5 times larger than the treated tumors. The data suggest that IL-13 toxin given by continuous daily IT injections (total = 10) can effectively arrest the growth of large established tumors.
Intratumoral treatment eradicates KS-imm tumors.
Our in vitro data showed that KS-imm tumors were also sensitive to IL-13 toxin. These cells expressed a higher numbers of IL-13R but a lower binding affinity (3.7 nmol/L) compared with KS Y-1 cells (0.9 nmol/L), thus IL-13 toxin was not very cytotoxic to KS-imm cells compared with KS Y-1 cells. The IC50 for KS-imm cells ranged from 380 to 520 ng/mL compared with 26 ng/mL for KS Y-1 cells. Nude mice bearing KS-imm tumors with an average size of 33 mm2 were IT injected with an effective dose 250 μg/kg per day of IL-13 toxin for 5 consecutive days (day 4-8). Tumors completely disappeared in 4 of 6 mice by day 8 (Figure 6D). The remaining small tumors in 2 of 6 mice disappeared by day 18. All 6 mice stayed tumor free for a total of 40 days; a small tumor (4 mm2) appeared in 1 of the 6 mice and reached a size of 25 mm2 by the last day of the experiment (day 63). These intratumor studies clearly suggest that the nontoxic dose of IL-13 toxin (250 μg/kg) administered using either the qod × 3 or qd × 5 schedule can yield durable CRs in both AIDS-KS tumor models.
Quantitation of IL-13 toxin at tumor site after intratumoral injection
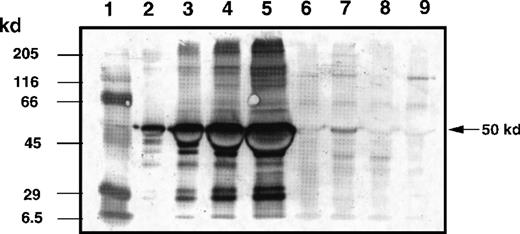
To evaluate the biodistribution of IL-13 toxin in the tumor bed, mice with experimentally established KS Y-1 tumors (86 ± 13 mm2) were IT injected with a single dose of 250 μg/kg of IL-13 toxin. The semiquantitative immunoblot analysis of tumor homogenates revealed that almost 70% of IL-13 toxin remained localized in the tumor bed up to 2 hours after injection (Figure7). Tumors harvested at 6 hours after injection still showed the presence of toxin (13%) in homogenates. Toxin was barely detectable (less than 1%) by 24 hours after injection. These results indicate that a substantial amount of IL-13 toxin remains localized in the tumor bed for at least 2 hours after administration.
Determination of IL-13 toxin at tumor bed by Western blot analysis.
The KS Y-1 tumors injected with IL-13 toxin (250 μg/kg, IT) were excised, homogenized, and supernatant collected as described in “Materials and methods.” Aliquots of standard IL-13 toxin (lanes 2, 0.1 μg; lane 3, 0.5 μg; lane 4, 1.0 μg; lane 5, 2.0 μg), supernatants containing 40 μg total protein from control tumor (lane 6), 2 hours (lane 7), 4 hours (lane 8), and 24 hours (lane 9) after injection were run on 10 % SDS-PAGE gel. Molecular weight markers (lane 1) are shown in kilodaltons (kd) on the left. The 50 kd arrow on the right indicates the approximate size of IL-13 toxin.
Determination of IL-13 toxin at tumor bed by Western blot analysis.
The KS Y-1 tumors injected with IL-13 toxin (250 μg/kg, IT) were excised, homogenized, and supernatant collected as described in “Materials and methods.” Aliquots of standard IL-13 toxin (lanes 2, 0.1 μg; lane 3, 0.5 μg; lane 4, 1.0 μg; lane 5, 2.0 μg), supernatants containing 40 μg total protein from control tumor (lane 6), 2 hours (lane 7), 4 hours (lane 8), and 24 hours (lane 9) after injection were run on 10 % SDS-PAGE gel. Molecular weight markers (lane 1) are shown in kilodaltons (kd) on the left. The 50 kd arrow on the right indicates the approximate size of IL-13 toxin.
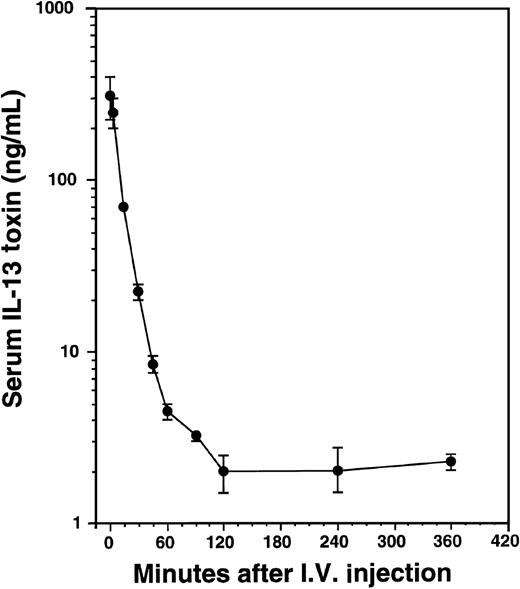
Pharmacokinetics of IL-13 toxin in AIDS-Kaposi's sarcoma xenografts
To determine serum concentrations of IL-13 toxin, mice implanted with KS Y-1 tumors received a single intravenous injection of 100 μg/kg of toxin (Figure 8). The levels of IL-13 toxin in serum collected at different time intervals were determined. The serum half-life of IL-13 toxin was calculated to be t1/2α = 9 minutes and t1/2β = 75 minutes. The short half-life of IL-13 toxin may be one reason that AIDS-KS tumors were not completely regressed when injected intravenously, indicating that continuous availability of the drug at higher level is necessary for complete KS tumor regression.
Pharmacokinetics of IL-13 toxin.
After a single intravenous injection of IL-13 toxin (100 μg/kg), the sera were collected at various times as indicated in “Material and methods.” The amount of IL-13 toxin in sera was calculated from cytotoxicity data generated by protein synthesis inhibition assay.
Pharmacokinetics of IL-13 toxin.
After a single intravenous injection of IL-13 toxin (100 μg/kg), the sera were collected at various times as indicated in “Material and methods.” The amount of IL-13 toxin in sera was calculated from cytotoxicity data generated by protein synthesis inhibition assay.
Detection of IL-13 toxin antibody in treated mice serum
To determine whether nude mice generate neutralizing antibodies against IL-13 toxin, sera were collected from mice in Figure 4A at the end of the experiment on day 47 (32 days after IL13-PE38QQR injection). None of the mice generated neutralizing antibody as indicated by the fact that sera from these animals did not neutralize the cytotoxic activity of recombinant IL13-PE38QQR (data not shown). Serum from a patient known to have developed neutralizing antibodies, did neutralize the cytotoxicity exerted by standard recombinant IL-13 toxin (data not shown).
Discussion
We have previously reported that AIDS-KS primary cell cultures express plasma membrane receptors for IL-13.19 Here, we demonstrate that IL-13R on KS cells are comprised of IL-13Rα, α′, and IL-4Rβ chains, indicating that these cells express type I IL-13R. These IL-13R on KS cells share 2 chains with the IL-4R system.35 36 The IL-13R on KS cells were targeted by chimeric fusion protein, IL-13 toxin, which was found to be specifically cytotoxic against 2 tumorigenic cell lines in vitro. The in vivo antitumor activity of IL-13 toxin was then evaluated in human KS xenografts. IL-13 toxin showed remarkable antitumor activities when flank tumor-bearing nude mice were injected with various doses of IL-13 toxin via different routes and schedules. The intravenous administration of IL-13 toxin produced a significant growth retardation of established tumors, but complete regressions were not observed in either tumor model. Because the half-life of IL-13 toxin in tumor-bearing mice was very short (t1/2α = 9 minutes and t1/2β = 75 minutes), it was hypothesized that the levels of IL-13 toxin at the tumor site were not sufficient to mediate complete and durable responses.
To overcome this problem, IL-13 toxin was administered by the intraperitoneal routes. Twice daily injections of 50 μg/kg dose for 5 days (total 10 injections) eradicated tumors completely in some animals; a total of 20 injections given by a similar schedule produced durable complete responses in all animals. These results suggest that prolonged and continuous exposure of IL-13 toxin is necessary for optimal and durable antitumor activity. Future studies will explore continuous infusion of IL-13 toxin via intraperitoneal micropumps for further enhancement of antineoplastic activity.
In contrast to intravenous and intraperitoneal routes, tumor-bearing mice tolerated higher doses of IL-13 toxins by the IT route. Intratumoral injections of 250 μg/kg of IL-13 toxin into KS Y-1 (qod × 3) and KS-imm (qd × 5) tumors produced CRs in all the treated mice without any visible toxicity (Figure 6A and D). Because IT distribution of the drug primarily depends on diffusion, it is possible that the agent may not distribute uniformly to the entire tumor bed. To address this heterogenous distributions, IL-13 toxin was injected in all 4 quadrants of KS tumors. By this mode of delivery, 70% of IL-13 toxin remained localized to the tumor bed 2 hours after injection (Figure 7). Because we observed that only 0.2% of toxin was present in mouse serum after 2 hours IT injection, the remaining IL-13 toxin at the tumor site may have undergone necessary proteolytic degradation to exert its cell killing effect.
It is of interest to note that IT injections of IL-13 toxin were also able to inhibit the growth of large, established tumors. Five daily injections of IL-13 toxin (250 μg/kg) arrested the growth of tumors approximately 124 mm2 in size, which represents more than 6% of the body weight of these tumor-bearing mice. In humans, a comparable size would corresponds to more than 4 kg of tumor, based on a 70 kg patient, which is a nonclinically achieved size. Thus, our results suggest that IL-13 toxin may be useful even for patients with high tumor burdens.
Of the 2 models studied, KS Y-1 tumors were more sensitive to IL-13 toxin in vivo than KS-imm tumors. This differential sensitivity may be directly attributed to higher in vitro sensitivity of KS Y-1 (IC50 26 ng/mL) toward IL-13 toxin than KS-imm (IC50 380 ng/mL). It is possible that the presence of lower affinity receptors on KS-imm cells (kd = 3.7 nmol/L) compared with KS Y-1 cells (0.9 nmol/L) resulted in lower sensitivity toward IL-13 toxin in vitro and in vivo. Nonetheless, IL-13 toxin was able to mediate remarkable antitumor activity against KS-imm tumors when administered by the IT route.
Recent studies have described the use of tumor-directed cytotoxin therapy in animal models of KS disease. The chimeric proteins composed of IL-2, IL-4, IL-6, VEGF, and diphtheria toxin were found to be highly cytotoxic in vitro.16-18,37 We have recently reported that IL4-PE toxin is highly cytotoxic against AIDS-KS in vivo.31Our current studies demonstrate that IL13-PE38 is also extremely active in AIDS-KS models. Whether IL13-PE38 is superior to other cytotoxins remains to be seen. However, it is important to note that all these therapeutic agents target different receptors expressed on KS tumor cells.
The major limitation of tumor-directed cytotoxins and immunotoxins has been its nonspecific toxicity, immunogenicity, and species specificity of the ligands. Because human IL-13 can bind to murine IL-13R,37 our approach overcame species-specific limitation. Because of this property, toxicity studies performed in the murine model will more likely be reflective of the situations in humans. Since a 50 μg/kg dose injected intravenously every other day for 3 or 9 injections only produced reversible grade I hepatic toxicity (Husain et al, unpublished data), we predict that IL-13 toxin would be well tolerated in a clinical trial. Although none of the mice produced a neutralizing antibody to IL13-PE38QQR, our course of therapy was able to mediate durable antitumor activity before antibodies were generated. Whether neutralizing antibodies will be generated in humans that will limit repeat administration of IL-13 toxin is not known. In this regard, it is important to note that in the presence of neutralizing antibodies, DAB389-IL-2 was able to cause tumor regression in patients with cutaneous T-cell lymphoma.38
In conclusion, our results suggest that local therapies can be used in the management of localized tumors, whereas systemic therapy may be appropriate for disseminated AIDS-KS. A combination of both therapies may be another option where tumors recur. A high dose of IT injection, followed by low-dose systemic infusion may inhibit recurrence of tumors without causing toxicity. Finally, our study is the first to establish that IL13 PE38QQR may be a potential therapeutic agent for the treatment of AIDS-KS and further clinical studies must be performed to explore its potential.
Acknowledgments
We thank Drs Malcolm Moos, Donald Fink, and Ms Mercedes Serabian for reviewing this manuscript; Ms Pamela Dover for technical assistance and procurement of laboratory supplies; Dr Bharat Joshi for providing the IL-13 toxin and assisting in the Western blot experiments; Drs Parkash Gill and Adriana Albini for the KS Y-1 and KS-imm cell lines, respectively. We also acknowledge Drs Robert Kreitman and Ira Pastan of NCI for the anti-PE antibody, PE38 plasmid, and fruitful discussions on tumor targeting.
Reprints:Raj K. Puri, Laboratory of Molecular Tumor Biology, Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research, Food and Drug Administration, National Institutes of Health-Bldg 29B, Rm 2NN10, 29 Lincoln Dr, MSC 4555, Bethesda, MD 20892; e-mail: puri@cber.fda.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal