Abstract
The human lymphocyte activation marker CD30 is highly overexpressed on Hodgkin/Reed–Sternberg cells and represents an ideal target for selective immunotherapy. We used the murine anti-CD30 hybridoma Ki-4 to construct a new recombinant immunotoxin (rIT) for possible clinical use in patients with CD30+ lymphoma. Hybridoma V genes were polymerase chain reaction-amplified, assembled, cloned, and expressed as a mini-library for display on filamentous phage. Functional Ki-4 scFv obtained by selection of binding phage on the CD30-expressing Hodgkin lymphoma cell line L540cy was inserted into the bacterial expression vector pBM1.1 and fused to a deletion mutant ofPseudomonas exotoxin A (ETA′). Periplasmically expressed Ki-4(scFv)–ETA′ demonstrated specific activity against a variety of CD30+ lymphoma cells as assessed by different in vitro assays. To evaluate in vivo antitumor activity, severe combined immunodeficient mice challenged with human lymphoma cell lines were treated with the immunotoxin. The blood distribution time t½ of Ki-4(scFv)–ETA′ was 19 minutes, and its serum elimination time t½ was 193 minutes. A single intravenous injection of 40 μg rIT 1 day after tumor inoculation rendered 90% of the mice tumor free, extending the mean survival time to more than 200 days compared with 38.1 days in the phosphate-buffered saline control group (P < .001). This new rIT is a promising candidate for further clinical evaluation in patients with Hodgkin lymphoma or other CD30+malignancies.
Although patients with Hodgkin lymphoma or anaplastic large-cell lymphoma can be cured by polychemotherapy and extended field radiotherapy, less than 30% of those whose lymphoma relapses remain disease-free after second-line treatment. The major reason for this poor outcome is the persistence of residual tumor cells surviving standard therapy.1-3 Thus, the selective elimination of minimal residual disease using monoclonal antibody-based immunoconjugates after standard treatment might improve the outcome in malignant lymphoma.
One of the most promising target antigens for immunotherapy of malignant lymphoma such as Hodgkin lymphoma or anaplastic large-cell lymphoma is the CD30 receptor. This antigen was originally discovered on cultured Hodgkin/Reed–Sternberg (H-RS) cells using the monoclonal antibody (mAb) Ki-1.4 The gene encoding for the CD30 receptor molecule5 is located on chromosome 1p36. The naturally occurring CD30 ligand also has been identified and cloned.6 The CD30/CD30 ligand system triggers cytolytic cell death in malignant lymphoma cell lines and induces proliferation and cytokine production in T cells or neutrophils.7Monoclonal antibodies against CD30 have been explored as vehicles for cytostatic drugs8 or plant toxins.9Immunotoxins constructed with anti-CD30 mAbs chemically linked to catalytically active toxins demonstrated specificity and potent anti-tumor activity against Hodgkin lymphoma cells in vitro and in mouse models.10-12 Twelve patients with refractory relapsed Hodgkin lymphoma were treated with an immunotoxin constructed by conjugating the anti-CD30 mAb BerH2 to Saporin-6 (Ber-H2-S6).13 Five patients exhibited a rapid reduction in tumor mass, underlining the validity of CD30 as a target antigen in this malignancy. The dose-limiting toxicity in this and other trials was related to vascular leak syndrome (VLS) consisting of decreased serum albumin level, weight gain, edema, pulmonary edema, and aphasia.14,15 Recently, it was reported that vascular epithelial cells are damaged by IL-2 and the catalytic proportions of applied toxic enzymes, which share a common (x)D(y) amino acid sequence motif where x = L, I, G, or V andy = V, L, or S and that this damage to epithelial cells might initiate VLS.16
To circumvent VLS, new therapeutics such as recombinant immunotoxin (rIT) with deleted (x)D(y) amino acid sequence motifs might be developed by DNA technology. Chimeric proteins composed of a truncated, binding-deficient toxin fused to a single-chain antibody fragment (scFv), rITs are defined, compact molecules that can be modified more easily and produced more economically than their chemical counterparts.
Our group has developed and evaluated a number of chemically linked immunotoxins for clinical application.14,15 One of the constructs, Ki-4.dgA,12 is based on the anti-CD30 mAb Ki-4, which has been demonstrated to inhibit the shedding of the extracellular part of the CD30 molecule.17 We subsequently used phage display technology for the selection of a specific, high-affinity Ki-4 scFv that was then genetically fused to a modifiedPseudomonas exotoxin A gene (ETA′).11Recombinant immunotoxins expressed so far under standard conditions remained primarily associated with inclusion bodies18 and were purified after careful denaturation and renaturation procedures.19 Under optimized conditions, only 5% to 10% of the input protein was properly folded and active. Thus, we developed a new protocol for the periplasmic expression of recombinant proteins under osmotic stress conditions in the presence of compatible solutes resulting in more than 95% functional activity in terms of specific in vitro binding and cytotoxic activity.20 In the current study we demonstrate the specific effects of the first recombinant anti-CD30 immunotoxin against disseminated human Hodgkin lymphoma in severe combined immunodeficiency (SCID) mice.
Materials and methods
Bacterial strains, oligonucleotides, and plasmids
Escherichia coli TG1 and E coli HB2151 were purchased from Pharmacia (Freiburg, Germany) and used as hosts for the synthesis of scFv-displaying phages or soluble scFv, respectively.E coli BL21(DE3), purchased from Novagen, (Abingdon, UK), were used for the synthesis of rIT. The phagemid vector pCANTAB621 is derived from pHEN122 and carries an additional His6 tag for immobilized metal-ion affinity chromatography (IMAC) purification. It is used for N-terminal fusion ofSfiI/NotI–scFv fragments to the minor coat protein p3 of filamentous phage M13. The plasmid pBM1.123 is derived from the pET27b vector (Novagen) and is used for N-terminal fusion of scFv to a modified deletion mutant of Pseudomonas aeruginosaexotoxin A.24 Synthetic oligonucleotides were synthesized by Eurogentec (Seraing, Belgium). Plasmids were prepared by the alkaline lysis method and purified using plasmid kits from Qiagen (Hilden, Germany). Restriction fragments or polymerase chain reaction products were separated by horizontal agarose gel electrophoresis and extracted with Qiaex II (Qiagen). Cloning into plasmid vectors was performed by standard methods.25
Cell culture
Cell lines including the Hodgkin-derived L540cy,26 its derivative L540rec (recultured from a subcutaneously growing tumor),27 HD-MyZ (kindly provided by B. Dörken),28 and the hybridoma cell line Ki-417 were cultivated in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated fetal calf serum, 50 μg/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine. All cells were cultured at 37°C in a 5% CO2atmosphere.
Cloning
Ki-4 VH and VL genes were amplified, assembled, and cloned into pCANTAB6 as previously described.11 Plasmids were transfected into 50 μL E coli TG-1 by electroporation as described elsewhere.29 The cells were grown for 1 hour in 2 × TY medium at 37°C before plating on 2 × TY agar medium containing 100 μg ampicillin/mL and 2% (wt/vol) glucose (2 × TY-Amp-Glu). Binding Ki-4(scFv) molecules were enriched on L540cy cells as reported.11 Their genes were released from phagemid vector pCANTAB6 by SfiI/NotI digestion and inserted into SfiI/NotI–restricted expression vector pBM1.1 containing the ETA′ gene.23 The resultant plasmids were transfected into E coli BL21(DE3).
Periplasmic expression and purification of the recombinant immunotoxins
As described recently,20 rITs were expressed under the control of the isopropyl β-thiogalactoside–inducibleTaq promotor in the E coli strain BL21(DE3).30 Briefly, bacteria were grown overnight at 26°C in Terrific broth containing 0.5 mmol/L ZnCl2 and 50 μg/mL kanamycin. The culture was diluted 30-fold in 200 mL of the same medium, at an OD600 of 2, and was supplemented with 0.5 mmol/L sorbitol, 4% NaCl, and 10 mmol/L betaine. Then it was incubated at 26°C for additional 30 to 60 minutes. Thereafter, immunotoxin production was induced by the addition of 2 mmol/L isopropylβ-thiogalactoside at 26°C. Fifteen hours later cells were harvested by centrifugation at 3700g for 10 minutes at 4°C. For all the following steps, tubes were kept on ice. The bacterial pellet was centrifuged, and its wet weight was determined. Cells were frozen at −196°C. After they thawed, the cells were resuspended in 75 mmol/L Tris HCl, pH 8, 300 mmol/L NaCl, 1 capsule protease inhibitors/50 mL NaCl (Complete; Roche Diagnostics, Mannheim, Germany), 5 mmol/L dithiothreitol, 10 mmol/L EDTA, and 10% glycerol, and they were sonicated 6 times for 30 seconds at 200 W. The periplasmic fraction was recovered as supernatant after centrifugation at 21 000g for 30 minutes at 4°C and transferred to 75 mmol/L Tris HCl, pH 8, 1 mol/L NaCl, and 10% glycerol using Hitrap desalting columns (Pharmacia). Recombinant immunotoxin was partially purified by immobilized IMAC using nickel–nitriloacetic acid (Ni2+-NTA) chelating Sepharose (Qiagen) on a BioLogic workstation (BioRad, München, Germany). Bound protein was eluted with 250 mmol/L imidazole in 75 mmol/L Tris HCl, pH 8, 1 mol/L NaCl, and 10% glycerol. Fractions containing RFT5(scFv)–ETA′ or Ki-4(scFv)–ETA′ were pooled and concentrated by ultrafiltration. Functional rIT was finally purified through size exclusion chromatography (SEC) using Bio-Prep SE-100/17 (BioRad) on the BioLogic workstation by separation in phosphate-buffered saline (PBS), pH 7.4, and 1 mol/L NaCl. Purified protein was analyzed by SDS-PAGE and quantified by densitometry (GS-700 Imaging Densitometer; BioRad) after Coomassie staining according to bovine serum albumin standards.
Binding analyses
The binding activity of Ki-4(scFv)–ETA′ was determined by CD30 receptor enzyme-linked immunosorbent assay (ELISA) as previously documented.11 Samples were incubated with anti-ETA′ mAb TC-1 for 1 hour. After washing, 100 μL (Fab′ 2) fragments of peroxidase-coupled goat–antimouse–IgG (Roche Diagnostics) 1:5000 in Tris-buffered saline (TBS) containing 0.5% bovine serum albumin (BSA) and 0.05% Tween 20 were added, and samples were incubated for 1 hour at room temperature. Bound Ki-4(scFv)–ETA′ was detected by the addition of 100 μLo-Phenylenediamine–dihydrochloride (Sigma, Deisenhofen, Germany) after incubation for 1 hour. Absorbance at 405 nm was measured with an ELISA reader (MWG Biotech, Ebersberg, Germany). Native mAbs were used as controls.
Cell-binding activity of Ki-4(scFv)–ETA′ expressed in E coli BL21(DE3) was evaluated by FACS analysis as described.11 Bound immunotoxin was documented using mAb TC-1 and fluorescein isothiocyanate-labeled goat–antimouse immunoglobulin on a FACScan (Becton Dickinson, Heidelberg, Germany).
Cytotoxicity assays
The effect of Ki-4(scFv)–ETA′ on the protein synthesis of the target cell lines was determined by measurement of [3H]-leucine incorporation as published recently.31 Incorporated [3H]-leucine was measured by liquid scintillation (LS/801; Beckman, Munich, Germany). The concentration required to achieve a 50% reduction in protein synthesis compared with untreated control cultures (IC50) was calculated. All measurements were made in triplicate. Competition experiments were performed with L540cy cells in the presence or absence of 10 μg/mL Ki-4 mAb as a competitor for Ki-4(scFv)–ETA′.
The cytotoxic effect was also determined by measuring the metabolism of yellow tetrazolium salt to a water-soluble orange formazan dye as previously reported.20 The spectrophotometric absorbances of the samples were measured at 450 nmol/L and 650 nmol/L (reference wavelengths) with an ELISA reader (MWG Biotech, Ebersberg, Germany).
Animals
The SCID mice were obtained from our own colony and were maintained under sterile conditions. No antibiotic prophylaxis was provided. ELISA was performed regularly to detect antibodies that might have leaked into the sera of the animals.
Antitumor experiments in mice
To evaluate the effects of immunotoxin treatment on the survival of SCID mice challenged with human Hodgkin lymphoma cells, animals were randomly divided into groups of 5. One day after inoculation of L540rec Hodgkin cells, mice received either a single intravenous injection of Ki-4(scFv)–ETA′, ETA′, or PBS in a volume of 400 μL PBS. The dose of immunotoxin (40 μg functional rIT) represented the maximum tolerated dose in SCID mice, determined in a dose-escalation study of groups of 3 mice.
Adult 4- to 6-week-old SCID mice were injected intravenously through the tail vein with 1 × 107 L540rec suspended in 600μL PBS as published.27 Vital signs were recorded daily, and weight was measured once per week. The SCID mice were killed when they showed signs of progressive disease such as weight loss, ruffled fur, and inactivity. After gross examination, organs that were macroscopically infiltrated with L540rec lymphoma cells were fixed in formalin. Cytocentrifuge slides were prepared from bone marrow extracted from the tibiae.
Animals surviving up to 200 days were killed at that time and full postmortem examinations were carried out. Spleen, liver, lungs, brain, kidney, ovary, and thymus were explanted, fixed in formalin, and taken for histopathologic examination and immunocytochemical staining for expression of human CD25 and CD30.
Immunohistochemical staining
Gross examination of various tissues of each animal was performed within 24 hours of death, as described recently.27 The tissues were removed, fixed in 4% formaldehyde buffered in TBS, dehydrated through a graded series of ethanol concentrations, and embedded in paraffin. Sections 4-μm thick were stained immunohistochemically with the monoclonal antibody Ber-H2 (anti-CD30) purchased from DAKO Diagnostica (Hamburg, Germany) and visualized by the biotin–streptavidin method using fast red as a chromogen.32 For detection of the CD25 receptor, an antigen-retrieval technique was applied33 using a commercially available antibody, 2A3 (Becton Dickinson, San Jose, CA). Tissue sections were counterstained with hematoxylin, mounted for light microscopy, and digitally documented using the Imaging System KS300 (Kontron Elektronik, München, Germany).
Detection of soluble CD30
CD30 receptor assays were performed in triplicate using clarified sera (1 μL/well) and photometric ELISA for quantitative determination of soluble human CD30 antigen, according to the manufacturer's instructions (DAKO Diagnostica).
Blood clearance study
SCID mice weighing approximately 20 g were injected intravenously through the tail vein with 40 μg rIT. At 20, 40, 60, 120, 240, and 360 minutes after injection, blood was drawn from the orbital sinus. For each time point, blood from 3 mice was drawn. Serum was stored at −80°C before immunotoxin levels were determined.
ELISA was performed according to standard methods in 96-well Polysorb microtiter plates (Nunc, Wiesbaden, Germany) coated at 4°C with 100 μL/well of 10 μg/mL antimouse–IgG (Sigma) in coating buffer (0.2 mol/L Na2CO3, 0.2 mol/L NaHCO3) (Merck, Darmstadt, Germany). Anti-ETA′ mAb TC-1 was bound for the detection of Ki-4(scFv)–ETA′ (1:100 in TBS containing 0.5% BSA and 0.05% Tween 20). Bound rIT was visualized with peroxidase-coupled goat–antimouse–IgG (1:1000 in TBS containing 0.5% BSA and 0.05% Tween 20).
Stability in mouse blood serum
The stability of 40 μg/mL rIT was evaluated by incubation in mouse serum at 37°C. Active immunotoxin remaining at different times was determined by cell proliferation and ELISA assays on L540rec cells.
Statistical analysis
Blood clearance was determined in the 2-compartment model after nonlinear regression of the obtained data with SPSS 8.0. Survival analyses were performed by the Kaplan-Meier method, and statistical significance was compared by the log-rank test. Using the ttest, the highest sCD30 concentrations of the PBS groups were correlated with those of the rIT-treated animals.
Results
In vitro characterization of Ki-4(scFv)–ETA′
After cloning the RFT5 scFv into the bacterial expression vector pBM1.1 and the transformation of E coli BL21(DE3), bacteria were grown under osmotic stress in the presence of betaine. The 70-kd Ki-4(scFv)–ETA′ was functionally accumulated in the periplasmic space and subsequently purified by combinations of IMAC and size-exclusion chromatography. The rIT was substantially stabilized during purification by 1 mol/L NaCl, eluted from Ni2+-NTA columns by 250 mmol/L imidazole, and separated by size-exclusion chromatography between 20 and 100 kd. Only intact Ki-4(scFv)–ETA′, but no degradation products or rIT aggregates, was collected. The purity of the prepared protein was determined by densitometry and by receptor ELISA. Functional Ki-4(scFv)–ETA′ was enriched to more than 95%. The rIT was purified by combinations of IMAC and size exclusion chromatography to a final concentration of approximately 1 mg/4 g cell paste from bacterial shaking cultures.20 In contrast to our former standard periplasmic expression and extraction protocol34 that resulted in some μg/4 g cell paste, the functionality of Ki-4(scFv)–ETA′ was preserved after the extraction procedure.
Binding properties of the Ki-4(scFv)–ETA′ against CD30+ target cell lines were measured by ELISA and flow cytometry.11,20 The rIT bound to CD30-positive cells tested, but not to the CD30− Burkitt lymphoma cell line BL38 or to the Hodgkin-derived cell line HD-MyZ. CD30 specificity was verified by competitive ELISA and flow cytometric experiments using 10 μg/mL Ki-4 mAb. The cytotoxic activity of Ki-4(scFv)–ETA′ was documented by [3H]-leucin uptake and yellow tetrazolium salt assays.11,20 CD30+ cells were killed by rIT purified from periplasm and from inclusion bodies with IC50′ of 3 to 6 ng/mL, whereas CD30−cell lines were not affected. Again, CD30 specificity was shown by competition experiments using 10 μg/mL Ki-4 mAb.20
Growth of L540rec Hodgkin cells in SCID mice
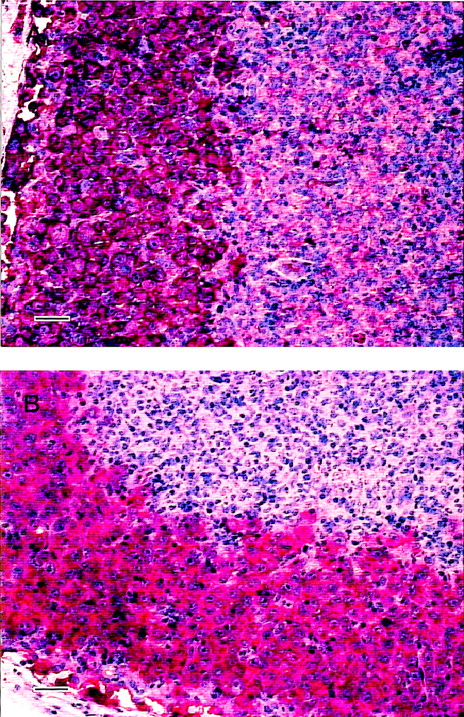
Inoculation of 1 × 107 L540rec cells through the tail vein induced tumor growth in 100% of untreated SCID mice. Untreated animals developed signs of progressive disease approximately 4 weeks after tumor cell challenge. Histologic examination revealed patterns of dissemination of L540rec tumors in various organs of the 32 female PBS-treated SCID mice, as previously published.27Tumors stained intensively with the mAbs 2A3 (anti-CD25) and Ber-H2 (anti-CD30), as demonstrated by immunohistochemistry. The histology of an involved lymph node is shown in Figure1.
Immunohistopathology of L540rec tumors in SCID mice.
Lymph node infiltrates visualized by 2A3 (A) and Ber-H2 (B) using the biotin–streptavidin method. Magnification ×200; scale bar, 50 μm.
Immunohistopathology of L540rec tumors in SCID mice.
Lymph node infiltrates visualized by 2A3 (A) and Ber-H2 (B) using the biotin–streptavidin method. Magnification ×200; scale bar, 50 μm.
Toxicity of Ki-4(scFv)–ETA′ in SCID mice
The MTD of Ki-4(scFv)–ETA′ was determined in a dose-escalation study of groups of 3 mice intravenously treated with 20, 30, 40, 50, and 60 μg rIT (Table 1). The MTD was reached at 40 μg per animal.
Maximum tolerated dose of rIT in SCID mice
| Days after injection . | PBS . | Ki-4(scFv)–ETA′ (μg) . | ||||
|---|---|---|---|---|---|---|
| 20 . | 30 . | 40 . | 50 . | 60 . | ||
| 2 | 2/2 | 3/3 | 3/3 | 3/3 | 1/3 | 0/3 |
| 4 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 7 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 14 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| Days after injection . | PBS . | Ki-4(scFv)–ETA′ (μg) . | ||||
|---|---|---|---|---|---|---|
| 20 . | 30 . | 40 . | 50 . | 60 . | ||
| 2 | 2/2 | 3/3 | 3/3 | 3/3 | 1/3 | 0/3 |
| 4 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 7 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
| 14 | 2/2 | 3/3 | 3/3 | 3/3 | 0/3 | 0/3 |
Numbers correspond to surviving animals/total.
Pharmacokinetics of Ki-4(scFv)–ETA′
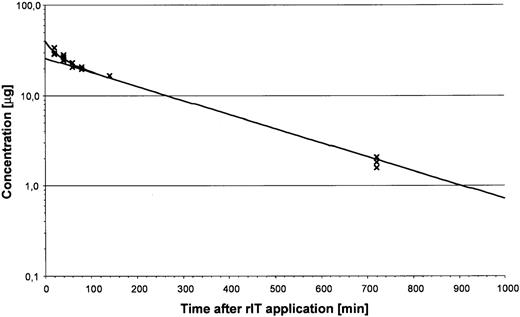
To determine the blood clearance of the rIT in SCID mice, animals were treated with a single intravenous dose of 40 μg Ki-4(scFv)–ETA′. Blood was drawn at different time points, and rIT presence was measured by ELISA. Kinetics of the 2-compartment model was determined after nonlinear regression. The dose/time relationship was calculated as: c = 13.95e−0.0371t + 26.05e−0.0036t, wherec = concentration (μg), and t = time (minutes). Thus, the distribution time t½α was 19 minutes, and the serum elimination time t½β was 193 minutes (Figure2).
Blood levels of Ki-4(scFv)–ETA′ in mice.
Female SCID mice were injected intravenously with 40 μg rIT. Blood samples were drawn at different times. Immunotoxin levels were determined by ELISA. Results are averaged from 3 animals for each time point.
Blood levels of Ki-4(scFv)–ETA′ in mice.
Female SCID mice were injected intravenously with 40 μg rIT. Blood samples were drawn at different times. Immunotoxin levels were determined by ELISA. Results are averaged from 3 animals for each time point.
Stability of Ki-4(scFv)–ETA′ in mouse serum
We examined the stability of Ki-4(scFv)–ETA′ by incubating 0.25 μg/mL purified protein at 37°C for different periods of time in mouse serum. The biologic activity of incubated rIT was determined by ELISA using rhCD30 and cell proliferation assays with CD30+ L540rec cells. Ki-4(scFv)–ETA′ was highly stable under these conditions, retaining 85% to 90% of initial binding activity for at least 24 hours (Table2). The IC50 dropped between 8.5 and 13 ng/mL after incubation in mouse serum (Table3). Mouse serum by itself was not cytotoxic to the cells. Thus, Ki-4(scFv)–ETA′ was relatively stable in mouse serum at physiological temperatures exceeding the time range calculated for the serum elimination time t½β.
Stability of Ki-4(scFv)–ETA′ in mouse serum at 37°C determined by ELISA
| Time (h) . | % Binding activity after incubation with Ki-4(scFv)–ETA′ (0.25 μg/mL) . |
|---|---|
| 0 | 100 (±3.6) |
| 1 | 99.7 (±1.5) |
| 2 | 97.5 (±0.98) |
| 24 | 81.4 (±1.2) |
| Time (h) . | % Binding activity after incubation with Ki-4(scFv)–ETA′ (0.25 μg/mL) . |
|---|---|
| 0 | 100 (±3.6) |
| 1 | 99.7 (±1.5) |
| 2 | 97.5 (±0.98) |
| 24 | 81.4 (±1.2) |
Effect of rIT treatment on the survival of tumor-bearing SCID mice
Female SCID mice were treated with the MTD for Ki-4(scFv)–ETA′, corresponding to 40 μg 1 day after tumor challenge by a singular intravenous injection. As documented by Kaplan-Meier analysis (Figure 3), the MST of the PBS- and ETA′-treated control groups was 38.1 ± 1.77 days and 39.8 ± 2.56 days, respectively. There was no difference between animals treated with PBS or ETA′ (P = .5804). Treatment of L540cy-challenged SCID mice with 40 μg Ki-4 mAb alone did not lead to tumor-free survival of all the animals tested.12 In contrast, 9 of 10 mice treated with Ki-4(scFv)–ETA′ did not show any tumor growth more than 200 days after treatment, extending the MST by a factor of 5 (201.6 ± 17.46 days). This result is statistically significant for both control groups (P < .001). None of the animals treated with 40 μg Ki-4(scFv)–ETA′ died of toxicity at this dose level. One of the treated animals died of unknown causes on day 36 after tumor challenge and showed no detectable L540rec cells on examination. The overall results are summarized in Table4.
Antitumor effects of Ki-4(scFv)–ETA′ on disseminated L540rec tumors in SCID mice as documented by Kaplan-Meier.
Groups of 5 animals were treated with PBS, ETA′, or Ki-4(scFv)–ETA′.
Antitumor effects of Ki-4(scFv)–ETA′ on disseminated L540rec tumors in SCID mice as documented by Kaplan-Meier.
Groups of 5 animals were treated with PBS, ETA′, or Ki-4(scFv)–ETA′.
Treatment of disseminated Hodgkin lymphoma in SCID mice with rIT4-150
| . | PBS . | ETA′ . | Ki-4(scFv)–ETA′ . |
|---|---|---|---|
| No. mice | 10 | 5 | 10 |
| L540rec tumors in | |||
| Superficial cervical LN | 5 | 2 | 0 |
| Posterior cervical LN | 1 | 2 | 0 |
| Facial LN | 5 | 1 | 0 |
| Iliac LN | 3 | 1 | 0 |
| Bone marrow | 1 | 0 | 0 |
| Brain | 10 | 5 | 1 |
| Muscle | 6 | 2 | 0 |
| Kidney | 2 | 2 | 0 |
| Ovary | 0 | 3 | 0 |
| Thymus | 2 | 3 | 0 |
| MST | 38.1 ± 1.77 | 39.8 ± 2.56 | 201.6 ± 17.46 |
| No. CR (%) | — | — | 9 (90%) |
| Significance (rel. to PBS) | — | P = .58 | P < .001 |
| . | PBS . | ETA′ . | Ki-4(scFv)–ETA′ . |
|---|---|---|---|
| No. mice | 10 | 5 | 10 |
| L540rec tumors in | |||
| Superficial cervical LN | 5 | 2 | 0 |
| Posterior cervical LN | 1 | 2 | 0 |
| Facial LN | 5 | 1 | 0 |
| Iliac LN | 3 | 1 | 0 |
| Bone marrow | 1 | 0 | 0 |
| Brain | 10 | 5 | 1 |
| Muscle | 6 | 2 | 0 |
| Kidney | 2 | 2 | 0 |
| Ovary | 0 | 3 | 0 |
| Thymus | 2 | 3 | 0 |
| MST | 38.1 ± 1.77 | 39.8 ± 2.56 | 201.6 ± 17.46 |
| No. CR (%) | — | — | 9 (90%) |
| Significance (rel. to PBS) | — | P = .58 | P < .001 |
Animals received a singular intravenous injection of 40 μg ETA′ or Ki-4(scFv)–ETA′ in PBS or PBS on day 1 after L540rec inoculation.
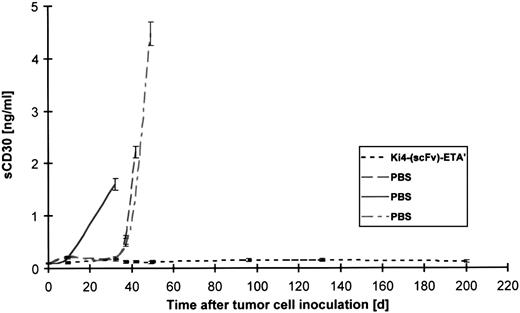
Because soluble CD30 has been shown to correlate with the tumor burden of mice with disseminated L540rec tumors, serum samples of animals were evaluated for circulating soluble CD30 receptor by ELISA. As documented in Figure 4, untreated animals showed increasing CD30 levels. An increase of sCD30 receptor was not detectable in mice treated by a single injection of 40 μg rIT 1 day after tumor challenge (P = .0036).
Soluble CD30R evaluated in 1 μL serum of SCID mice inoculated with L540rec cells using human IL-2 receptor ELISA.
Serum was drawn from 3 control mice treated with PBS and from all mice treated with Ki-4(scFv)–ETA′ at different time points after tumor cell challenge.
Soluble CD30R evaluated in 1 μL serum of SCID mice inoculated with L540rec cells using human IL-2 receptor ELISA.
Serum was drawn from 3 control mice treated with PBS and from all mice treated with Ki-4(scFv)–ETA′ at different time points after tumor cell challenge.
Discussion
In this study, we report the antitumor activity of a recombinant anti-CD30 immunotoxin against disseminated, growing Hodgkin tumors in SCID mice. The major findings to emerge from our study are that (1) Ki-4(scFv)–ETA′ isolated from the periplasmic space of E coli, cultured under osmotic stress in the presence of compatible solutes, can easily be purified in large amounts of functionally active protein by a combination of immobilized metal affinity and molecular size chromatography; (2) in vitro characteristics of the recombinant protein were documented by ELISA, flow cytometry, and cytotoxic activity against a variety of CD30+ lymphoma cells; (3) purified protein was stable to 85% to 90% of its biologic activity in mouse serum for more than 24 hours; (4) Ki4(scFv)–ETA′ showed a blood distribution time t½α of 19 minutes and a serum elimination time t½β of 193 minutes; and (5) a single injection of rIT 1 day after tumor cell inoculation rendered 90% of animals tumor-free when they were examined 200 days after treatment.
The CD30 antigen is overexpressed on hematologic malignancies such as Hodgkin lymphoma, anaplastic large-cell lymphoma, peripheral T-cell lymphoma,35 virally transformed cell lines,35,36 and latently HIV-infected CD4+ T cells.37 After the initial report by our group,10 CD30 has attracted increasing attention for immunotherapy.38 39 This is at least in part attributable to the fact that CD30 is expressed in high copy number on malignant cells compared with its low expression on normal cells.
Initially our group, like most others, used denaturation and renaturation procedures19 to isolate recombinant immunotoxins from E coli.31 Because this method is associated with the major disadvantage of low yields of biologically active protein after renaturation, we developed a new strategy for the periplasmic expression of recombinant ETA′-based fusion proteins using osmotic stress in the presence of compatible solutes.20 This method was successfully applied to different immunotoxins and yielded approximately 1 mg protein/4 g cell paste from bacterial shaking cultures for each construct tested. This expression system has been further standardized, delivering functional protein within 3 weeks from cloning to expression. Using the periplasmic expression strategy followed by a 2-step purification of Ki-4(scFv)–ETA′, we can now easily produce large amounts of functional rIT in a short time.
We isolated Ki-4(scFv)–ETA′ from the periplasmic space with a substantial proportion of biologically active protein (more than 95%). In accordance with published data for other rITs,20,40,41purified Ki-4(scFv)–ETA′ was demonstrated to be stable in terms of binding and cytotoxic activity in mouse serum at 37°C for at least 24 hours. Generally, rIT has a much shorter half-life in mouse serum than in IgG-based chemical conjugates.40 Blood distribution times range from 20 to 30 minutes after challenge with 10 μg rIT or more.24,41 42 The calculated distribution timet½α of 19 minutes determined for Ki-4(scFv)–ETA′ in this study matches these results. The stability in mouse serum indicates the functional activity of the protein during serum elimination timet½β of 193 minutes resulting in excellent antitumor activity against disseminated growing human tumors in SCID mice.
The effects of Ki-4(scFv)–ETA′ on disseminated growing L540rec lymphomas was evaluated by gross macroscopy, immunohistopathology, and measurement of soluble CD30 receptor in serum. A high degree of correlation in detecting L540rec tumors was documented by these methods. Tumors identified by gross macroscopy were confirmed by immunohistology of tumor sections stained for human CD25 and CD30. In addition, soluble CD30 directly correlated with tumor burden.
Our results compare favorably with those obtained using chemically linked immunotoxins. When applied 1 day after tumor challenge, 90% of animals treated with 40 μg recombinant protein were disease-free more than 200 days after tumor cell inoculation (P < .001). In contrast, only 50% of animals treated with the IgG-based ricin A-chain construct, Ki-4.dgA, reached tumor-free survival.12 Although not a statistically verified comparison, these data indicate the good efficacy of our newly developed recombinant construct.
In summary, our results demonstrate the highly potent activity of the first recombinant anti-CD30 immunotoxin isolated from E coli periplasma against disseminated Hodgkin lymphoma in SCID mice.
Acknowledgments
We thank Gisela Schön for assistance with cloning and Silke Drillich for performing the cytotoxicity assays.
Supported in part by Deutsche Forschungsgemeinschaft grants SFB502 and TFB6-98.
S.B. and M.H. contributed equally to this work.
Reprints:S. Barth, Labor fuer Immuntherapie, Medizinische Klinik I der Universitaet zu Koeln, Joseph–Stelzmann–Strasse 9, D-50931 Koeln, Germany; e-mail:stefan.barth@uni-koeln.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal