Abstract
We tested the hypothesis that estrogen acutely stimulates constitutive nitric oxide synthase activity in human granulocytes by acting on a cell surface estrogen receptor (ER). The release of nitric oxide was measured in real time with an amperometric probe. Exposure of granulocytes to 17β-estradiol stimulated NO release within seconds in a concentration-dependent manner. The NO release was also stimulated by 17β-estradiol conjugated to bovine serum albumin (E2-BSA), which suggests mediation by a cell surface receptor. Tamoxifen, an ER inhibitor, antagonized the action of both 17β-estradiol and E2-BSA, whereas ICI 182,780, an inhibitor of the nuclear ER, had no effect. Using dual emission microfluorometry in a calcium-free medium, the 17β-estradiol–stimulated release of NO from granulocytes was shown to be dependent on intracellular calcium ([Ca2+]i) transients in a tamoxifen-sensitive process. Exposure to BAPTA-AM (1,2bis-(-aminophenoxy)ethans-N,N,N′,N′-tetraacetic acid tetra(acetoxyymethyl) ester), a [Ca2+]i chelator, reduced [Ca2+]i in response to E2-BSA, and depleting [Ca2+]i stores abolished the effect of 17β-estradiol on NO release. Confocal photomicrographs using E2-BSA–FITC (fluorescein isothiocyanate) revealed cell membrane reactivity. Estrogen-stimulated NO release had an immunosuppressive effect, and it initiated granulocyte rounding and loss of adherence in a tamoxifen-sensitive manner. Finally, using reverse transcriptase–polymerase chain reaction, human neutrophil granulocytes expressed ER but not ERβ, suggesting that ER may be the membrane receptor for 17β-estradiol. The study demonstrated that a physiological dose of estrogen down-regulates granulocyte activity by acutely stimulating NO release via the activation of a cell surface ER which is coupled to increases in [Ca2+]i.
Estrogen signaling exerts a cellular immunosuppressive action against various chemotactic agents in immune cells such as neutrophils.1-10 Estrogen also diminishes leukocyte phagocytosis11,12 and the potential of leukocytes to adhere to the endothelial lining of the vasculature.3,13-18 Recent reports of estrogen receptor (ER) expression on monocytes, polymorphonuclear leukocytes, T cells, and B cells complement this literature.19-22 Nevertheless, to our knowledge, ER expression by human neutrophils has never been positively demonstrated. Only indirect evidence has been provided: binding experiments at the ultrastructural level with 3H-estradiol have shown an accumulation of the radio label on granules of rat neutrophil granulocytes.23
The recent demonstration that neutrophils express constitutive nitric oxide synthase (cNOS) messenger RNA (mRNA)24 suggests that NO could be one of the immunosuppressive agents released by these cells as a result of estrogen stimulation.25 In this regard, in systems other than blood, estrogen has been shown to increase cNOS expression26-28 and activity29through a cytosolic receptor-mediated system. Recently, Prevot et al30 and Chen et al31 have shown that estrogen could also stimulate rapid NO release through a pathway that did not require transcription. In addition, it has been shown that when Chinese hampster ovary (CHO) cells are transfected with ERα and ERβ complementary DNA (cDNA), the receptors are localized to both membrane and nuclear fractions.32 This suggests that the nontranscriptional stimulation of NO release by estradiol could be mediated via cell membrane ERα and/or ERβ of cNOS-expressing cells.
The incidence of cardiovascular events in women increases after menopause, suggesting that estrogen deficiency may play a role in cardiovascular disease.33 The mechanisms by which estrogen influences coronary arteries and protects blood vessels against atherosclerotic development are unclear, but recent evidence suggests that NO production may play an important role in this event.34-36 In addition, macrophages and granulocytes with the ability to release NO have been found in atherosclerotic lesions, which also suggests their involvement.25 Thus, estrogen-stimulated NO release may down-regulate these cells, thereby contributing to the beneficial effects of this signaling molecule and underscoring the significance of estrogen signaling as a hematological phenomenon.
Given these recent findings, we examined human peripheral neutrophils to determine if (1) acute estrogen exposure is able to stimulate rapid NO release via cNOS activation; (2) 17β-estradiol–stimulated NO release is dependent on the initial stimulation of intracellular calcium ([Ca2+]i) transients because cNOS is Ca2+ dependent; (3) estrogen, through NO production, has immunosuppressive effects on granulocytes; and (4) neutrophil granulocytes express ERα and/or ERβ.
Materials and methods
Materials
For NO measurements, we used human peripheral blood cells (Long Island Blood Services, Melville, NY). Red cells were removed by dextran sedimentation, and Ficoll-Hypaque (Pharmacia Biotech, Orsay, France) density separation was used to isolate 1.077-1.080 g/mL granulocytes. The granulocytes were washed in Roswell Park Memorial Institute medium (RPMI 1640) for 5 minutes, centrifuged twice at 600g, and then washed in 25 mmol/L HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (Life Technologies, Gaithersburg, MD) as previously described.37-39 The cells were stained with Wright's stain and checked for purity by microscopic examination, which revealed 94%-97% neutrophils. More than 95% of the cells were viable, as determined by trypan blue exclusion.
For reverse transcriptase–polymerase chain reaction (RT-PCR) studies, we used human monocytes and neutrophil granulocytes (Pasteur Institute, Lille, France). Human peripheral blood cells were purified by Ficoll-Hypaque separation. Fresh anticoagulant-treated human venous blood (3.5 mL) was layered onto a discontinuous gradient of Ficoll-Hypaque solution (2.5 mL of 1.065 g/L onto 2.5 mL of 1.070 g/L). After centrifugation at 400g for 30 minutes in a swinging bucket rotor at room temperature, 2 fractions were obtained: a top leukocyte band containing 94%-98% mononuclear cells (lymphocytes and monocytes) and a lower band containing 96%-99% polymorphonuclear leukocytes (granulocytes). Both bands were recovered and washed gently with balanced salt solution comprising 0.01 mol/L phosphate buffered saline (PBS), 0.132 mmol/L sodium chloride (NaCL), and 0.132 mmol/L ammonium bicarbonate (NH4HCO3) (pH 7.2) supplemented with 0.5% bovine serum albumin (BSA). The bands were then washed with 2 mmol/L ethylenediamine tetraacetic acid (EDTA) to remove the separation medium and resuspended in 80 μL PBS.
The cells were further purified using magnetic cell sorting (MACS) microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) as described by the manufacturer. Briefly, CD14 and CD16 microbeads were developed for human cell separation based on the expression of the CD14 and CD16 antigens. The CD14 antigen is expressed in large quantities on monocytes and/or macrophages and in low amounts on granulocytes. The CD16 antigen is expressed on virtually all natural killer cells and eosinophils. Briefly, for MACS separation, cells were magnetically labeled with CD14 or CD16 microbeads and separated on a column that was placed in the magnetic field of a MACS separator. The unlabeled cells were depleted from CD14+ or CD16+cells by washing. After removal of the column from the magnetic field, the magnetically retained CD14+ or CD16+ cells were eluted as the positively selected cell fraction.
For monocyte purification, 20 μL MACS CD14 microbeads were added to 80 μL monocyte-enriched mononuclear cells (top band) and incubated for 15 minutes at 10°C. Cells were washed by adding 10-20 mL PBS buffer and centrifuged at 300g for 10 minutes. The supernatant was removed, and the pellet was resuspended in 500 μL PBS buffer. The cell suspension was applied onto the column that was then rinsed with 3 × 500 μL PBS buffer to allow CD14− cells to pass through. The column was removed from the separator and placed on a collection tube. Positive cells (up to 106 CD14+ cells) were flushed out with 1 mL PBS using the plunger supplied with the column.
For granulocyte purification, 20 μL MACS CD14 microbeads were added to 80 μL polynuclear-enriched leukocyte cells (upper band) to remove remaining mononuclear cells as described earlier. As a second step, the CD14− eluent was then incubated with CD16 microbeads and applied onto a column. After column separation, the eluent was collected as the CD16− fraction containing the purified neutrophil granulocyte fraction (up to 107 cells). After MACS separation, monocyte and neutrophil purity and viability were greater than 98%, as determined by the trypan blue dye exclusion method.
Confocal microscopic analysis
Venous blood (30 mL) treated with EDTA was diluted in an equal volume of a balanced salt solution, 0.9% NaCl. The diluted blood (2.5 mL) was carefully layered on 2.5 mL of Ficoll Hypaque solution and centrifuged at 600g for 30 minutes. Using a Pasteur pipette, the upper layer of plasma and the mononuclear cell layer were eliminated. The pellets containing granulocytes and erythrocytes were then transferred to a 50-mL tube to perform erythrocyte lysis using 30 mL lysis solution containing ammonium chloride with ammonium bicarbonate (pH 7.2). Lysis did not affect the leukocytes provided the temperature was maintained at 4°C to minimize diffusion. Polynuclear cells were washed twice in PBS. Purity was evaluated by microscopic examination after May-Grünwald-Giemsa staining on cytocentrifuged cells and demonstrated more than 95% neutrophils.
Aliquots containing 3 × 106 granulocytes in PBS were centrifuged, and the cell pellets were incubated with 200 μL E2-BSA-FITC (Sigma Chemical, St Quentin Fallavier, France) at a concentration of 1.5 × 10−5 mol/L for 1 hour in the dark, at room temperature. The control had the following concentrations: 10−2 mol/L E2, 2.84 × 10−4 mol/L E2-BSA, and 1.5 × 10−5 mol/L E2-BSA-FITC. After 2 washings in PBS for 5 minutes, the cells were again centrifuged at 600g and fixed in 25 μL 0.05% paraformaldehyde (Sigma, St Louis, MO) for 15 minutes. The suspension was placed on silicated microscope slides. When the slides were dried, the cells were washed in PBS before being mounted and observed. Analysis was performed on a confocal laser (argon krypton) microscope, and sections were viewed at 2 μm.
Direct measurement of NO release
The release of NO from the incubated granulocytes (106cells per chamber) was measured directly using a NO-specific amperometric probe (World Precision Instruments [WPI], Sarasota, FL) as described by Stefano and colleagues.37 40 Briefly, the cells were placed in a superfusion chamber in 2 mL RPMI containing 25 mmol/L HEPES. A micromanipulator (Zeiss-Eppendorff) attached to the stage of an inverted microscope (Diaphot; Nikon, Melville, NY) was employed to position the amperometric probe 15 μm above the cell surface. Cells were washed just prior to measurement initiation to remove any materials that could be released by the cells. The system was calibrated daily by adding potassium nitrite to a solution of potassium iodide, thereby resulting in the liberation of a known quantity of NO (WPI). Baseline levels were determined by evaluating NO release in PBS. Cells were stimulated with the respective ligand, and the concentration of NO gas in solution was measured in real time with the DUO 18 computer data acquisition system (WPI). The amperometric probe was allowed to equilibrate for at least 12 hours in RPMI prior to being transferred to the superfusion chamber containing the cells, and manipulation of the cells was performed only with glass instruments. Each experiment was repeated 4 times and was simultaneously performed with a control from the same tissue source (vehicle alone) to exclude experimental drift in NO release unrelated to the study drugs.
To evaluate NO release, cells were exposed to a concentration gradient of each of the various ligands. If an antagonist or a NOS inhibitor was used, it was administered 2 minutes prior to administration of the various estrogen ligands. The NOS inhibitor N omega-nitro-L-arginine methyl ester (L-NAME) was used in these studies. (Unless noted, all drugs were purchased from Sigma, St Louis, MO.) From the concentration curves of the estrogen agonist–stimulated NO release, we determined the concentration at which a half-maximal effect (EC50) of stimulated NO release occurred. From antagonist concentration curves (10−11 to 10−7mol/L) against 10−9 mol/L E2-BSA, the concentration at which half-maximal inhibition (IC50) of stimulated NO release occurred was also determined.
Computer-interfaced DUO-18 software (WPI) was used for data acquisition. The experimental values were then transferred to Sigma-Plot and Sigma-Stat (Jandel, San Rafael, CA) for graphic representation and evaluation. The differences between the data before and after treatment were tested for normal distribution. All data were normally distributed and subsequently evaluated by Student ttest for paired samples. Data gatherers were unaware of the experimental treatments.
Ligands
Granulocytes were stimulated with various concentrations of 10−13 to 10−7 mol/L 17β-estradiol or E2-BSA (with the same 17β-estradiol concentration). The granulocytes were also stimulated with one of the following (n = 4): (1) 10−9 mol/L 17α-estradiol, tamoxifen, or ICI 182,780 (Zeneca Pharmaceuticals, Costa Mesa, CA) ER antagonists; (2) 10−9 mol/L tamoxifen plus 10−9 mol/L 17β-estradiol; (3) 10−9 mol/L tamoxifen plus E2-BSA (10−13 to 10−7 mol/L or 10−9 mol/L 17β-estradiol); or (4) 10−9 mol/L ICI 182,780 plus E2-BSA (10−9 mol/L 17β-estradiol).
Tamoxifen and ICI 182,780 were added to the milieu 2 minutes before 17β-estradiol or E2-BSA. To determine that there was no dissociation between 17β-estradiol and BSA, we used a radioimmune assay (RIA) kit (ICN, Costa Mesa, CA) optimized for the direct quantitative determination of very low concentrations of free 17β-estradiol. Measurements were taken of 17β-estradiol in the cytosolic fraction of granulocytes (106 cells per mL) treated with 10−9 and 10−8 mol/L E2-BSA. After washing the cells, they were put through a freeze-thaw cycle; in PBS, cells were frozen instantly at −70°C for 5 minutes and thawed in a 37°C water bath for 1 minute. This process was repeated 5 times. The supernatant fluids were harvested after centrifugation for 10 minutes at 12 000g in a refrigerated centrifuge, and then the pellet containing cell debris was discarded. Estradiol was not detected in the cytosolic material upon evaluation. The assay sensitivity was 0.2 pg/mL.
[Ca2+]i levels monitored by Ca2+imaging
Granulocytes, diluted to approximately 100 cells per chamber slide (Nunc, Naperville, IN), were allowed to adhere in RPMI supplemented with 1% fetal calf serum (FCS) at 37°C in 5% carbon dioxide (CO2).41 To promote rapid adherence, the chambers were rinsed with 1% BSA. The cells were left under these conditions for 30 minutes before experimentation commenced. We estimated that at the end of this period, approximately 45% of the cells were lost due to the presence of dimethyl sulfoxide, which caused some cells not to adhere.
[Ca2+]i levels were measured by dual emission microfluorometry using the fluorescent indicator dye fura-2/acetoxymethyl (fura-2/AM). The cells were washed twice in the incubation medium minus Ca2+, balanced with sucrose to maintain osmolarity,41 and then incubated with 5 μmol/L fura-2/AM for 30 minutes at room temperature. In experiments designed to deplete [Ca2+]i, cells were maintained in the same medium, with more frequent changes over a 1-hour period. The nonionic and nondenaturing detergent Pluronic F-127 helped disperse the AM esters of fura-2 in the loading buffer. Cells were washed twice with RPMI, and then test drugs were added.
The concentration of [Ca2+]i was calculated from the fluorescence ratio (excitation, 340 and 380 nm; emission wavelength, 510 nm) according to the following equation42 43: [Ca2+]i = (R − Rmin) kdβ/(Rmax − R) , where R is the fluorescence ratio recorded from the cell; Rmin, the fluorescence ratio of fura-2 free acid recorded in the absence of Ca2+; Rmax, the fluorescence ratio of fura-2 free acid recorded in the saturating concentration of Ca2+; kd, the Ca2+ dissociation constant of the dye; and β, the ratio of the fluorescence of fura-2 free acid in the Ca2+ free form to the Ca2+ saturated form recorded at the wavelength used in the denominator of the ratio.
Images were acquired every 0.4 seconds with an image processing system (COMPIX C-640 SIMCA; Compix, Mars, PA) and an inverted Nikon microscope. Experiments were carried out at room temperature in PBS without Ca2+ or magnesium. When the respective receptor antagonists were used, they were administered 2 minutes prior to the respective agonist. The antagonists did not stimulate an increase in [Ca2+]i at the test concentrations. Furthermore, in our hands, the cells exhibited a basal level of Ca2+ sparkling in the 82-85 nmol/L range. This basal level of Ca2+mobility was set to 0 for the experimental protocols.
The [Ca2+]i chelator BAPTA-AM (1,2bis-[-aminophenoxy]ethans-N,N,N′,N′-tetraacetic acid tetra[acetoxyymethyl] ester) (Molecular Probes, Eugene, OR) was used to determine dependence of estrogen signaling on Ca2+mobility.44 Granulocytes were incubated with BAPTA-AM at the indicated concentrations for 30 minutes prior to estrogen exposure. An additional control experiment was performed in the presence of 1 μmol/L TPEN (N,N,N′,N′-tetrakis(2-pyridylmethylethylenediamine) for 30 minutes because BAPTA can chelate other cations, and TPEN prefers heavy metals to Ca2+. Here, TPEN did not block the 17β-estradiol–induced increases in [Ca2+]i (data not shown).
A 2-way analysis of variance (ANOVA) was used for statistical analysis at the time of peak [Ca2+]i, 7 seconds after agonist exposure to the cells. Each experiment was simultaneously performed with up to 8 cells. The mean value was combined with the mean value taken from 4 other replicates, and the SEM was determined.
Analysis of cellular activity
The pharmacological effects of granulocyte exposure to drugs was determined as previously described.37 45 Briefly, the cells were allowed to adhere to a portion of a glass slide previously coated with 0.1% BSA surrounded by a petroleum jelly ring. Approximately 400 cells were added to this slide in 100 μL buffer at 37°C and maintained on a microscope stage slide warmer. Effective concentrations of test substances in 100 μL physiological saline were added to the cells. Cell conformation changes due to the test substances were directly observed 10 minutes following drug exposure. Antagonists were added 5 minutes prior to the agonists. Approximately 24-34 cells were observed for each 400-μm viewing diameter, and 4 viewing diameters were observed per slide. The entire process was repeated 3 more times, and the resulting mean plus or minus SEM was graphed.
Changes in granulocyte conformation were analyzed by phase-contrast microscopy using a Nikon inverted microscope. The granulocytes were observed for up to 30 minutes. Changes in cellular conformation, ranging from inactive rounded (diameter range, 10-14 μm) to active amoeboid (diameter, greater than 15 μm) were determined by measurements of cellular area and perimeter. They were expressed mathematically using the shape-factor formula of the Compix Cell Analysis System (the lower the shape factor, the higher the perimeter and the more amoeboid the cellular shape) as noted elsewhere in detail.37,45 The degree of cellular activation was determined as noted elsewhere.37,45,46 Briefly, under phase contrast optics, round cells appear light, and amoeboid cells appear dark. The system, manually tuned, identifies these color differences, and the proportion of each is used as an index of activation. It is important to note that amoeboid-activated cells not only change their conformation in response to a pharmacological stimulus, but they also become motile, are capable of phagocytosis, secrete cytokines, and exhibit changes in adhesion molecule expression.47
RT-PCR analysis
Total RNA from monocytes or neutrophils was extracted using Trizol (GIBCO/BRL, Strasbourg, France). RNA (3 μg) was reverse transcribed into cDNA using random hexamers and Moloney murine leukemia virus RT (GIBCO/BRL) as previously described.48 One-sixth of the first strand synthesis reaction was amplified for 40 cycles using 1 unit Taq polymerase (Eurogentec, Liege, Belgium) and 100 pmol of each forward and reverse primer. The cycling parameters were 94°C for 90 seconds, 65°C for 90 seconds, and 72°C for 120 seconds. Negative control RT-PCR reactions were performed by omitting RT or RNA from the reaction mixture. In both pairs, the priming sites were separated by an intron, thus preventing amplification of any contaminating genomic DNA (data not shown). For ERα amplification, the primer pair (25-mer) was designed to amplify a 281–base pair (281-bp) cDNA fragment (residues, 83-17749). For ERβ amplification, the primer pair (25-mer) was designed to amplify a 265-bp cDNA product (residues, 381-46950). As an internal control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA also was amplified using a primer pair (26-mer) designed to amplify a 470-bp cDNA (residues, 36-19251). Monocytes were used as a positive control because they have been shown to express ER material.52
PCR products were subcloned as described in detail elsewhere48 using the TA cloning vector system (Stratagene, Paris, France) and sequenced to verify the specificity of the amplification. Briefly, the PCR products were ligated into the PCR-II vector (Invitrogen, Carlsbad, CA) and transformed into competentEscherichia coli JM109 cells (Promega, Charbonnieres, France). Plasmid DNA was sequenced with a T7 sequencing kit (Pharmacia Biotech) according to the manufacturer's instructions.
Results
17β-estradiol and NO release
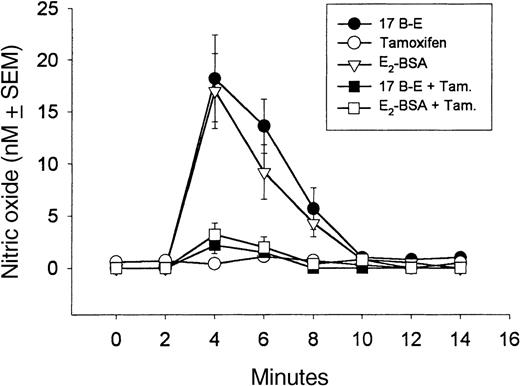
Normally, granulocytes release low levels of cNOS-derived NO (range, 0-1 nmol/L37). The NO release from granulocytes is stimulated by 17β-estradiol in a concentration-dependent manner with a saturable effective dose of 10−9 mol/L 17β-estradiol (Figure 1). Testosterone and progesterone do not cause release of granulocyte-derived NO (Figure1). In real time, 10−9 mol/L 17β-estradiol–stimulated NO release (peak value, 17 nmol/L) occurs rapidly, within the initial 60-second exposure period (Figure2B). This release then diminishes over a 10-minute period (Figure 3). However, 10−9 mol/L 17β-estradiol did not stimulate NO release from the granulocytes (data not shown). Tamoxifen (10−9 mol/L), an estradiol receptor inhibitor, blocked 17β-estradiol–stimulated NO release (P < .005) (Figure 3). L-NAME (100 μmol/L), a NOS inhibitor, substantially reduced the NO stimulating activities of 17β-estradiol (Table1). Confocal laser microscopy, using E2-BSA-FITC, revealed immunoreactivity only on the granulocyte membrane, thereby demonstrating its cell surface presence (Figure 2D).
Dose-dependent release of NO after in vitro stimulation of granulocytes (106 cells per mL) by 17β-estradiol and E2-BSA but not by progesterone or testosterone.
The graphed values represent peak values obtained 2 minutes after drug exposure. The cells were exposed to the agents for the entire observation period (15 minutes). Each experiment was repeated four times, and the resulting mean value plus or minus SEM was graphed. Control cells were those exposed just to the vehicle.
Dose-dependent release of NO after in vitro stimulation of granulocytes (106 cells per mL) by 17β-estradiol and E2-BSA but not by progesterone or testosterone.
The graphed values represent peak values obtained 2 minutes after drug exposure. The cells were exposed to the agents for the entire observation period (15 minutes). Each experiment was repeated four times, and the resulting mean value plus or minus SEM was graphed. Control cells were those exposed just to the vehicle.
Real-time representation of 10−9 mol/L 17β-estradiol–stimulated [Ca2+]i and NO release from human granulocytes.
(A) Real time representation of 10−9 mol/L 17β-estradiol–stimulated NO release from peripheral granulocytes and its blockade by 10−9 mol/L tamoxifen (Tam) but not 10−8 mol/L ICI-182,780 (ICI). Tamoxifen and ICI-182,780 were administered 2 minutes prior to 17β-estradiol. (B) 17β-Estradiol–stimulated [Ca2+]i (Est) and its antagonist. Tamoxifen (Tam; 10−9 mol/L) antagonizes 17β-estradiol–stimulated [Ca2+]i, but 10−8 mol/L ICI-182,780 (ICI) does not. Drugs were administered as in (A). (C) 17β-Estradiol–stimulated [Ca2+]i (Est) and its BAPTA-AM concentration-dependent decrease, which demonstrates that [Ca2+]i transients are associated with estradiol's action. (D) Confocal laser photomicrograph of E2-BSA-FITC reactivity of the cell surface of granulocytes (right) and control granulocyte (left).
Real-time representation of 10−9 mol/L 17β-estradiol–stimulated [Ca2+]i and NO release from human granulocytes.
(A) Real time representation of 10−9 mol/L 17β-estradiol–stimulated NO release from peripheral granulocytes and its blockade by 10−9 mol/L tamoxifen (Tam) but not 10−8 mol/L ICI-182,780 (ICI). Tamoxifen and ICI-182,780 were administered 2 minutes prior to 17β-estradiol. (B) 17β-Estradiol–stimulated [Ca2+]i (Est) and its antagonist. Tamoxifen (Tam; 10−9 mol/L) antagonizes 17β-estradiol–stimulated [Ca2+]i, but 10−8 mol/L ICI-182,780 (ICI) does not. Drugs were administered as in (A). (C) 17β-Estradiol–stimulated [Ca2+]i (Est) and its BAPTA-AM concentration-dependent decrease, which demonstrates that [Ca2+]i transients are associated with estradiol's action. (D) Confocal laser photomicrograph of E2-BSA-FITC reactivity of the cell surface of granulocytes (right) and control granulocyte (left).
Estrogen stimulation of NO release by 17β-estradiol and E2-BSA and its antagonism by tamoxifen.
17β-Estradiol (17 B-E; 10−9 mol/L) stimulates NO release from granulocytes within 2 minutes of its application. The release of NO is stimulated by 17β-estradiol coupled to 10−9 mol/L BSA (E2-BSA) with the same kinetic profile as 17β-estradiol (P < .01), which indicates that estradiol acts at the membrane surface. The NO release stimulated by 17β-estradiol and E2-BSA was antagonized by tamoxifen (Tam), an antiestrogen. We added 17β-estradiol and E2-BSA to the milieu at 2 minutes, whereas 10−9 mol/L tamoxifen was added at 0 minutes. Each experiment was repeated 4 times, and the resulting mean value plus or minus SEM was graphed.
Estrogen stimulation of NO release by 17β-estradiol and E2-BSA and its antagonism by tamoxifen.
17β-Estradiol (17 B-E; 10−9 mol/L) stimulates NO release from granulocytes within 2 minutes of its application. The release of NO is stimulated by 17β-estradiol coupled to 10−9 mol/L BSA (E2-BSA) with the same kinetic profile as 17β-estradiol (P < .01), which indicates that estradiol acts at the membrane surface. The NO release stimulated by 17β-estradiol and E2-BSA was antagonized by tamoxifen (Tam), an antiestrogen. We added 17β-estradiol and E2-BSA to the milieu at 2 minutes, whereas 10−9 mol/L tamoxifen was added at 0 minutes. Each experiment was repeated 4 times, and the resulting mean value plus or minus SEM was graphed.
L-NAME inhibits estrogen-stimulated NO release from granulocytes
| Cells . | Estrogen agonist, 10−9 mol/L . | L-NAME, μmol/L . | NO level, nmol/L . |
|---|---|---|---|
| Granulocytes | 17β-estradiol | — | 18.1 + 2.3 |
| Granulocytes | 17β-estradiol | 100 | 1.9 + 0.8 |
| Cells . | Estrogen agonist, 10−9 mol/L . | L-NAME, μmol/L . | NO level, nmol/L . |
|---|---|---|---|
| Granulocytes | 17β-estradiol | — | 18.1 + 2.3 |
| Granulocytes | 17β-estradiol | 100 | 1.9 + 0.8 |
The NO level is given as the mean plus or minus SEM. Each experiment was replicated 4 times. P < .01 in comparing NO release in the presence of L-NAME to that in the appropriate tissues without it.
17β-estradiol acts as a surface receptor
E2-BSA, which does not penetrate the cellular membrane due to its size, also stimulates granulocyte NO release in a tamoxifen-sensitive manner (Figures 1 and 3). E2-BSA–stimulated NO release occurred in a dose-dependent manner, with a saturable effect at the same concentration (10−9 mol/L) as 17β-estradiol (Figure 1), which demonstrates that E2-BSA is as potent as 17β-estradiol.
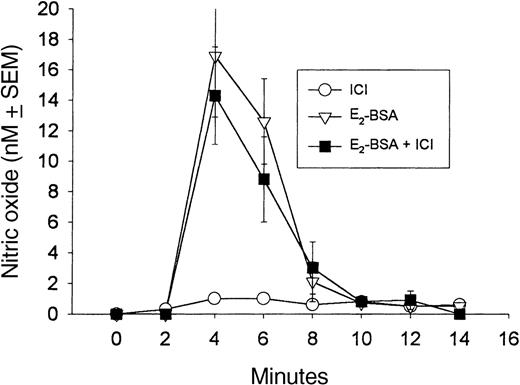
To further establish the specificity of estradiol and E2-BSA action, we attempted to inhibit NO release using ICI 182,780, a nuclear ER antagonist.27 53 Unlike tamoxifen, the addition of 10−9 to 10−7 mol/L ICI 182,780 did not cause the neutralization of NO release stimulated by E2-BSA or 17β-estradiol (Figures 2,4). This supports the conclusion that the receptor that couples NO release to estrogen binding is located on the cell surface.
Estrogen stimulation of granulocyte NO release by E2-BSA is not antagonized by 10−8 mol/L ICI 182,780.
E2-BSA (10−9 mol/L) stimulates NO release from granulocytes within 2 minutes of its application, and this release is not antagonized by ICI 182,780 (ICI), an antiestrogen nuclear binding protein. ICI 182,780 was added to the milieu at 0 minutes, and E2-BSA was added at a 2-minute interval. Each experiment was repeated 4 times, and the resulting mean value plus or minus SEM was graphed.
Estrogen stimulation of granulocyte NO release by E2-BSA is not antagonized by 10−8 mol/L ICI 182,780.
E2-BSA (10−9 mol/L) stimulates NO release from granulocytes within 2 minutes of its application, and this release is not antagonized by ICI 182,780 (ICI), an antiestrogen nuclear binding protein. ICI 182,780 was added to the milieu at 0 minutes, and E2-BSA was added at a 2-minute interval. Each experiment was repeated 4 times, and the resulting mean value plus or minus SEM was graphed.
Direct evaluation of [Ca2+]i release
We have previously demonstrated that in endothelial cells, morphine, anandamide, and estrogen stimulate NO release by a mechanism that involves cNOS and is also dependent on [Ca2+]i transients.41,54 Therefore, we performed these same experiments with granulocytes in a Ca2+-free medium. In real time, 10−9 mol/L 17β-estradiol stimulated rapid [Ca2+]i transients within 8 seconds of its exposure to these cells (Figure 2B). This event could be blocked by prior exposure to 10−9 tamoxifen but not to 10−12 to 10−7 mol/L ICI 182,780 (Figure 2B). The EC50 for 17β-estradiol is 7 × 10−10 mol/L, and the IC50 for tamoxifen is 8 × 10−10 mol/L, indicating a similar receptor-mediated process. In addition, BAPTA-AM, a [Ca2+]i chelator,44 inhibits the effects of 10−9 mol/L E2-BSA in a concentration-dependent manner (Figure 2C).
Relationship between [Ca2+]i and NO
In comparing the sequence of events during 17β-estradiol–stimulated increases in both [Ca2+]i and NO production in granulocytes, we found that the Ca2+transients precede NO release by approximately 40 seconds (from 4 experiments, as shown in Figure 5). Given the fact that Ca2+ is required for NOS,55 we attempted to determine if these events were linked in granulocytes. During a 1-hour period we changed the Ca2+-free incubation medium of the cells 5 times in an attempt to deplete [Ca2+]i stores.41 After the 1-hour incubation, 10−9 mol/L 17β-estradiol increased [Ca2+]i to 2.5 ± 0.9 nmol/L (SEM), a level significantly lower than that previously observed under nondepleting conditions (Figure 4; P < .01). Furthermore, NO release was barely above background in the granulocytes depleted of [Ca2+]i following 17β-estradiol exposure (3.3 ± 0.9 nmol/L NO compared with a peak value of 17.8 ± 2.7 nmol/L NO; P < .01). This strongly suggests that [Ca2+]i originates from the coupling of ER to [Ca]i.
Sequence of events regarding real-time E2-BSA–stimulated [Ca2+]i transients and NO release from human granulocytes.
Addition of 10−9 mol/L E2-BSA to the medium results in immediate calcium transients (application added at the base of the steep increase) that are then followed by a progressive decrease lasting about 2 minutes. Approximately 40 seconds later, an increase in NO release (peak level, 13 nmol/L E2-BSA) occurs and lasts for 10 minutes. The mean values (n = 4) were graphed with spline curves so that the precise times could be better visualized.
Sequence of events regarding real-time E2-BSA–stimulated [Ca2+]i transients and NO release from human granulocytes.
Addition of 10−9 mol/L E2-BSA to the medium results in immediate calcium transients (application added at the base of the steep increase) that are then followed by a progressive decrease lasting about 2 minutes. Approximately 40 seconds later, an increase in NO release (peak level, 13 nmol/L E2-BSA) occurs and lasts for 10 minutes. The mean values (n = 4) were graphed with spline curves so that the precise times could be better visualized.
Estrogen alters granulocyte activity via NO release
Morphine and anandamide cause the release of about 17-40 nmol/L NO from monocytes, granulocytes, and endothelial cells.37,40,46,56,57 This level of NO, also determined by exposing these cells to various concentrations of S-nitroso-N-acetyl-DL-penicillamine (SNAP), a stable NO donor, alters cell shape and cell adherence.37,40,46,56,57 As a result, cells in an amoeboid conformation (shape factor, 0.48) become round (shape factor, 0.80-0.94) and immobile. In vitro, estrogen signaling exerts a cellular immunosuppressive action in granulocytes, as was noted for SNAP, and round and immobile cells cannot respond to antigenic challenge.25,39,47,57 We exposed granulocytes to 10−9 mol/LE2-BSA for 10 minutes, then examined them for the percent of cells that was spontaneously active (shape factor, less than 0.49). As in previous studies,37,40,46,56 57 before adding E2-BSA, the percent of spontaneously active cells was 8.2% ± 1.4% SEM. Following exposure to E2-BSA (10 minutes later), this level of activity dropped to 1.5% ± 0.4% SEM (comparing both groups,P < .005).
Preexposure of the cells for 5 minutes to 100 μmol/L L-NAME followed by E2-BSA only diminished the inhibitory action of the estrogen agonist to 7.4% ± 1.2% SEM, which demonstrates that E2-BSA exerts its inhibitory action via nitric oxide. Furthermore, pretreatment with 10−9 mol/L tamoxifen followed by E2-BSA reduced the activation level to 2.1% ± 0.8% SEM (P < .01), whereas 10−9 mol/L ICI 182,780 only slightly reduced it to 7.8% ± 1.7% SEM (nonsignificant compared to control). Additionally, 10−9 mol/L E2-BSA exposure caused 69% of previously adherent cells (field, 175% ± 12% SEM) to flow off a slide after gentle washing, whereas only 21% (P < .005) of cells exposed to tamoxifen and then 2 minutes later to 10−9 mol/L E2-BSA were removed from the slide. This indicates that the rounding is associated with a loss of adherence47 and that estrogen acts through a cell surface receptor to release NO, which then has an immunosuppressive effect in granulocytes.
ER and ERβ gene expression in human monocytes and neutrophils
To determine which ER genes are expressed in granulocytes, we performed RT-PCR analysis of RNA extracted from 2 independent blood samples. The presence of GAPDH transcripts was also assessed as a control. As shown in Figure 6, single bands of the correct predicted sizes of 281-bp (ERα), 265-bp (ERβ), and 470-bp (GAPDH) were detected in these cells including monocytes.52 An amplification signal for ERα but not ERβ was observed in granulocytes, whereas ERβ is only detectable in monocytes. Both cell lines displayed a predominant ERα expression. Subcloning and sequencing the specific bands further confirmed the identity of the PCR products; control ERα and ERβ cDNA sequences obtained from PCR products of RNA from human breast cell lines (MCF7 and MDA MB231) were identical to those from monocytes and granulocytes.
ER gene expression in human monocytes and neutrophils.
RT-PCR was performed using either no RNA (negative control, lane 1) or 3 μg RNA from human breast cell lines (positive control, lane 2), monocytes (lane 3), or neutrophils (lane 4). MCF7 and MDA MB231 cell lines were used for ERα and ERβ amplification controls, respectively. PCR amplification was also performed using a primer pair specific for human GAPDH as a cDNA control. The PCR products (one-fifth of the ERα and ERβ amplification, one-tenth of the GAPDH amplification) were separated by electrophoresis on a 2% agarose gel. DNA markers (1-kb ladder) were run in parallel. The sizes of the amplified products are indicated in the base pair on the right.
ER gene expression in human monocytes and neutrophils.
RT-PCR was performed using either no RNA (negative control, lane 1) or 3 μg RNA from human breast cell lines (positive control, lane 2), monocytes (lane 3), or neutrophils (lane 4). MCF7 and MDA MB231 cell lines were used for ERα and ERβ amplification controls, respectively. PCR amplification was also performed using a primer pair specific for human GAPDH as a cDNA control. The PCR products (one-fifth of the ERα and ERβ amplification, one-tenth of the GAPDH amplification) were separated by electrophoresis on a 2% agarose gel. DNA markers (1-kb ladder) were run in parallel. The sizes of the amplified products are indicated in the base pair on the right.
Discussion
The present study demonstrates that at physiological concentrations, 17β-estradiol rapidly stimulates NO release from human granulocytes. Our results suggest that rapid estrogen-mediated NO release is also mediated by a specific ER on the cell surface; this NO release can be stimulated by E2-BSA, which cannot enter the cell. The location of this ER on the surface of cells expressing cNOS is also consistent with the observation that ICI 182,780 cannot block this stimulation. Furthermore, 17β-estradiol– and E2-BSA–stimulated NO release is inhibited by L-NAME, a NOS inhibitor, indicating that the effect of the agonists on NO release is mediated by coupling the membrane ER to cNOS. We further demonstrate that the surface ER-mediated NO release is dependent on [Ca2+]i transients and leads to down-regulation of granulocyte function. In addition, confocal microscopy using E2-BSA-FITC demonstrated cell surface labeling. Finally, RT-PCR analysis showed expression of ERα mRNA in granulocytes, suggesting that ERα could mediate estradiol's action at the plasma membrane.
The mechanisms by which estrogen influences coronary arteries and protects blood vessels are unknown, but our findings might explain, in part, the mechanism that underlies some of the clinical benefits of estrogens, such as their effects of diminishing the risk of atherosclerosis36,58-63 in premenopausal women and as estrogen replacement therapy in postmenopausal women. Estrogen-stimulated NO release may act to down-regulate the activity of monocytes and granulocytes (making them round and nonadherent) as well as the endothelial lining because their combined increased activity is implicated in this pathological process.25,52,54 64
Previous studies have shown that within minutes of estrogen exposure, endothelial cNOS (ecNOS) and [Ca2+]i are stimulated in a tamoxifen-sensitive or ICI 182,780–sensitive manner in fetal pulmonary artery endothelium via a nuclear ER.53 Recently, however, Chen and colleagues31 reported that in ovine endothelial cells, both tamoxifen and ICI 182,780 antagonized estradiol-stimulated NO release in a transcription-independent manner, thereby suggesting action through a cell surface receptor. Tamoxifen, although often considered an antagonist of the nuclear ER, is also able to antagonize the effect of estradiol on a membrane receptor.30,52,54,65In the study by Chen et al,31 micromolar concentrations of ICI 182,780 were antagonistic; in our study, this estrogen cell surface receptor was insensitive to ICI 182,780 at nanomolar concentrations.
This apparent discrepancy may be explained by the following: Razandi and colleagues32 have shown that the binding of 17β-estradiol to the ER membrane is competitively inhibited by micromolar concentrations of ICI 182,780. While in our study nanomolar concentrations of tamoxifen (8.7 × 10−10 nmol/L IC50) inhibited estradiol-stimulated NO release, whereas 10−9 to 10−7 mol/L ICI 182,780 did not. The affinity constant of the ER membrane for 17β-estradiol is in the 0.2-nmol/L range.32 Therefore, the use of high (micromolar) concentrations of ICI 182,780 to inhibit the actions of 17β-estradiol may lead to the observation of nonspecific effects. Supporting this conclusion, unpublished data show that 10−5 mol/L tamoxifen or ICI 182,780 reduced morphine-stimulated release of NO from human endothelial cells by 20% or 22%, respectively.54This suggests that at high doses, these ER antagonists become less selective.
In other recent studies, 17β-estradiol stimulated NO release from human monocytes and endothelial cells by acting on an estrogen surface membrane receptor.52 54 In our studies, nanomolar concentrations of ICI-182,780 did not block the stimulatory action of estradiol on cNOS, whereas tamoxifen was inhibitory at nanomolar concentrations.
In regard to leukocytes, estrogen down-regulates many cell functions including chemotaxis and phagocytosis.1-12,66,67 This conclusion is supported in the present study, which demonstrated that estrogen-stimulated NO release initiates granulocyte rounding, a condition that inhibits the cells from moving and changing conformation in order to initiate phagocytosis. NO-stimulated cell rounding has been associated with an inhibition of adherence, thus further inhibiting a cell's ability to respond to chemotactic signals.68 The NO-associated down-regulation can also be initiated by inhibiting the adhesion potential of the leukocytes and endothelial lining of the vasculature.3,13-15 It is interesting to note that morphine and anandamide, via their respective cell surface receptors, release the same level of NO and initiate the same level of monocyte and granulocyte down-regulation as estradiol.47,52,68 Thus, estrogen exerts its immunocyte down-regulation1-12 67 via the NO mechanism.
While our studies demonstrate a reduction in cell adherence, others have found that in contrast, 17β-estradiol enhances cellular adherence via a cytokine association.69,70 In these studies, however, endothelial cells were exposed to 17β-estradiol for 3-24 hours. In the past we have demonstrated that exposure of endothelial cells or leukocytes to NO donors, SNAP, cNOS stimulators, or morphine results in a biphasic effect.46,68,71 First, NO exposure (at the levels achieved here with estrogen stimulation) inhibits the cells, which become round, nonadherent, and immobile. After some time, depending on the concentration of NO, there is a rebound from NO-stimulated inhibition. In this rebound stage the cells are hyperactivated, exhibiting amoeboid conformations, enhanced adherence, and motility.46,68,71,72 This last stage occurs hours after the initial exposure to NO or NO-stimulating agents. Therefore, we suggest that in the studies demonstrating 17β-estradiol–enhanced cytokine-associated adherence, this last stage results from a rebound of NO-stimulated cellular down-regulation. This hypothesis is supported by the fact that morphine and anandamide, an endocannabinoid, both cause cellular down-regulation through the release of NO in the same tissues, at NO concentrations similar to those released by 17β-estradiol.46,68 71
Our results are further supported by studies demonstrating that cells expressing ERα and ERβ target the protein to both membrane and nuclear fractions.32 While our RT-PCR results suggest the presence of only ERα transcripts in granulocytes, we do not know if these are the receptors that mediate NO release in response to 17β-estradiol. While recent studies have identified several variant ER transcripts,73 Chen et al31 have shown that expression of ERα and ecNOS in COS cells can reconstitute the acute response of NOS to 17β-estradiol. (The COS-7 fibroblast-like cell line is SV40 transformed and established from a kidney cell line, CVI, of the African green monkey.) This shows that ERα alone can mediate this effect.
Finally, our results are supported by other functional studies that demonstrate a rapid-acting vasodilatory role for estrogen-mediated NO release59 and the potential74 to diminish granulocyte and monocyte adherence.68 The significance of these processes may correlate with the beneficial activities reported for estrogen in vascular tissues and those pathologies associated with immunocyte activation such as atherosclerosis.75 Indeed, the vascular protection afforded premenopausal women may well be due to estrogen's capacity to down-regulate leukocyte and endothelial excitation and thus interaction. These findings promise to open new areas of investigation to better understand the mechanisms by which estrogen and the drugs that inhibit its action mediate their clinical effects. These studies further demonstrate the significance of granulocytes and monocytes in their potential to affect other tissues via common signaling molecules.
Supported by a grant from the Research Foundation and Central Administration of the State University of New York, Old Westbury, NY; a grant from the University of Lille II, Lille, France; a grant from the FEDER, France; and the following grants from the National Institutes of Health, Bethesda, MD: NIMH COR 17138, the National Institute of Mental Health; NIDA09010, the National Institute on Drug Abuse; and NIH Fogarty INT 00045 (G.B.S.).
Reprints:G. B. Stefano, Neuroscience Research Institute, State University of New York at Old Westbury, Old Westbury, NY 11568-0210; e-mail: stefanog@surg.som.sunysb.edu orgbs11@banet.net.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 2. Real-time representation of 10−9 mol/L 17β-estradiol–stimulated [Ca2+]i and NO release from human granulocytes. / (A) Real time representation of 10−9 mol/L 17β-estradiol–stimulated NO release from peripheral granulocytes and its blockade by 10−9 mol/L tamoxifen (Tam) but not 10−8 mol/L ICI-182,780 (ICI). Tamoxifen and ICI-182,780 were administered 2 minutes prior to 17β-estradiol. (B) 17β-Estradiol–stimulated [Ca2+]i (Est) and its antagonist. Tamoxifen (Tam; 10−9 mol/L) antagonizes 17β-estradiol–stimulated [Ca2+]i, but 10−8 mol/L ICI-182,780 (ICI) does not. Drugs were administered as in (A). (C) 17β-Estradiol–stimulated [Ca2+]i (Est) and its BAPTA-AM concentration-dependent decrease, which demonstrates that [Ca2+]i transients are associated with estradiol's action. (D) Confocal laser photomicrograph of E2-BSA-FITC reactivity of the cell surface of granulocytes (right) and control granulocyte (left).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3951/8/m_bloo01221002w.jpeg?Expires=1767769021&Signature=AWdOqYNEKErUtBRJSTVIZCRawchluALze~n2DhK~0Xj8bZaXnDvl5qsxNAjOntRROryUBszxxw2G2IWwdbolIsIHhmd~rQ7OIBPgRPOevRjHQWZuuwruNohNu1RazC3HiAT6tRj-eqfUeUF9kL4aJM3Tf4VVudhm2bkuOdXmSGxkmc97~E4ydZRd7KmA--p6F7V1oBkOCo8twc63GWQGDHbBnZBO5DkWCY-ECF5PPSDYQrb~xMpGgyewQ~aQHjHGUZJ6kteHBBVLd8gapI43Zo6DIOhPQoocoaKPrkq2H8vTxYnd8hMjOUEbd3d47nwxLtJnfz7QDxDa9~5dUnfLZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Sequence of events regarding real-time E2-BSA–stimulated [Ca2+]i transients and NO release from human granulocytes. / Addition of 10−9 mol/L E2-BSA to the medium results in immediate calcium transients (application added at the base of the steep increase) that are then followed by a progressive decrease lasting about 2 minutes. Approximately 40 seconds later, an increase in NO release (peak level, 13 nmol/L E2-BSA) occurs and lasts for 10 minutes. The mean values (n = 4) were graphed with spline curves so that the precise times could be better visualized.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/12/10.1182_blood.v95.12.3951/8/m_bloo01221005x.jpeg?Expires=1767769021&Signature=mvEe3czNCiQgAc0UWnT4WhID5Q65FlOSVhfzZByRSO8kEEaNEXOq0sXQDx7Hji5CPsXY7QfT5Ld6u8TdvZTtGgnDPdFJSUUoH3C47AARHD-esWIewTeQL4P1pKVGd~-J2miv1KkKqWtzgqKbcZrfte0z0x4ZwVsL~A~CBtjJBGPbR~lnS91Zty86P0Ma3bhDcKbXMl2K4l86lpvV1F1s0EyPj4I4WKehUgkXGrH96mZ8pmo74zuUsK4EyCiCjlgx013A1-Lz~jzNLUVHERIHn6YQB55IB65ut7bMegZN5InTLKRW4LVI9gajD-FpojwKd7PA8AJ2DcB5M52~HPoVRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal