Abstract
Human T-cell leukemia virus type-I (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL). This study examined the status of the oncogenic transcription factor AP-1 in leukemic cells freshly isolated from patients with ATL. Leukemic cells from peripheral blood of all patients with ATL exhibited constitutive AP-1 DNA binding activity, whereas mononuclear cells from normal individuals did not. In agreement with previous studies, HTLV-I transforming protein, Tax, was found to stimulate the DNA binding activity of AP-1 in a T-cell line. However, HTLV-I genes, including Tax, were not significantly expressed in leukemic cells freshly obtained from patients with ATL. Moreover, all T-cell lines derived from leukemic cells of patients with ATL also displayed constitutive AP-1 DNA binding activity, but expressed little Tax protein. Thus, leukemic cells of patients with ATL appear to have Tax-independent mechanisms that induce AP-1 activity, both in vivo and in vitro. In antibody supershift experiments, AP-1 in fresh leukemic cells and ATL-derived cell lines were found to contain JunD. Consistently, all primary ATL cells and ATL-derived cell lines expressed high levels of JunD messenger RNA. Our results suggest that AP-1 is activated in leukemic cells of patients with ATL through a Tax-independent mechanism and this may play a role in the deregulated phenotypes of ATL leukemic cells.
Adult T-cell leukemia (ATL) is an aggressive and fatal T-cell malignancy caused by infection with human T-cell leukemia virus type I (HTLV-I).1-3 HTLV-I is also associated with the development of a variety of chronic inflammatory diseases such as HTLV-I-associated myelopathy/tropical spastic paraparesis and uveitis. In addition to structural proteins, the HTLV-I genome encodes several regulatory proteins, including Tax,4 a 40-kd transcriptional transactivator of HTLV-I gene expression. There is increasing evidence that Tax plays a crucial role in cellular transformation.5 For instance, Tax transforms fibroblast cell lines and immortalizes primary human T cells in vitro, in the presence of interleukin (IL)-2. Tax also induces various tumor types, including neurofibromas and sarcomas, in addition to large granular lymphocytic leukemia in transgenic animals.
Tax activates a number of cellular genes, including those encoding cytokines,6-8 their receptors,9 and nuclear proto-oncogenes.10-12 Some of these genes are thought to contribute to T-cell immortalization or transformation. Tax activates the transcription of cellular genes through selective enhancer elements such as cyclic adenosine monophosphate-responsive element (CRE), the NF-κB element, and the CArG box. Tax does not directly interact with these enhancers, but with cellular transcription factors or transcriptional modulators such as CRE-binding protein,13,14 serum-responsive factor (CArG box binding protein),15 IκB, IκB kinase, and NF-κB.5,9 Previous studies have shown that T-cell lines transformed by HTLV-I express high levels of AP-1 activity.8,11,16 AP-1 is a transcription factor complex composed of members of the Fos family (c-Fos, FosB, Fra-1, and Fra-2) and the Jun family (c-Jun, JunB, and JunD).17 T-cell lines infected with HTLV-I express high levels of messenger RNA (mRNA) encoding c-Fos, Fra-1, c-Jun, JunB, and JunD.11,18 Some of these genes may also be induced by Tax.10-12,19 We and others have identified an AP-1-like site in several cellular genes as a responsive element for Tax.8,16 19-23 AP-1 has been implicated in carcinogenesis and transformation of T cells. Thus, activation of AP-1 by Tax is thought to contribute to the deregulated phenotypes of T cells infected with HTLV-I.
The development of leukemia in individuals infected with HTLV-I is preceded by a long clinical latent period of 40 to 50 years. In addition, only 5% of HTLV-I carriers develop ATL. Thus, it seems that several events in HTLV-I-infected cells are required for the development of the full malignant phenotype. Most leukemic cells do not express significant levels of Tax in vivo. Indeed, the expression of Tax can only be detected using a highly sensitive reverse transcriptase-polymerase chain reaction (RT-PCR) method.24 Thus, the expression of viral proteins, including Tax, does not seem to be necessary for the proliferation of leukemic cells in the late stages of the disease. However, the cellular events that cause deregulated proliferation of leukemic cells in vivo, have not yet been elucidated.
In this study, we investigated AP-1 activity in peripheral blood mononuclear cells (PBMC), freshly obtained from patients with ATL. Results showed that AP-1 complexes containing JunD were highly expressed in PBMC from all patients with ATL, although no viral gene expression was detected. Furthermore, all T-cell lines derived from patients with ATL displayed constitutive AP-1 DNA binding activity involving JunD, but expressed little Tax protein. These findings suggest that Tax is not the only mechanism for constitutive activation of AP-1 in HTLV-I-infected T cells. Hence, a Tax-independent mechanism appears to exist for constitutive AP-1 activation in leukemic cells of patients with ATL. These findings are discussed in the context of deregulated proliferation of leukemic cells in the absence of detectable viral gene expression in vivo.
Materials and methods
Cells
Jurkat, MOLT-4, and CCRF-CEM are HTLV-I-negative human T-cell lines. ST-1, SO-4, and KK-1 are IL-2-dependent T-cell lines of leukemic cell origin, established from patients with ATL.25 The leukemic cell origin of these 3 cell lines was confirmed by identification of the same provirus integration sites or T-cell receptor β-chain gene rearrangement profiles found in their original leukemic cells, using Southern blot analysis. These 3 cell lines were maintained in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS), containing 10 ng/mL recombinant human IL-2 (a kind gift from Takeda Chemical Industries, Osaka, Japan). MT-126 and TL-OmI27are leukemic T-cell lines derived from patients with ATL. HUT-10228 was established from a patient with cutaneous T-cell lymphoma originally diagnosed as Sézary syndrome but later considered as lymphoma-type ATL. Unfortunately, the clonal origin of this line is unclear and can no longer be determined. MT-2,29 MT-4,30 C5/MJ,31 and SLB-132 are HTLV-I transformed T-cell lines, established by an in vitro coculture protocol. MT-2, MT-4, and C5/MJ are umbilical cord blood T-cell lines, whereas SLB-1 is an adult peripheral blood T-cell line (Table 1). These cell lines, in addition to control, HTLV-I-negative cell lines, were maintained in RPMI 1640 supplemented with 10% FBS. JPX-933 is a derivative of Jurkat cells, which carries the transfected Tax gene under the control of a metallothionein promoter. Expression of Tax was induced by adding 20 μmol/L CdCl2 into the medium. PBMC were analyzed from 11 patients, including 7 with acute-type ATL (patients 1, 2, 3, 5, 6, 9, and 10) and 4 with chronic-type ATL (patients 4, 7, 8, and 11).34 The diagnosis of ATL was based on clinical features, hematologic characteristics, presence of serum antibodies to ATL-associated antigens, and presence of HTLV-I proviral genome in DNA from leukemic cells. PBMC were isolated by Ficoll/Hypaque (Pharmacia LKB, Uppsala, Sweden) using density gradient centrifugation. Each patient had more than 90% leukemic cells in the blood at the time of analysis.
Characteristics of T-cell lines
| Cell line . | Origin . | IL-2 dependency* . | HTLV-I provirus . | HTLV-I mRNA† . | Tax protein† . | JunD mRNA† . | AP-1 DNA binding† . |
|---|---|---|---|---|---|---|---|
| Jurkat | ALL‡ | — | — | — | — | — | — |
| MOLT-4 | ALL‡ | — | — | — | — | — | — |
| CCRF-CEM | ALL‡ | — | — | — | — | — | — |
| MT-2 | Cord1-153 | — | + | +++ | ++ | + | +++ |
| MT-4 | Cord1-153 | — | + | ++ | ++ | ++ | ++ |
| C5/MJ | Cord1-153 | — | + | + | + | ++ | ++ |
| SLB-1 | Normal1-153 | — | + | +++ | ++ | + | +++ |
| HUT-102 | ? | — | + | +++ | ++ | ++ | +++ |
| MT-1 | ATL | — | + | — | — | + | ++ |
| TL-OmI | ATL | — | + | — | — | + | ++ |
| ST-1 | ATL | + | + | — | — | + | + |
| SO-4 | ATL | + | + | — | — | + | + |
| KK-1 | ATL | + | + | — | — | + | ++ |
| Cell line . | Origin . | IL-2 dependency* . | HTLV-I provirus . | HTLV-I mRNA† . | Tax protein† . | JunD mRNA† . | AP-1 DNA binding† . |
|---|---|---|---|---|---|---|---|
| Jurkat | ALL‡ | — | — | — | — | — | — |
| MOLT-4 | ALL‡ | — | — | — | — | — | — |
| CCRF-CEM | ALL‡ | — | — | — | — | — | — |
| MT-2 | Cord1-153 | — | + | +++ | ++ | + | +++ |
| MT-4 | Cord1-153 | — | + | ++ | ++ | ++ | ++ |
| C5/MJ | Cord1-153 | — | + | + | + | ++ | ++ |
| SLB-1 | Normal1-153 | — | + | +++ | ++ | + | +++ |
| HUT-102 | ? | — | + | +++ | ++ | ++ | +++ |
| MT-1 | ATL | — | + | — | — | + | ++ |
| TL-OmI | ATL | — | + | — | — | + | ++ |
| ST-1 | ATL | + | + | — | — | + | + |
| SO-4 | ATL | + | + | — | — | + | + |
| KK-1 | ATL | + | + | — | — | + | ++ |
Dependency on IL-2 (10 ng/mL) in the culture medium for growth support.
Northern analysis of HTLV-I and JunD transcripts, Western analysis of Tax expression, and AP-1 DNA binding activity in cell lines, scored on a scale of 0 to 3+, with 0 being negative and 3+ being strongly positive.
ALL, acute lymphoblastic leukemia.
Established by cocultivation of normal cord blood or peripheral blood lymphocytes with HTLV-I infected cells.
Northern blot analysis
Total cellular RNA was extracted from the cells using Trizol as described by the supplier (GIBCO-BRL, Gaithersburg, MD). Total RNA (20 μg) was electrophoresed on a formaldehyde-agarose gel and transferred onto a nylon filter. Filters were prehybridized (in 0.5 mol/L sodium phosphate, 0.1% bovine serum albumin, 7% sodium dodecyl sulfate, 100 μg/mL salmon testis DNA, and 100 μg/mL yeast RNA) for 2 hours at 65°C, and then hybridized overnight with the following α-32P radiolabeled probes: complementary DNA (cDNA) of human JunD,35 glyceraldehyde-3-phosphate dehydrogenase (GAPDH),36 and HTLV-I Tax.37 Radiolabeled probes were generated using a Megaprime DNA Labeling system (Amersham, Arlington Heights, IL).
Western blotting
Cell lysates prepared from T-cell lines were resolved by electrophoresis on 10% polyacrylamide gels and transferred to polyvinylidine difluoride membranes. The blots were incubated with the mouse monoclonal anti-Tax antibody, Lt-4,38 rinsed, and incubated with antimouse immunoglobulin conjugated to horseradish peroxidase. Proteins recognized by the antibodies were visualized using the enhanced chemiluminescence Western blotting detection system (Amersham).
Oligonucleotides
The sequence of the oligonucleotide corresponding to the AP-1 motif, derived from IL-8 gene was 5′-gatcGTGATGACTCAGGTT-3′. Underlined sequences represent the AP-1 motif. The oligonucleotide, 5′-gatcTGTCGAATGCAAATCACTAGAA-3′, containing the consensus sequence of the octamer binding motif (underlined) was used to identify specific binding of the transcription factor, Oct-1. This transcription factor regulates transcription of a number of so-called housekeeping genes. For competition studies, oligonucleotides containing a mutated AP-1 binding site, a consensus AP-1 binding site, and a TPA (12-O-tetradecanoylphorbol 13-acetate)-responsive element (TRE) sequence, derived from the human metallothionein gene promoter,39 were used. The sequences were 5′-gatcGaaACTCAGCGCG-3′, 5′-gatcCGCTTGATGAGTCAGCCGGAA-3′, and 5′-gatcGTGACTCAGCGCG-3′, respectively. For preparation of the probe used in the electrophoretic mobility shift assay (EMSA), a radiolabeled double-stranded oligonucleotide was prepared by annealing and filling the overhang using the Klenow fragment of DNA polymerase I in the presence of α-32P-deoxycytidine triphosphate and α-32P-deoxyadenosine triphosphate.
Preparation of nuclear extracts
Nuclear extracts were prepared as described by Antalis et al,40 with some modifications. Cells (107) were washed twice with cold phosphate-buffered saline and the cell pellet was suspended in 400 μL hypotonic buffer A (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 1 mmol/L dithiothreitol [DTT], 2 mmol/L aminoethyl-benzene sulfonyl fluoride [AEBSF], and 0.2% Nonidet P-40) for 10 minutes at 4°C. Nuclei were prepared by microcentrifugation for 5 minutes at 4°C. The nuclear pellet was suspended in 75 μL buffer C (20 mmol/L HEPES, pH 7.9, 0.4 mol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L DTT, 2 mmol/L AEBSF, 33 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL E-64, and 10 μg/mL pepstatin A) and incubated for 30 minutes at 4°C with brief mixing. The mixture was microcentrifuged (15 000 cpm) for 15 minutes at 4°C. The protein concentration was measured using the Bradford assay (Bio-Rad, Richmond, CA).
EMSA
As previously described,7 nuclear extracts (5 μg of protein) were preincubated in 20 μL total reaction volume, containing 10 mmol/L Tris-HCl, pH 7.5, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 5% glycerol, and 1 μg of poly-deoxy-inosinic-deoxy-cytidilic acid (Pharmacia, Piscataway, NJ) for 15 minutes at room temperature. The reaction mixture was then incubated with the radiolabeled oligonucleotide (50,000 cpm) for 15 minutes at room temperature. Samples were analyzed by electrophoresis using 4%, nondenaturing polyacrylamide gel with 0.25× TBE buffer (22.3 mmol/L Tris, 22.2 mmol/L boric acid, and 0.5 mmol/L EDTA). The gels were dried and analyzed by autoradiography. Supershifts were performed by adding antibodies to the incubating mixture of nuclear extracts and the labeled DNA probe. One microgram of anti-c-Fos, Fra-1, c-Jun, JunB, and JunD antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and 2 μg of anti-FosB and Fra-2 antibodies (Santa Cruz Biotechnology) were used per lane.
Plasmids and transfection
A reporter plasmid 2× AP-1 site LUC (a kind gift from Dr N. Mukaida, Kanazawa University, Kanazawa, Japan) is a luciferase expression plasmid controlled by 2 copies of the AP-1 binding site from the IL-8 promoter.41 The Tax expression plasmid, pH2R40M,42 was a gift from Dr M. Hatanaka (Shionogi Institute for Medical Science, Osaka, Japan). The JunD expression plasmid, pSG-JunD, was prepared by subcloning a cDNA fragment of human JunD into the EcoRI site of pSG5. Transfections were performed by electroporation.7 In all cases, the reference plasmid, pRL-TK, an internal control renilla luciferase expression vector (Toyo Ink Co, Tokyo, Japan), was cotransfected to correct for transfection efficiency. For the luciferase assay, transfected cells were lysed in lysis reagent (Toyo Ink Co), and luciferase activity was measured according to the protocol provided by the manufacturer. Each assay was independently repeated at least 3 times.
Results
ATL-derived cell lines exhibit little expression of viral genes, including Tax
Three HTLV-I-negative and 10 HTLV-I-positive cell lines were used for these experiments. HTLV-I-positive cell lines can be classified into 2 groups (Table 1). The 4 cell lines (MT-2, MT-4, C5/MJ, and SLB-1) were established by the in vitro transformation of normal T cells with HTLV-I. Five cell lines (MT-1, TL-OmI, SO-4, ST-1, and KK-1) originated from leukemic cells of patients with ATL. The leukemic cell origin of these cell lines was previously confirmed by having the same provirus integration sites or T cell receptor β-chain gene rearrangement profiles as those of their original leukemic cells, by Southern blot25 or chromosomal analysis. HUT-102 could not be classified by this criterion because its clonal origin had not been examined.
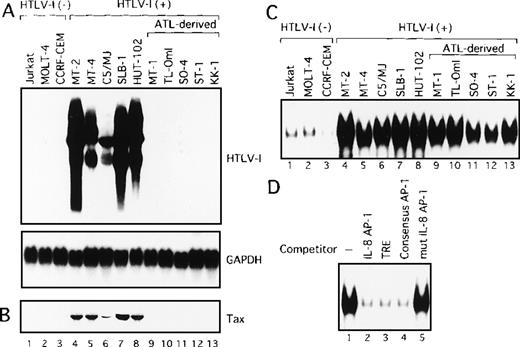
We initially analyzed the expression of HTLV-I mRNA in these T-cell lines by Northern blot analysis. No association between IL-2 dependency for growth and HTLV-I mRNA expression was observed (Table 1). Surprisingly, only HTLV-I transformed cell lines originating from normal T cells expressed detectable levels of viral mRNA (Figure1A). In sharp contrast, HTLV-I mRNA was not detectable in any of the ATL-derived T-cell lines. Consistent with RNA analysis, Tax protein was detected by immunoblot analysis, in the 5 cell lines, including HTLV-I transformed cell lines originating from normal T cells, but not in those derived from leukemic cells (Figure1B). These results indicate that cell lines derived from ATL exhibit little expression of viral genes, including Tax. We have previously established OMT, an IL-2-dependent T-cell line from a patient with ATL.43 This line expressed high levels of both HTLV-I mRNA and Tax protein. The cell origin was confirmed by Southern blot analysis, but it was derived from a nonleukemic cell clone. HUT-102 also expressed both HTLV-I mRNA and Tax protein. HUT-102 shows viral gene expression features similar to the HTLV-I transformed T-cell lines originating from normal T cells. Although HUT-102 was established from a patient with ATL, this line may be derived from the nonleukemic cells infected with HTLV-I, as suggested in OMT.
HTLV-I-infected T-cell lines.
(A) Determination of HTLV-I mRNA expression by Northern blot analysis in HTLV-I-infected T-cell lines. GAPDH expression was used as a control. Predominant 2.1, 4.2, and 8.5 kb viral mRNA species were detected in MT-2, MT-4, C5/MJ, SLB-1, and HUT-102 cell lines (lanes 4-8). (B) Expression of Tax protein in HTLV-I-infected T-cell lines determined by Western blotting using the monoclonal antibody, Lt-4. (C) Increased AP-1 binding activity in HTLV-I-infected T-cell lines. Labeled IL-8 AP-1 oligonucleotide was incubated with nuclear extracts of T-cell lines and the mixture was separated by polyacrylamide gel. Jurkat, MOLT-4, and CCRF-CEM are HTLV-I-negative cells and the remaining cell lines are HTLV-I infected. (D) Sequence specificity of AP-1 binding activity in ATL-derived cell line, MT-1. The binding reaction was carried out in the presence of 100 ng of the indicated cold oligonucleotides as competitors.
HTLV-I-infected T-cell lines.
(A) Determination of HTLV-I mRNA expression by Northern blot analysis in HTLV-I-infected T-cell lines. GAPDH expression was used as a control. Predominant 2.1, 4.2, and 8.5 kb viral mRNA species were detected in MT-2, MT-4, C5/MJ, SLB-1, and HUT-102 cell lines (lanes 4-8). (B) Expression of Tax protein in HTLV-I-infected T-cell lines determined by Western blotting using the monoclonal antibody, Lt-4. (C) Increased AP-1 binding activity in HTLV-I-infected T-cell lines. Labeled IL-8 AP-1 oligonucleotide was incubated with nuclear extracts of T-cell lines and the mixture was separated by polyacrylamide gel. Jurkat, MOLT-4, and CCRF-CEM are HTLV-I-negative cells and the remaining cell lines are HTLV-I infected. (D) Sequence specificity of AP-1 binding activity in ATL-derived cell line, MT-1. The binding reaction was carried out in the presence of 100 ng of the indicated cold oligonucleotides as competitors.
Constitutive AP-1 binding activities containing JunD in HTLV-I-infected cell lines
We next examined AP-1 activity by EMSA, in T-cell lines characterized above, using the oligonucleotide containing the AP-1 binding site of IL-8 gene as a probe. Three of the 5 HTLV-I-infected cell lines of leukemic cell origin required IL-2 for growth (Table 1). However, because IL-2 is known to induce AP-1 activity, they were cultured in the absence of IL-2 for 24 hours before the preparation of nuclear extracts. As shown in Figure 1C, low levels of the protein-DNA complex were detected in nuclear extracts from HTLV-I-negative T-cell lines (lanes 1-3). In contrast, high levels of the complex were detected in all HTLV-I-infected T-cell lines, including ATL-derived cell lines not expressing Tax (Figure 1C, lanes 4-13). The complex was specific for the AP-1 site, because efficient competition was provided by a 100-fold excess of 3 unlabeled AP-1 binding oligonucleotides: the AP-1 site from the IL-8 promoter (Figure 1D, lane 2), TRE from the metallothionein promoter (lane 3), and the consensus AP-1 site (lane 4). No competition was observed using an oligonucleotide containing mutations within the AP-1 site (lane 5). The high AP-1 activity of the metallothionein TRE oligonucleotide was also observed in nuclear extracts from all HTLV-I-infected cell lines (data not shown). These results demonstrated that AP-1 is activated not only in cell lines exhibiting Tax expression but also in ATL-derived cell lines that do not express Tax.
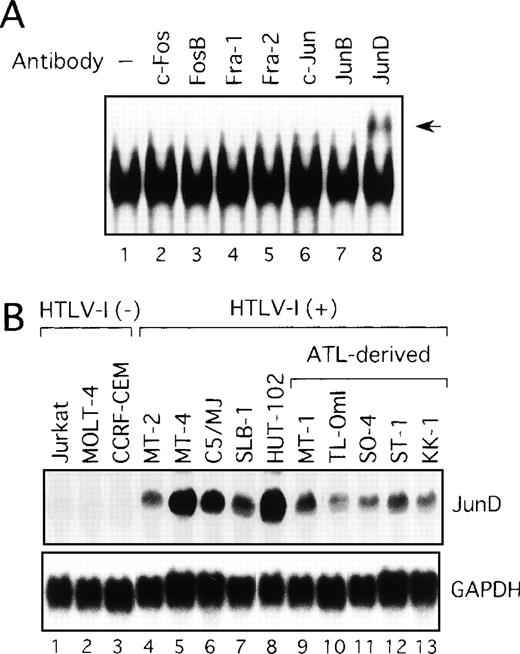
Activation of JunD in HTLV-I-infected T-cell lines
We next examined components of the AP-1 complex in the ATL-derived cell line, MT-1. The antibody supershift assay showed that the AP-1 complex in MT-1 contained JunD, because the anti-JunD antibody induced a slowly migrating (supershift) complex when added to the assay reaction (Figure 2A). No obvious changes were found when antibodies against c-Fos, FosB, Fra-1, Fra-2, c-Jun, and JunB were used (Figure 2A). The complex was found to contain JunD in the remaining HTLV-I-infected cell lines (data not shown). Therefore, we examined by Northern blot analysis the expression of JunD mRNA in these T-cell lines, which either did or did not express Tax. All HTLV-I-infected T-cell lines, including the 5 ATL-derived cell lines, expressed high levels of JunD mRNA, whereas JunD mRNA was not detectable in 3 HTLV-I-negative cell lines (Figure 2B). Thus, ATL-derived cell lines overexpressed JunD mRNA, even though they expressed little Tax.
Activation of JunD in HTLV-I-infected T-cell lines.
(A) Nuclear extracts of the ATL-derived cell line, MT-1, contain AP-1 binding activity, involving JunD. The antisera indicated above lanes were incubated with nuclear extracts from MT-1 cells and subjected to EMSA using IL-8 AP-1 as a probe. A supershift band was observed on addition of antisera against JunD (arrow). (B) Expression of JunD mRNA determined by Northern blot analysis of HTLV-I-infected T-cell lines. GAPDH expression was used as a control.
Activation of JunD in HTLV-I-infected T-cell lines.
(A) Nuclear extracts of the ATL-derived cell line, MT-1, contain AP-1 binding activity, involving JunD. The antisera indicated above lanes were incubated with nuclear extracts from MT-1 cells and subjected to EMSA using IL-8 AP-1 as a probe. A supershift band was observed on addition of antisera against JunD (arrow). (B) Expression of JunD mRNA determined by Northern blot analysis of HTLV-I-infected T-cell lines. GAPDH expression was used as a control.
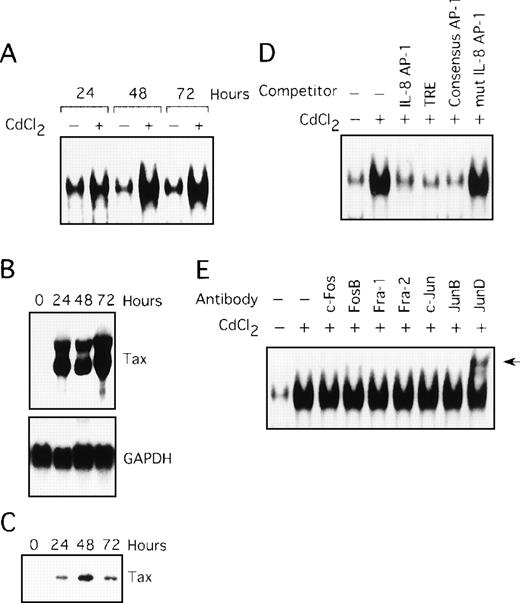
Involvement of Tax in induction of AP-1 binding activity
In the next series of experiments, we used the JPX-9 cell line to obtain direct evidence for the induction of AP-1 activity by Tax. JPX-9 is derived from the Jurkat T-cell line and has an inducible Tax gene under the control of a metallothionein promoter. Treatment of JPX-9 cells with CdCl2 (Figure 3A, lanes 2, 4, and 6) resulted in a significant increase in IL-8 gene complex formation with the AP-1 site. The level of expression of Tax mRNA and protein in these cells was determined by Northern and Western blot analyses (Figure 3B and C). The specificity of the complex was confirmed by performing competition assays with the autologous oligonucleotide (Figure 3D, lane 3), TRE oligonucleotide (lane 4), the consensus AP-1 site (lane 5), and the mutated AP-1 site oligonucleotide (lane 6). We further analyzed the composition of the AP-1 complex using the antibody supershift assay. As shown in Figure 3E, the complex in JPX-9 stimulated with CdCl2 contained JunD, because antisera specific for JunD induced a supershift complex (lane 9). Thus, Tax can lead to activation of AP-1, containing the JunD protein, in a T-cell line.
Tax induces AP-1 binding activity, involving JunD.
(A) Nuclear extracts isolated from JPX-9 cells, treated with CdCl2 (20 μmol/L) for the indicated time periods, were subjected to EMSA. (B) Northern blot analysis for expression of Tax mRNA in JPX-9 cells treated with CdCl2. (C) Western blot analysis for expression of Tax protein in JPX-9 cells treated with CdCl2. (D) Nuclear extracts from JPX-9 cells treated with CdCl2 for 48 hours were mixed with the labeled IL-8 AP-1 probe in the absence or presence of 100 ng of the cold competitor, indicated on top of each lane. (E) JPX-9 nuclear extracts obtained after 48 hours of CdCl2 treatment were incubated with the indicated antibodies, before addition of the labeled IL-8 AP-1 probe, and analyzed by EMSA. A supershift band was observed on addition of antisera against JunD (arrow).
Tax induces AP-1 binding activity, involving JunD.
(A) Nuclear extracts isolated from JPX-9 cells, treated with CdCl2 (20 μmol/L) for the indicated time periods, were subjected to EMSA. (B) Northern blot analysis for expression of Tax mRNA in JPX-9 cells treated with CdCl2. (C) Western blot analysis for expression of Tax protein in JPX-9 cells treated with CdCl2. (D) Nuclear extracts from JPX-9 cells treated with CdCl2 for 48 hours were mixed with the labeled IL-8 AP-1 probe in the absence or presence of 100 ng of the cold competitor, indicated on top of each lane. (E) JPX-9 nuclear extracts obtained after 48 hours of CdCl2 treatment were incubated with the indicated antibodies, before addition of the labeled IL-8 AP-1 probe, and analyzed by EMSA. A supershift band was observed on addition of antisera against JunD (arrow).
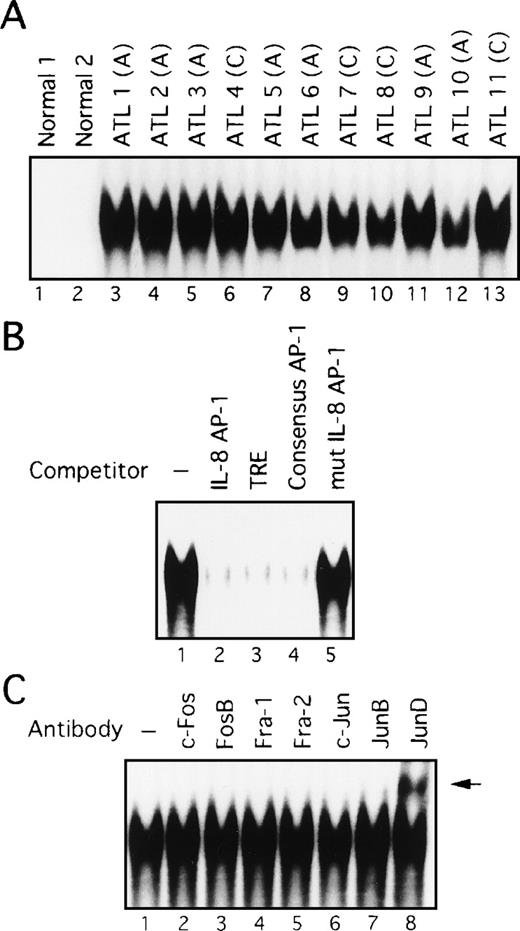
Constitutive activation of AP-1 in freshly prepared primary leukemic cells from patients with ATL
The above data showed that cell lines originating from ATL cells express high levels of AP-1 activity. We next examined whether AP-1 is indeed activated in vivo in primary leukemic cells of patients with ATL. Nuclear extracts were prepared from PBMC of 11 patients, including 7 with acute-type ATL and 4 with chronic-type ATL. High levels of binding to the AP-1 site of the IL-8 gene were detected in nuclear extracts prepared from all patients (Figure4A, lanes 3-13). However, no such activity was found in PBMC extracts from 2 healthy volunteers (lanes 1 and 2). The specificity of this activity was verified by competition with an excess of wild-type and mutant oligonucleotides (Figure 4B). However, no differences between patients with ATL and healthy volunteers were observed when the octamer motif sequence was used as a probe for EMSA.44 Furthermore, no quantitative or qualitative differences in AP-1 binding activity were observed between patients with acute and chronic disease (Figure 4A). It should be noted that Tax expression was not detectable by Northern and Western blot analyses of primary cells isolated from these patients, although it could be detected by RT-PCR (references 24 and 45, and data not shown). Thus, these results indicate a high level of AP-1 activity is present in primary leukemic cells of ATL patients in vivo and that this activity is likely to be independent of Tax.
AP-1 binding activity in nuclear extracts from leukemic cells of patients with ATL involves JunD.
(A) Increased AP-1 binding activity in nuclear extracts of leukemic cells of patients with ATL. A, acute-type; C, chronic type. (B) Specificity of the DNA-protein complex formation at the AP-1 site in competition EMSA. Nuclear extracts from leukemic cells of patient 9 were mixed with the labeled IL-8 promoter containing the AP-1 site, in the absence (lane 1) or presence of 100 ng of IL-8 AP-1 site (lane 2), TRE (lane 3), consensus AP-1 (lane 4), or mutated IL-8 AP-1 site (lane 5) competitors. (C) The AP-1 binding complex was recognized by anti-JunD antibody. Nuclear extracts from leukemic cells of patient 9 were preincubated without or with the antisera indicated on top of each lane, before adding the IL-8 AP-1 site probe to the binding mixtures. A supershift band was observed on addition of antisera against JunD (arrow).
AP-1 binding activity in nuclear extracts from leukemic cells of patients with ATL involves JunD.
(A) Increased AP-1 binding activity in nuclear extracts of leukemic cells of patients with ATL. A, acute-type; C, chronic type. (B) Specificity of the DNA-protein complex formation at the AP-1 site in competition EMSA. Nuclear extracts from leukemic cells of patient 9 were mixed with the labeled IL-8 promoter containing the AP-1 site, in the absence (lane 1) or presence of 100 ng of IL-8 AP-1 site (lane 2), TRE (lane 3), consensus AP-1 (lane 4), or mutated IL-8 AP-1 site (lane 5) competitors. (C) The AP-1 binding complex was recognized by anti-JunD antibody. Nuclear extracts from leukemic cells of patient 9 were preincubated without or with the antisera indicated on top of each lane, before adding the IL-8 AP-1 site probe to the binding mixtures. A supershift band was observed on addition of antisera against JunD (arrow).
Activation of JunD in leukemic cells of patients with ATL
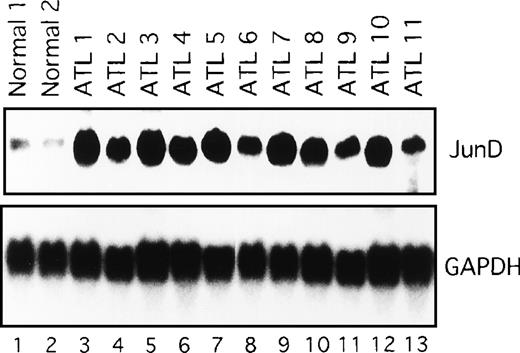
We next examined the components of the AP-1 complex in fresh primary leukemic cells obtained from patients with ATL, by supershift experiments using appropriate antibodies. The AP-1 complex in all 11 ATL samples contained JunD (Figure 4C and data not shown). No obvious supershift complex was found when antibodies against c-Fos, FosB, Fra-1, Fra-2, c-Jun, and JunB were used (Figure 4C). Thus, AP-1 in primary leukemic cells of patients with ATL contains the JunD protein. Northern blot experiments showed that JunD mRNA was highly expressed in leukemic cells of all patients, but only in trace amounts in normal PBMC (Figure 5). Thus, high expression of JunD mRNA is likely to be involved in the activation of AP-1 in primary ATL cells in vivo.
Expression of JunD mRNA in primary leukemic cells from patients with ATL, by Northern blot analysis.
GAPDH expression served as control. Molecular sizes of the transcripts were as follows. JunD, 1.8 kb; GAPDH, 1.8 kb.
Expression of JunD mRNA in primary leukemic cells from patients with ATL, by Northern blot analysis.
GAPDH expression served as control. Molecular sizes of the transcripts were as follows. JunD, 1.8 kb; GAPDH, 1.8 kb.
JunD transactivates AP-1 element and can potentially work with Tax
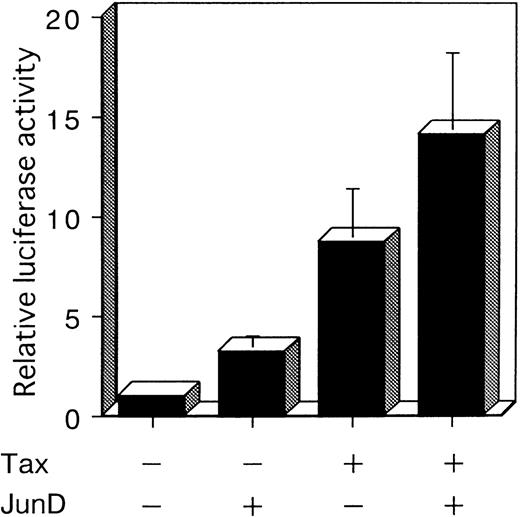
To investigate whether JunD is involved in AP-1 site-mediated transcription in T cells, we performed a cotransfection experiment with a luciferase reporter plasmid containing 2 AP-1 elements (2× AP-1 site LUC), using the human T-cell line, Jurkat. Jurkat exhibited a low level of endogenous AP-1 activity (Figure 1C). Cotransfection experiments showed that Tax stimulated the expression of the luciferase gene regulated by IL-8 AP-1 sites, by 8.7-fold. JunD stimulated expression 3.2-fold; and JunD and Tax together stimulated expression 14.1-fold (Figure 6). These results indicate that JunD as well as Tax can stimulate the transcription of cellular genes regulated by the AP-1 site in T cells, both alone and synergistically.
JunD can potentially act synergistically with Tax.
Jurkat cells were cotransfected with 3 μg of 2× AP-1 site LUC and 5 μg of the indicated expression vectors. The total amount of transfected plasmid was maintained at 13 μg by the addition of expression vector without inserts. Bars represent the mean ± SD of 3 independent experiments.
JunD can potentially act synergistically with Tax.
Jurkat cells were cotransfected with 3 μg of 2× AP-1 site LUC and 5 μg of the indicated expression vectors. The total amount of transfected plasmid was maintained at 13 μg by the addition of expression vector without inserts. Bars represent the mean ± SD of 3 independent experiments.
Discussion
AP-1 is a pleiotropic regulator of inducible expression of several genes. These genes encode proteins involved in the modulation of inflammatory and host defense processes in eucaryotic cells. The protein components of AP-1 are encoded by a set of genes called “immediate early genes” whose transcription is rapidly induced following cell stimulation, independent of de novo protein synthesis. AP-1 has been shown to alter gene expression in response to growth factors, cytokines, tumor promoters, and carcinogens. It is believed that deregulation of these early genes or an imbalance between oncoproteins of the AP-1 complex may contribute in part to the malignant transformation.
In the present study, we showed that the transcription factor, AP-1, is activated in vivo in PBMC (< 90% were leukemic cells) from all 11 patients with ATL (7 with acute-type and 4 with chronic-type). All cell lines originating from ATL cells expressed high AP-1 activity, indicating that AP-1 is indeed activated by factors in the leukemic cells from the peripheral blood of these patients. AP-1 plays various roles in cell proliferation and transformation. Thus, activation of AP-1 in ATL cells in vivo may be involved in their deregulated phenotypes, including those associated with leukemic proliferation.
We examined HTLV-I viral gene expression of ATL-derived cell lines compared with that of HTLV-I transformed cell lines. HTLV-I viral products were not detected in ATL-derived cell lines by Northern and Western blot analyses. Interestingly, Imada et al46 have reported that cell lines derived from the leukemic cell clone but not HTLV-I transformed cell lines could engraft in severe combined immunodeficiency mice, suggesting that HTLV-I-infected cell lines of nonleukemic cell origin may not have enough leukemogenic changes to acquire the tumorigenic potential. Taken together, ATL-derived cell lines should be distinguished from in vitro immortalized cell lines.
Tax induces the expression of various members of the AP-1 family, such as c-Fos, Fra-1, c-Jun, JunB, and JunD, at the RNA level.11,12We also observed that Tax induces AP-1 DNA binding activity in a T-cell line (Figure 3A). However, activation of AP-1 in ATL cells in vivo was likely to be Tax independent. Thus, Northern and Western blot analyses showed that HTLV-I mRNA and Tax protein expressions were not detectable in ATL cells in vivo (data not shown) or in ATL-derived cell lines (Figure 1A and B). The expression of Tax mRNA could be detected in ATL cells and in ATL-derived cell lines except for TL-OmI by RT-PCR (references 24 and 45, and data not shown). Alternatively, a trace amount of Tax may be sufficient for AP-1 activation in ATL cells in vivo. However, we think this possibility unlikely, from the following observations. The ATL-derived cell line, TL-OmI, expressed high levels of AP-1 (Figure 1C), whereas Tax mRNA expression could not be detected in this cell line, even using the highly sensitive RT-PCR.45,47 The induction of AP-1 DNA binding activity was seen in CdCl2-treated JPX-9 cells, which produced Tax at relatively high levels (Figure 3A-C). It is likely that activation of AP-1 requires a sufficient level of Tax for detection by Northern and Western blot analyses in T cells. Thus, activation of AP-1 in ATL cells in vivo mainly occurs through a Tax-independent mechanism. In the present study, we identified JunD as an active component of AP-1 in primary leukemic cells from ATL patients as well as in ATL-derived cell lines. Activation of JunD binding to the AP-1 site was mediated, at least in part, by increased expression of JunD mRNA in both primary leukemic cells from ATL patients and ATL-derived cell lines (Figures 2B and 5). In addition, we showed that AP-1 activated by Tax in a T-cell line also contained the JunD protein. Thus, JunD may play important roles in both Tax-dependent and Tax-independent steps involved in HTLV-I-induced leukemogenesis. JunD was initially considered as an inhibitor of cellular proliferation and transformation.48,49 However, it has since been reported that JunD can transform rat embryo fibroblasts in cooperation with Ras50 and that mutant forms of JunD have a transforming potential.51 It remains to be determined whether the JunD molecules found in ATL cells represent wild-type or mutant versions. JunD appears to be important for cell-cycle progression, because antibodies against this protein delay the transition from G1 to the S phase.52 Thus, JunD may act both as a positive and negative regulator of cell proliferation and transformation, depending on the cellular context.
Activation of AP-1 is regulated at the transcriptional and posttranslational level by components of certain kinase cascades in response to a variety of physiologic stress stimuli and inflammatory cytokines. AP-1 becomes activated through phosphorylation of Jun family transcription factors by members of the c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK) family.53Previously, constitutive JNK activation has been reported in HTLV-I-infected T-cell lines as well as leukemic cells from ATL patients.54 These findings suggest that HTLV-I-induced JNK activation may also contribute to the activation of AP-1 in T cells.
In the context of human hematopoietic malignancies, continuous AP-1 DNA binding activity involving JunD has been found in 3 cell lines of cutaneous T-cell lymphoma and 2 patients with Sézary syndrome.55 Sézary syndrome is a disease involving CD4+ T cells, which has a similar pathology to ATL, including leukemic T cells in the blood and infiltration of leukemic cells into the skin. Thus, it is likely that the AP-1 pathway may also be involved in this type of leukemogenesis. Further characterization of AP-1 in HTLV-I-induced transformation should contribute to our understanding of oncogenic mechanisms and may also provide a unique therapeutic target for this unusual type of leukemia.
Acknowledgments
We thank Drs N. Mukaida and M. Hatanaka for providing plasmids, Dr M. Nakamura for providing JPX-9, and Fujisaki Cell Center, Hayashibara Biochemical Laboratories Inc (Okayama, Japan) for providing Jurkat, HUT-102, MT-1, and C5/MJ. Recombinant human IL-2 was kindly provided by Takeda Chemical Industries (Osaka, Japan). We also thank M. Yamamoto and M. Sasaki for excellent technical assistance.
Supported in part by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture of Japan, and a Cooperative Research Grant No. 1999-10-A-14 from the Institute of Tropical Medicine, Nagasaki University.
Reprints:Naoki Mori, Department of Preventive Medicine and AIDS Research, Institute of Tropical Medicine, Nagasaki University, 1-12-4, Sakamoto, Nagasaki 852-8523, Japan; e-mail:n-mori@net.nagasaki-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal