Abstract
T-cell depletion of donor marrow decreases graft–versus–host disease resulting from transplants from unrelated and human leukocyte antigen (HLA)-mismatched related donors. However, there are diverse strategies for T-cell–depleted transplantation, and it is uncertain whether any improve leukemia-free survival (LFS). To compare strategies for T-cell–depleted alternative donor transplants and to compare T-cell depleted with non-T-cell–depleted transplants, we studied 870 patients with leukemia who received T-cell–depleted transplants from unrelated or HLA-mismatched related donors from 1982 to 1994. Outcomes were compared with those of 998 non-T-cell–depleted transplants. We compared LFS using different strategies for T-cell–depleted transplantation considering T-cell depletion technique, intensity of pretransplant conditioning, and posttransplant immune suppression using proportional hazards regression to adjust for other prognostic variables. Five categories of T-cell depletion techniques were considered: narrow-specificity antibodies, broad-specificity antibodies, Campath antibodies, elutriation, and lectins. Strategies resulting in similar LFS were pooled to compare T-cell–depleted with non-T-cell–depleted transplants. Recipients of transplants T-cell depleted by narrow-specificity antibodies had lower treatment failure risk (higher LFS) than recipients of transplants T-cell depleted by other techniques. Compared with non-T-cell–depleted transplants (5-year probability ± 95% confidence interval [CI] of LFS, 31% ± 4%), 5-year LFS was 29% ± 5% (P = NS) after transplants T-cell depleted by narrow-specificity antibodies and 16% ± 4% (P < .0001) after transplants T-cell depleted by other techniques. After alternative donor transplantation, T-cell depletion of donor marrow by narrow-specificity antibodies resulted in LFS rates that were higher than those for transplants T-cell depleted using other techniques but similar to those for non-T-cell–depleted transplants.
Extensive data indicate that donor T cells cause graft–versus–host disease (GVHD), a major cause of mortality after allogeneic bone marrow transplantation.1,2 Removing T cells from the graft reduces the risk for GVHD. Many techniques for T-cell depletion are available.3-16 Early studies of human leukocyte antigen (HLA)-identical sibling transplants showed that although T-cell depletion decreased GVHD, T-cell–depleted transplants had higher risks for graft failure and leukemia relapse. Leukemia-free survival (LFS) rates were not improved compared with rates in non-T-cell–depleted transplants.17-26 Newer strategies for T-cell–depletedtransplants address problems of graft failure and relapse by removing either fewer or selected subsets of T cells, intensifying pretransplant immune suppression (conditioning), and adding posttransplant immune suppression.
Transplants from unrelated or HLA-mismatched related donors (alternative donors) differ from HLA-identical sibling transplants in that they carry a higher risk for GVHD and have associated complications.27,28 There are conflicting reports about whether T-cell depletion improves LFS after alternative donor transplantation.28 29
It was the purpose of this analysis to evaluate different strategies for T-cell–depleted alternative donor transplantation in patients with leukemia by considering techniques for depletion, intensity of the pretransplant conditioning regimen, and posttransplant immune suppression and to determine whether LFS after T-cell–depleted alternative donor transplantation differed from LFS after non-T-cell depleted transplantation.
Patients and methods
Patients
The study included 1868 transplant recipients reported to the International Bone Marrow Transplant Registry (IBMTR) who met the following criteria: (1) diagnosis of chronic myelogenous leukemia (CML), acute myelogenous leukemia (AML), or acute lymphoblastic leukemia (ALL); (2) transplantation between 1982 and 1994; (3) unrelated or HLA-mismatched related donor (alternative donor); (4) transplant T-cell depleted by 1 of the 5 techniques described in Table1 or transplant non-T-cell depleted using posttransplant methotrexate and cyclosporine for GVHD prophylaxis.30 Donor–recipient histocompatibility was determined by results of serologic typing for HLA-A, HLA-B, and HLA-DR, as reported by the transplant center. The study population included 870 recipients of T-cell–depleted transplants reported to the IBMTR by 78 teams and 998 recipients of non-T-cell–depleted transplants reported by 161 teams.31 32 Excluded were recipients of T-cell–depleted transplants using a technique that could not be placed in 1 of the 5 categories in Table 1 (n = 23) and patients with missing information regarding relapses (n = 18). Median follow-up time of survivors was 24 months (range, 4 to 132 months).
Techniques of T-cell depletion
| Group . | Technique of T-cell depletion . |
|---|---|
| Narrow-specificity antibodies N = 450 (52%) | Antibodies targeting T10B9 (α/β T-cell receptor), CD3 ± CD7, CD3 + CD6 + CD8, CD4 ± CD8, CD4 + CD5 + CD8, CD5 ± CD8, CD6 ± CD7, CD6 + CD8, CD6 + CD7 + CD8, CD8 ± CD7 |
| Broad-specificity antibodies N = 73 (8%) | Antibodies targeting CD2 ± CD3, CD2 + CD5, CD2 + CD7, CD2 + CD8, CD2 + CD4 + CD8, CD2 + CD5 + CD7, CD2 + CD5 + CD8, CD2 + CD3 + CD4 + CD8, CD2 + CD3 + CD4 + CD5 + CD6 + CD8 + CD28; ATG incubation |
| Campath 1 N = 131 (15%) | Antibodies targeting CD52 including Campath 1G (IgG-2b), Campath 1M (IgM) |
| Elutriation N = 75 (9%) | Elutriation; density-gradient centrifugation (DGR) |
| Lectins/SRBC N = 141 (16%) | Lectins + CD5 + CD8; lectins ± sheep red blood cell; rosetting (SRBC); SRBC ± DGR |
| Group . | Technique of T-cell depletion . |
|---|---|
| Narrow-specificity antibodies N = 450 (52%) | Antibodies targeting T10B9 (α/β T-cell receptor), CD3 ± CD7, CD3 + CD6 + CD8, CD4 ± CD8, CD4 + CD5 + CD8, CD5 ± CD8, CD6 ± CD7, CD6 + CD8, CD6 + CD7 + CD8, CD8 ± CD7 |
| Broad-specificity antibodies N = 73 (8%) | Antibodies targeting CD2 ± CD3, CD2 + CD5, CD2 + CD7, CD2 + CD8, CD2 + CD4 + CD8, CD2 + CD5 + CD7, CD2 + CD5 + CD8, CD2 + CD3 + CD4 + CD8, CD2 + CD3 + CD4 + CD5 + CD6 + CD8 + CD28; ATG incubation |
| Campath 1 N = 131 (15%) | Antibodies targeting CD52 including Campath 1G (IgG-2b), Campath 1M (IgM) |
| Elutriation N = 75 (9%) | Elutriation; density-gradient centrifugation (DGR) |
| Lectins/SRBC N = 141 (16%) | Lectins + CD5 + CD8; lectins ± sheep red blood cell; rosetting (SRBC); SRBC ± DGR |
Strategies for T-cell depletion
Depletion techniques.
Five categories of T-cell depletion techniques (described in Table 1) were considered: narrow-specificity antibodies targeting T cells or T-cell subsets; broad-specificity antibodies targeting T cells and other immune cells; Campath antibodies with very broad specificity; counterflow elutriation separating cells based on size and density; and lectin fractionation, agglutinating T cells, used commonly in combination with sheep red blood cell rosetting.
Intensification of pretransplant conditioning.
Pretransplant conditioning regimens were considered in 3 categories: standard intensity radiation regimens, corresponding to 12 Gy fractionated or 10 Gy unfractionated total body irradiation and 100 to 120 mg/kg cyclophosphamide; high-intensity radiation regimens with either higher total body irradiation doses or drugs in addition to cyclophosphamide; and busulfan and cyclophosphamide without radiation.
Posttransplant immune suppression to prevent GVHD.
Two categories were considered: any or none. Eighty-three percent of patients receiving posttransplant immune suppression were administered cyclosporine with or without other drugs.
Outcomes
The primary outcome of this study was LFS. Other outcomes analyzed were graft failure, acute and chronic GVHD, transplant-related mortality (TRM), relapse, and survival. Results of survival analyses were virtually identical to LFS analyses and are not presented. Graft failure was analyzed in patients surviving 21 days after transplantation using published criteria.33 Primary nonengraftment and transient engraftment were considered graft failures. Acute GVHD was defined as moderate to severe (grades 2 to 4) disease using published criteria; patients surviving more than 21 days with engraftment were considered at risk.34,35 Chronic GVHD was determined by clinical criteria in patients surviving more than 90 days with engraftment.36 TRM was defined as death before relapse. Relapse was defined as hematologic or clinical recurrence at any site or as initiation of treatment for relapse, whichever occurred first; patients in continuous complete remission were censored at death or, for survivors, at last follow-up. LFS was defined as survival in continuous complete remission; relapse and death from causes other than leukemia were considered treatment failures, whereas patients alive and in remission were censored at last follow-up.
The International Bone Marrow Transplant Registry
The International Bone Marrow Transplant Registry is a voluntary working group of more than 350 transplant teams worldwide that contribute detailed data on their allogeneic bone marrow transplants to the Statistical Center at the Medical College of Wisconsin. Participants are required to report all consecutive transplants; compliance is monitored by on-site audits. Approximately two thirds of all active transplant centers register their transplants with the IBMTR. The IBMTR database includes 40% to 45% of all recipients of allogeneic transplants since 1970. Patients are followed up longitudinally each year. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality. To participate in this study, centers had to provide current follow-up on more than 90% of all eligible patients.
Statistical methods
Cox proportional hazards regression was used to assess the association of T-depletion technique with treatment failure (inverse of LFS).37 38 Potential confounding factors considered were leukemia type (ALL, AML, CML), pretransplant disease state (1st remission or chronic phase, 2nd remission or accelerated phase, not in remission or blast phase), patient age (by decade), Karnofsky performance score (90% vs less than 90%), year of transplantation (1982-1988 vs 1989-1994; the breakpoint was determined by using the regression model with the largest partial likelihood), white blood cell count at diagnosis (more than vs less than or equal to 50 × 109/L for acute leukemia, more than vs less than or equal to 200 × 109/L for CML), donor age (by decade), donor–recipient sex match, donor–recipient relationship, and HLA histocompatibility (related, 0 to 1 antigen mismatch; related, ≥2 antigen mismatch; unrelated, matched; unrelated mismatched), intensity of conditioning regimen (see Table2), cell dose (greater than or equal to vs less than median), use of posttransplant immune suppression (any vs none), and prophylactic use of growth factors (any vs none). Forward stepwise variable selection was used to determine which of these covariates were associated with outcome (P = .05 was considered statistically significant). Covariates for T-cell depletion technique were included in each stepwise model. Tests of the appropriateness of the proportional hazards model were made by adding a time-dependent covariate for each significant covariate. Interactions between technique of T-cell depletion and other significant covariates in the model were considered. There were no significant interactions between technique of T-cell depletion, intensity of conditioning regimen, and use of posttransplant immune suppression. Pairwise comparisons between T-cell depletion techniques were made from a final multivariate Cox model, adjusting for relevant risk factors.
Patient, disease, and transplant characteristics
| . | T-cell depleted . | Non-T-cell depleted . | P . |
|---|---|---|---|
| N | 870 | 998 | |
| Median age, y (range) | 24 (0.4-57) | 28 (0.5-61) | .0001 |
| Performance score 90%, n/n eval (%) | 706/869 (81%) | 735/988 (74%) | .0001 |
| Disease and disease state, n/n eval (%) | .001 | ||
| ALL, 1st remission | 45/851 (5%) | 64/987 (6%) | |
| ALL, 2nd remission | 162/851 (19%) | 141/987 (14%) | |
| ALL, not in remission | 86/851 (10%) | 62/987 (6%) | |
| AML, 1st remission | 53/851 (6%) | 90/987 (9%) | |
| AML, 2nd remission | 51/851 (6%) | 66/987 (7%) | |
| AML, not in remission | 101/851 (12%) | 107/987 (11%) | |
| CML, 1st chronic phase | 203/851 (24%) | 292/987 (30%) | |
| CML, accelerated phase | 110/851 (13%) | 115/987 (12%) | |
| CML, blast phase | 40/851 (5%) | 50/987 (5%) | |
| Year of transplantation, n/n eval (%) | .0001 | ||
| 1982-1987 | 316/870 (36%) | 133/998 (14%) | |
| 1988-1994 | 554/870 (63%) | 865/998 (86%) | |
| Median interval diagnosis-transplant, mo (range) | |||
| ALL, 1st remission | 6 (2-21) | 7 (2-19) | NS |
| ALL, 2nd remission | 28 (5-148) | 34 (4-151) | NS |
| ALL, not in remission | 18 (3-73) | 13 (3-97) | NS |
| AML, 1st remission | 6 (2-17) | 6 (2-19) | NS |
| AML, 2nd remission | 21 (7-67) | 17 (5-88) | NS |
| AML, not in remission | 10 (3-54) | 10 (1-61) | NS |
| CML, 1st chronic phase | 20 (2-124) | 19 (2-123) | NS |
| CML, accelerated phase | 26 (2-107) | 27 (2-126) | NS |
| CML, blast phase | 27 (6-123) | 20 (2-116) | NS |
| Median WBC at diagnosis, ×109/L (range) | |||
| Acute leukemia | 16 (0.5-850) | 16 (0.4-882) | NS |
| Chronic leukemia | 164 (4-880) | 158 (4-760) | NS |
| Median donor age, y (range) | 35 (1-81) | 35 (1-75) | NS |
| Donor recipient sex-match, n/n eval (%) | NS | ||
| Male-male | 283/859 (33%) | 292/987 (30%) | |
| Male-female | 173/859 (20%) | 215/987 (22%) | |
| Female-male | 242/859 (28%) | 282/987 (28%) | |
| Female-female | 161/859 (19%) | 198/987 (20%) | |
| Donor-recipient CMV status, n/n eval (%) | .0001 | ||
| Negative-negative | 289/726 (40%) | 265/912 (29%) | |
| Negative-positive | 151/726 (21%) | 197/912 (21%) | |
| Positive-negative | 116/726 (16%) | 152/912 (17%) | |
| Positive-positive | 170/726 (23%) | 298/912 (33%) | |
| Median cell dose, ×108/kg (range) | 1.1 (0.1-12) | 3 (0.3-25) | .0001 |
| Conditioning regimen*, n/n eval (%) | .0001 | ||
| High intensity | 664/863 (77%) | 339/986 (34%) | |
| Standard intensity | 83/863 (10%) | 374/986 (38%) | |
| Busulfan + cyclophosphamide | 25/863 (3%) | 213/986 (22%) | |
| Other | 91/863 (10%) | 60/986 (6%) | |
| Donor-recipient relationship and HLA-match, n/n eval (%) | .0001 | ||
| Phenotypically matched related donor | 56/870 (6%) | 96/998 (10%) | |
| 1-Antigen mismatched related donor | 180/870 (21%) | 332/998 (33%) | |
| 2-Antigen mismatched related donor | 153/870 (18%) | 78/998 (8%) | |
| 3-Antigen mismatched related donor | 98/870 (11%) | 12/998 (1%) | |
| Phenotypically matched unrelated donor | 267/870 (31%) | 408/998 (41%) | |
| Mismatched unrelated donor | 116/870 (13%) | 72/998 (7%) | |
| Prophylactic use of growth factors†, n/n eval (%) | 79/870 (9) | 103/998 (10) | NS |
| Posttransplant immune suppression, n/n eval (%) | — | ||
| Methotrexate (MTX) | 39/869 (4%) | ||
| Cyclosporine A (CsA) | 399/869 (46%) | ||
| CsA + MTX | 40/869 (5%) | 998/998 (100%) | |
| CSA + Corticosteroids | 120/869 (14%) | ||
| Corticosteroids | 91/869 (10%) | ||
| Other | 25/869 (3%) | ||
| None | 155/869 (18%) |
| . | T-cell depleted . | Non-T-cell depleted . | P . |
|---|---|---|---|
| N | 870 | 998 | |
| Median age, y (range) | 24 (0.4-57) | 28 (0.5-61) | .0001 |
| Performance score 90%, n/n eval (%) | 706/869 (81%) | 735/988 (74%) | .0001 |
| Disease and disease state, n/n eval (%) | .001 | ||
| ALL, 1st remission | 45/851 (5%) | 64/987 (6%) | |
| ALL, 2nd remission | 162/851 (19%) | 141/987 (14%) | |
| ALL, not in remission | 86/851 (10%) | 62/987 (6%) | |
| AML, 1st remission | 53/851 (6%) | 90/987 (9%) | |
| AML, 2nd remission | 51/851 (6%) | 66/987 (7%) | |
| AML, not in remission | 101/851 (12%) | 107/987 (11%) | |
| CML, 1st chronic phase | 203/851 (24%) | 292/987 (30%) | |
| CML, accelerated phase | 110/851 (13%) | 115/987 (12%) | |
| CML, blast phase | 40/851 (5%) | 50/987 (5%) | |
| Year of transplantation, n/n eval (%) | .0001 | ||
| 1982-1987 | 316/870 (36%) | 133/998 (14%) | |
| 1988-1994 | 554/870 (63%) | 865/998 (86%) | |
| Median interval diagnosis-transplant, mo (range) | |||
| ALL, 1st remission | 6 (2-21) | 7 (2-19) | NS |
| ALL, 2nd remission | 28 (5-148) | 34 (4-151) | NS |
| ALL, not in remission | 18 (3-73) | 13 (3-97) | NS |
| AML, 1st remission | 6 (2-17) | 6 (2-19) | NS |
| AML, 2nd remission | 21 (7-67) | 17 (5-88) | NS |
| AML, not in remission | 10 (3-54) | 10 (1-61) | NS |
| CML, 1st chronic phase | 20 (2-124) | 19 (2-123) | NS |
| CML, accelerated phase | 26 (2-107) | 27 (2-126) | NS |
| CML, blast phase | 27 (6-123) | 20 (2-116) | NS |
| Median WBC at diagnosis, ×109/L (range) | |||
| Acute leukemia | 16 (0.5-850) | 16 (0.4-882) | NS |
| Chronic leukemia | 164 (4-880) | 158 (4-760) | NS |
| Median donor age, y (range) | 35 (1-81) | 35 (1-75) | NS |
| Donor recipient sex-match, n/n eval (%) | NS | ||
| Male-male | 283/859 (33%) | 292/987 (30%) | |
| Male-female | 173/859 (20%) | 215/987 (22%) | |
| Female-male | 242/859 (28%) | 282/987 (28%) | |
| Female-female | 161/859 (19%) | 198/987 (20%) | |
| Donor-recipient CMV status, n/n eval (%) | .0001 | ||
| Negative-negative | 289/726 (40%) | 265/912 (29%) | |
| Negative-positive | 151/726 (21%) | 197/912 (21%) | |
| Positive-negative | 116/726 (16%) | 152/912 (17%) | |
| Positive-positive | 170/726 (23%) | 298/912 (33%) | |
| Median cell dose, ×108/kg (range) | 1.1 (0.1-12) | 3 (0.3-25) | .0001 |
| Conditioning regimen*, n/n eval (%) | .0001 | ||
| High intensity | 664/863 (77%) | 339/986 (34%) | |
| Standard intensity | 83/863 (10%) | 374/986 (38%) | |
| Busulfan + cyclophosphamide | 25/863 (3%) | 213/986 (22%) | |
| Other | 91/863 (10%) | 60/986 (6%) | |
| Donor-recipient relationship and HLA-match, n/n eval (%) | .0001 | ||
| Phenotypically matched related donor | 56/870 (6%) | 96/998 (10%) | |
| 1-Antigen mismatched related donor | 180/870 (21%) | 332/998 (33%) | |
| 2-Antigen mismatched related donor | 153/870 (18%) | 78/998 (8%) | |
| 3-Antigen mismatched related donor | 98/870 (11%) | 12/998 (1%) | |
| Phenotypically matched unrelated donor | 267/870 (31%) | 408/998 (41%) | |
| Mismatched unrelated donor | 116/870 (13%) | 72/998 (7%) | |
| Prophylactic use of growth factors†, n/n eval (%) | 79/870 (9) | 103/998 (10) | NS |
| Posttransplant immune suppression, n/n eval (%) | — | ||
| Methotrexate (MTX) | 39/869 (4%) | ||
| Cyclosporine A (CsA) | 399/869 (46%) | ||
| CsA + MTX | 40/869 (5%) | 998/998 (100%) | |
| CSA + Corticosteroids | 120/869 (14%) | ||
| Corticosteroids | 91/869 (10%) | ||
| Other | 25/869 (3%) | ||
| None | 155/869 (18%) |
Standard intensity: cyclophosphamide + 12 Gy fractionated or 10 Gy unfractionated total body irradiation.
High intensity: >12 Gy total body irradiation or cyclophosphamide, total body irradiation and other drugs.
G-CSF or GM-CSF started within 1 week of transplantation.
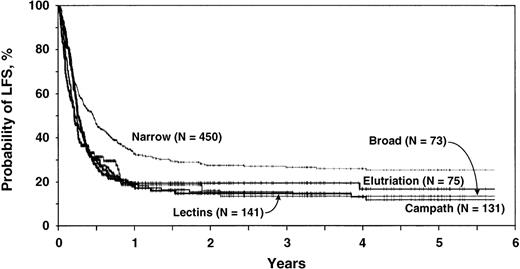
Recipients of bone marrow T-cell depleted by narrow-specificity antibodies had a lower treatment failure risk than recipients of marrow T-cell depleted by other techniques. Pairwise comparisons showed treatment failure risks to be similar in recipients of transplants T-cell depleted by broad-specificity antibodies, Campath antibodies, elutriation, or lectins (Table3). In-depth comparisons of other outcomes according to specific T-cell depletion technique were not performed. For subsequent comparison with non-T-cell–depleted transplants, T-cell depletion techniques were partitioned into narrow-specificity antibodies and other T-cell depletion techniques.
Comparison of T-cell-depletion methods in multivariate analysis of treatment failure3-150
| . | Relative risk (95% CI) . | P . | Adjusted 5-y LFS3-151 (95% CI) . |
|---|---|---|---|
| Narrow-specificity antibodies | 1.00 | 3-152 | 25% ± 5% |
| Broad-specificity antibodies | 1.37 (1.04-1.81)3-153 | .03 | 13% ± 10% |
| Campath antibodies | 1.58 (1.26-1.99)3-153 | .0001 | 12% ± 6% |
| Elutriation | 1.65 (1.23-2.19)3-153 | .0007 | 17% ± 10% |
| Lectins | 1.47 (1.18-1.84)3-153 | .0007 | 15% ± 7% |
| . | Relative risk (95% CI) . | P . | Adjusted 5-y LFS3-151 (95% CI) . |
|---|---|---|---|
| Narrow-specificity antibodies | 1.00 | 3-152 | 25% ± 5% |
| Broad-specificity antibodies | 1.37 (1.04-1.81)3-153 | .03 | 13% ± 10% |
| Campath antibodies | 1.58 (1.26-1.99)3-153 | .0001 | 12% ± 6% |
| Elutriation | 1.65 (1.23-2.19)3-153 | .0007 | 17% ± 10% |
| Lectins | 1.47 (1.18-1.84)3-153 | .0007 | 15% ± 7% |
Other significant covariates were pretransplant disease state, patient age, performance score, donor-recipient relationship and HLA histocompatibility, year of transplantation; intensity of conditioning regimen and posttransplant immune suppression were not significantly associated with treatment failure. There was no significant interaction between prognostic factors.
Five-year probability of leukemia-free survival estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Reference group.
These groups are not significantly different from each other.
Patient, disease, and transplant characteristics of patients receiving T-cell–depleted and non-T-cell–depleted transplants were compared using the χ2 statistic for categorical variables and the Mann–Whitney U test for continuous variables (Table 2).
Univariate (unadjusted) probabilities of outcomes were calculated using the Kaplan–Meier estimator, and 95% confidence limits (CI) were calculated using the standard error of the survivor function by Greenwood formula. Adjusted probabilities of survival were calculated using the multivariate Cox models described below, stratified on T-cell depletion and weighted by the sample proportion value for each prognostic factor; 95% CI for adjusted survival probabilities andP values of pairwise comparisons were derived from pointwise estimates and were calculated using standard techniques.39These adjusted probabilities estimate the likelihood of outcomes in populations with similar prognostic factors.
Comparisons of the effects of T-cell depletion by narrow-specificity antibodies, T-cell depletion by other techniques, and non-T-cell–depleted transplants on graft failure, acute GVHD, chronic GVHD, relapse, survival, and LFS were made using Cox regression model building procedures as described above. The proportionality assumption did not hold for T-cell depletion effects in models of relapse and LFS, indicating that the effect of T-cell depletion differed in various posttransplant time intervals. To determine periods in which the relative risks of treatment failure and death between T-cell–depleted and T-cell–nondepleted transplants were constant, a series of Cox models with different cut-off points for time-dependent T-cell depletion effects were fit. The final model chosen was the one giving the largest partial likelihood.
Results
Patient, disease, and transplant characteristics of the study population are shown in Table 2. There were statistically significant differences between recipients of T-cell–depleted and non-T-cell–depleted transplants in distributions of patient age, pretransplant performance score, leukemia type and stage, year of transplantation, intensity of pretransplant conditioning, donor–recipient relationship, and HLA histocompatibility. Patients receiving T-cell–depleted transplants were more likely to have the favorable characteristics of younger age, performance score at least 90%, and negative donor and recipient CMV serology. However, T-cell–depleted transplant recipients also were more likely to have advanced leukemia and greater donor–recipient HLA disparity. There were relatively few 3-antigen mismatched-related donor transplants in the study population (n = 110, 6%), but most (n = 98) received T-cell–depleted transplants. Recipients of T-cell–depleted transplants more frequently received high-intensity conditioning regimens.
Table 3 shows results of the multivariate regression model of treatment failure comparing the 5 techniques of T-cell depletion. T-cell depletion using narrow-specificity antibodies was used as baseline and was assigned a relative risk of treatment failure (inverse of LFS) of 1.00. Treatment failure risks for the other 4 techniques were significantly higher (RR, 1.37 to 1.65) but were comparable to each other. Adjusted 5-year probabilities of LFS are shown in Table 3and Figure 1. Other significant covariates were pretransplant leukemia state, recipient age, pretransplant Karnofsky performance score and donor–recipient relationship and histocompatibility. Neither intensity of pretransplant conditioning nor posttransplant immune suppression were significantly associated with treatment failure and so were not included in the final models. For subsequent comparison with non-T-cell–depleted transplants, T-cell depletion techniques were therefore considered in 2 groups: those depleted using narrow-specificity antibodies and those depleted with other techniques.
Adjusted 5-year probabilities of LFS by T-cell–depletion technique.
Adjusted probability of leukemia-free survival by T-cell–depletion technique, estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Adjusted 5-year probabilities of LFS by T-cell–depletion technique.
Adjusted probability of leukemia-free survival by T-cell–depletion technique, estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Table 4 shows results of the multivariate analysis of graft failure, grades 2 to 4 acute and chronic GVHD, TRM, relapse, and LFS in recipients of T-cell–depleted transplants compared to recipients of non-T-cell–depleted transplants adjusted for relevant prognostic variables. Analyses of survival gave results almost identical to those of LFS and are not shown. Graft failure risk was increased in recipients of T-cell–depleted transplants, less so in those depleted with narrow-specificity techniques than in those depleted using other techniques. Both T-cell depletion groups had lower risks for grades 2 to 4 acute GVHD compared to recipients of non-T-cell–depleted transplants. Risks for grades 3 and 4 acute GVHD were also decreased. Unadjusted 100-day probabilities of grades 3 and 4 acute GVHD were 35% ± 4% after non-T- cell–depleted transplantation, 19% ± 4% after T-cell–depleted transplantation using narrow-specificity antibodies (P < .0001 forcomparison with non-T-cell–depleted transplants), and 21% ± 5% after other T-cell–depleted transplants (P < .0001 for comparison with non-T-cell–depleted transplants; P = NS for comparison with T-cell–depleted transplants using narrow-specificity antibodies).
Multivariate analysis comparing graft failure, acute and chronic GVHD, relapse, and treatment failure after T-cell-depleted versus non-T-cell-depleted alternative donor transplants4-150
| . | Relative risk4-150 (95% CI) . | P . | Adjusted probability4-151 . | |
|---|---|---|---|---|
| (95% CI) . | P‡ (pointwise) . | |||
| Graft failure (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 6 ± 2% | |
| 2. Narrow specificity antibody | 1.65 (1.01-2.71) | .05 | 10 ± 4% | 1 vs 2: .04 |
| 3. Other T-depleted | 3.37 (2.26-5.02) | .0001 | 19 ± 5% | 1 vs 3: .0001 |
| Grades 2-4 acute GVHD (100 days) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 57 ± 3% | |
| 2. Narrow specificity antibody | 0.57 (0.47-0.68) | .0001 | 38 ± 5% | 1 vs 2: .0001 |
| 3. Other T-depleted | 0.50 (0.41-0.61) | .0001 | 34 ± 5% | 1 vs 3: .0001 |
| Chronic GVHD (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 52 ± 5% | |
| 2. Narrow specificity antibody | 1.50 (1.20-1.88) | .0003 | 61 ± 7% | 1 vs 2: .05 |
| 3. Other T-depleted | 0.86 (0.64-1.14) | NS | 47 ± 10% | 1 vs 3: NS |
| Transplant related mortality (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | 53 ± 4% | — | |
| 2. Narrow specificity antibody | 1.18 (.99-1.39) | NS | 64 ± 6% | 1 vs 2 .003 |
| 3. Other T-depleted | 1.60 (1.36-1.88) | .0001 | 71 ± 6% | 1 vs 3 <.0001 |
| Relapse (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 31 ± 5% | — |
| 2. Narrow specificity antibody | ||||
| < 2 months4-155 | 0.53 (0.31-0.92) | .02 | ||
| ≥ 2 months | 1.06 (0.77-1.45) | NS | 28 ± 7% | 1 vs 2: NS |
| 3. Other T-depleted | ||||
| < 2 months4-155 | 0.68 (0.40-1.15) | NS | ||
| ≥ 2 months | 1.87 (1.38-2.54) | .0001 | 51 ± 11% | 1 vs 3: .001 |
| Treatment failure (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | 31 ± 4%4-154 | ||
| 2. Narrow specificity antibody | 1.01 (0.87-1.16) | NS | 29 ± 5%4-154 | 1 vs 2: NS |
| 3. Other T-depleted | ||||
| < 2 months4-155 | 1.15 (0.94-1.42) | NS | ||
| ≥ 2 months | 1.79 (1.50-2.12) | .0001 | 16 ± 4%4-154 | 1 vs 3: .0001 |
| . | Relative risk4-150 (95% CI) . | P . | Adjusted probability4-151 . | |
|---|---|---|---|---|
| (95% CI) . | P‡ (pointwise) . | |||
| Graft failure (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 6 ± 2% | |
| 2. Narrow specificity antibody | 1.65 (1.01-2.71) | .05 | 10 ± 4% | 1 vs 2: .04 |
| 3. Other T-depleted | 3.37 (2.26-5.02) | .0001 | 19 ± 5% | 1 vs 3: .0001 |
| Grades 2-4 acute GVHD (100 days) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 57 ± 3% | |
| 2. Narrow specificity antibody | 0.57 (0.47-0.68) | .0001 | 38 ± 5% | 1 vs 2: .0001 |
| 3. Other T-depleted | 0.50 (0.41-0.61) | .0001 | 34 ± 5% | 1 vs 3: .0001 |
| Chronic GVHD (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 52 ± 5% | |
| 2. Narrow specificity antibody | 1.50 (1.20-1.88) | .0003 | 61 ± 7% | 1 vs 2: .05 |
| 3. Other T-depleted | 0.86 (0.64-1.14) | NS | 47 ± 10% | 1 vs 3: NS |
| Transplant related mortality (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | 53 ± 4% | — | |
| 2. Narrow specificity antibody | 1.18 (.99-1.39) | NS | 64 ± 6% | 1 vs 2 .003 |
| 3. Other T-depleted | 1.60 (1.36-1.88) | .0001 | 71 ± 6% | 1 vs 3 <.0001 |
| Relapse (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | — | 31 ± 5% | — |
| 2. Narrow specificity antibody | ||||
| < 2 months4-155 | 0.53 (0.31-0.92) | .02 | ||
| ≥ 2 months | 1.06 (0.77-1.45) | NS | 28 ± 7% | 1 vs 2: NS |
| 3. Other T-depleted | ||||
| < 2 months4-155 | 0.68 (0.40-1.15) | NS | ||
| ≥ 2 months | 1.87 (1.38-2.54) | .0001 | 51 ± 11% | 1 vs 3: .001 |
| Treatment failure (5 y) | ||||
| 1. Non-T-depleted | 1.004-153 | 31 ± 4%4-154 | ||
| 2. Narrow specificity antibody | 1.01 (0.87-1.16) | NS | 29 ± 5%4-154 | 1 vs 2: NS |
| 3. Other T-depleted | ||||
| < 2 months4-155 | 1.15 (0.94-1.42) | NS | ||
| ≥ 2 months | 1.79 (1.50-2.12) | .0001 | 16 ± 4%4-154 | 1 vs 3: .0001 |
GVHD, graft-versus-host disease.
Other significant risk factors for graft failure were disease, donor age, Karnofsky performance score, donor type and degree of HLA histocompatibility, conditioning regimen, drugs given for GVHD prophylaxis, year of transplantation, cell dose.
Other significant risk factors for acute GVHD were disease, donor age, donor type and degree of HLA histocompatibility, year of transplantation.
Other significant risk factors for chronic GVHD were disease, donor age, year of transplantation, GVHD prophylaxis with drugs, Karnofsky performance score, donor type and degree of HLA histocompatibility, prophylactic use of growth factors.
Other significant risk factors for transplant-related mortality were pretransplant disease state, patient age, donor type and degree of HLA histocompatibility, donor recipient cytomegalovirus status, Karnofsky performance score.
Other significant risk factors for relapse were pretransplant disease state, Karnofsky performance score, white blood cell count at diagnosis, donor type and degree of HLA histocompatibility.
Other significant risk factors for LFS were pretransplant disease state, patient age, Karnofsky performance score, donor type and degree of HLA histocompatibility, year of transplantation and white cell count.
Adjusted probabilities estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Significance test based on the 95% CI of the difference in at graft failure, chronic GVHD, relapse and LFS at 5 y, and for acute GVHD at 100 d.
Reference group.
Because of nonproportional hazards in the Cox proportional hazards regression models, indicating different risks of treatment failure for the first 2 months after transplantation and the period thereafter, models were fit with a time-varying covariate for T-cell depletion.
Probability of leukemia-free survival.
Recipients of marrow T-cell depleted by narrow-specificity antibodies had higher risks for chronic GVHD than patients receiving non-T-cell–depleted transplants. Recipients of marrow T-cell depleted by other techniques had similar risks for chronic GVHD as did recipients of non-T-cell–depleted transplants.
Early TRM is often used to assess the impact of multiple transplant-related complications, such as slow or incomplete engraftment, GVHD, and infections. Unadjusted probabilities of 100-day TRM were 33% ± 3% after non-T-cell– depleted transplantation, 35% ± 5% after T-cell–depleted transplantation using narrow-specificity antibodies (P = NS), and 48% ± 5% after other T-cell–depleted transplantation (P < .0001 for comparison with non-T-cell–depleted transplantation;P < .0007 for comparison with transplants T-cell depleted with narrow-specificity antibodies). TRM was similar in recipients of T-cell–depleted transplants using narrow-specificity antibodies compared with non-T-cell–depleted transplants (adjusted RR, 1.18; 95% CI, 0.99 to 1.39; P = NS) but higher in recipients of marrow T-cell depleted by other methods (adjusted RR, 1.60; 95% CI, 1.36 to 1.88; P < .0001).
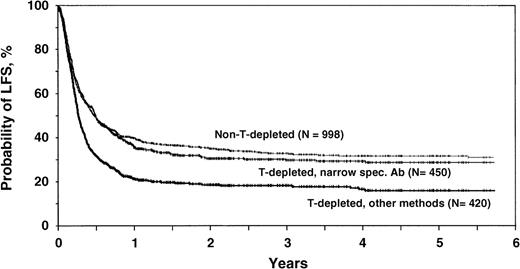
The effect of T-cell depletion on relapse and LFS was time dependent. Effects differed in the first 2 months after transplantation and thereafter. Outcomes are therefore best compared using adjusted probabilities of relapse and LFS. After adjustment for significant covariates, 5-year probabilities (95% CI) of relapse were 31% ± 5% for recipients of non-T-cell–depleted transplants, 28% ± 7% (P = NS) for recipients of transplants T-cell depleted by narrow-specificity antibodies, and 51% ± 11% (P < .001) for recipients of transplants T-cell depleted by other techniques. Adjusted 5-year probabilities of LFS were 31% ± 4% for recipients of non-T-cell–depleted transplants, 29% ± 5% (P = NS) for recipients of transplants T-cell depleted by narrow-specificity antibodies, and 16% ± 4% (P < .0001) for recipients of transplants T-cell depleted by other techniques (Figure2).
Adjusted 5-year probabilities of LFS after T-cell depleted and non-T-cell–depleted transplants.
Adjusted probabilities of LFS for patients receiving non-T-cell–depleted transplants, transplants T-cell depleted by narrow-specificity antibodies, or transplants T-cell depleted by other techniques, estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Adjusted 5-year probabilities of LFS after T-cell depleted and non-T-cell–depleted transplants.
Adjusted probabilities of LFS for patients receiving non-T-cell–depleted transplants, transplants T-cell depleted by narrow-specificity antibodies, or transplants T-cell depleted by other techniques, estimated from the multivariate model assuming a distribution of prognostic factors equal to that in the entire study population.
Table 5 shows univariate outcome probabilities by pretransplant disease state and degree of donor–recipient HLA disparity. Patterns of graft failure and GVHD were similar regardless of donor type. There were no differences in relapse probabilities between recipients of non-T-cell–depleted transplants and recipients of transplants T-cell depleted by narrow-specificity antibodies, regardless of donor type. Relapse rates were higher in recipients of transplants T-cell depleted by other techniques. Probabilities of LFS were similar between non-T-depleted– and narrow-specificity antibody-depleted–transplants and transplants T-cell depleted by other techniques with significantly lower probabilities of LFS, regardless of donor type. There was no significant difference in these results when each leukemia type was considered separately or when related and unrelated transplants were considered separately.
Univariate outcome probabilities by donor type and disease state
| . | 0-1 HLA-antigen mismatched related or HLA-matched unrelated donor . | 2,3-HLA antigen mismatched related or HLA-mismatched unrelated donor . | ||||
|---|---|---|---|---|---|---|
| NonT-depleted . | Narrow Sp antibody . | Other T-depletion technique . | NonT-depleted . | Narrow Sp antibody . | Other T-depletion technique . | |
| Graft failure (5 y) | 7 ± 2% | 5 ± 3% | 22 ± 6% | 7 ± 4% | 16 ± 6% | 28 ± 8% |
| Grades 2-4 acute GVHD (100 d) | 54 ± 4% | 35 ± 7% | 35 ± 6% | 61 ± 8% | 50 ± 7% | 37 ± 9% |
| Chronic GVHD (5 y) | 57 ± 8% | 54 ± 9% | 47 ± 11% | 54 ± 13% | 68 ± 11% | 37 ± 16% |
| TRM (5 y) | 51 ± 4% | 58 ± 8% | 62 ± 7% | 57 ± 10% | 68 ± 8% | 88 ± 9% |
| Relapse (5 y) | ||||||
| 1st CR or 1st CP | 17 ± 6% | 23 ± 14% | 40 ± 15% | 32 ± 20% | 18 ± 16% | 42 ± 38% |
| ≥ 2nd CR or AP | 32 ± 9% | 17 ± 12% | 60 ± 24% | 27 ± 16% | 21 ± 13% | 46 ± 28% |
| Relapse or BP | 74 ± 12% | 74 ± 17% | 49 ± 22% | 47 ± 22% | 35 ± 15% | 67 ± 28% |
| LFS (5 y) | ||||||
| 1st CR or 1st CP | 43 ± 6% | 40 ± 12% | 25 ± 8% | 39 ± 15% | 42 ± 16% | 17 ± 15% |
| ≥2nd CR or AP | 33 ± 7% | 33 ± 11% | 12 ± 9% | 32 ± 13% | 22 ± 10% | 4 ± 7% |
| Relapse or BP | 13 ± 7% | 8 ± 7% | 16 ± 12% | 10 ± 15% | 15 ± 10% | 2 ± 4% |
| . | 0-1 HLA-antigen mismatched related or HLA-matched unrelated donor . | 2,3-HLA antigen mismatched related or HLA-mismatched unrelated donor . | ||||
|---|---|---|---|---|---|---|
| NonT-depleted . | Narrow Sp antibody . | Other T-depletion technique . | NonT-depleted . | Narrow Sp antibody . | Other T-depletion technique . | |
| Graft failure (5 y) | 7 ± 2% | 5 ± 3% | 22 ± 6% | 7 ± 4% | 16 ± 6% | 28 ± 8% |
| Grades 2-4 acute GVHD (100 d) | 54 ± 4% | 35 ± 7% | 35 ± 6% | 61 ± 8% | 50 ± 7% | 37 ± 9% |
| Chronic GVHD (5 y) | 57 ± 8% | 54 ± 9% | 47 ± 11% | 54 ± 13% | 68 ± 11% | 37 ± 16% |
| TRM (5 y) | 51 ± 4% | 58 ± 8% | 62 ± 7% | 57 ± 10% | 68 ± 8% | 88 ± 9% |
| Relapse (5 y) | ||||||
| 1st CR or 1st CP | 17 ± 6% | 23 ± 14% | 40 ± 15% | 32 ± 20% | 18 ± 16% | 42 ± 38% |
| ≥ 2nd CR or AP | 32 ± 9% | 17 ± 12% | 60 ± 24% | 27 ± 16% | 21 ± 13% | 46 ± 28% |
| Relapse or BP | 74 ± 12% | 74 ± 17% | 49 ± 22% | 47 ± 22% | 35 ± 15% | 67 ± 28% |
| LFS (5 y) | ||||||
| 1st CR or 1st CP | 43 ± 6% | 40 ± 12% | 25 ± 8% | 39 ± 15% | 42 ± 16% | 17 ± 15% |
| ≥2nd CR or AP | 33 ± 7% | 33 ± 11% | 12 ± 9% | 32 ± 13% | 22 ± 10% | 4 ± 7% |
| Relapse or BP | 13 ± 7% | 8 ± 7% | 16 ± 12% | 10 ± 15% | 15 ± 10% | 2 ± 4% |
sp, specificity; GVHD, graft-versus-host disease; TRM, transplant-related mortality; CR, complete remission; CP, chronic phase; AP, accelerated phase; BP, blast phase; LFS, leukemia-free survival.
Discussion
Previous studies of transplantation using HLA-identical sibling donors showed that T-cell depletion of donor marrow decreased GVHD but increased graft failure and relapse and did not improve LFS. Recipients of transplants from donors other than HLA-identical siblings have higher risks for GVHD,27,28 leading to the hypothesis that T-cell depletion may be more beneficial in this setting. A number of uncontrolled studies have addressed these issues,40-47 and a randomized multicenter trial of T-cell depletion in unrelated donor transplants is ongoing.
Various T-cell depletion techniques have been studied. Depending on the method, a different spectrum of T, B, NK, and accessory cells is depleted, with varying effects on engraftment and immune reconstitution. We found that recipients of transplants T-cell depleted by narrow-specificity antibodies had lower treatment failure risks than recipients of transplants T-cell depleted by other techniques. The narrow-specificity antibody techniques were defined by their relative specificity for T-cells versus other immune cells, though, depending on the antibody used, small proportions of NK or other cells may also be removed with these methods. Other T-cell–depletion techniques tend to remove larger numbers and a more diverse population of lymphocytes from the graft. Recipients of transplants depleted by narrow-specificity antibodies generally were at lower risk for relapse than were recipients of transplants depleted by other methods (Table 4). Because we lacked data on T-cell dose and phenotype in the grafts, we were unable to address whether this difference resulted from quantitative or qualitative differences, or both, in donor T cells. The absence of significant interactive effects of T-cell–depletion techniques and the intensity of the conditioning regimen or posttransplant immune suppression indicated that estimated effects were independent.
We compared the 870 recipients of T-cell–depleted alternative donor transplants with 998 concurrently treated recipients of non-T-cell–depleted transplants using methotrexate and cyclosporine for GVHD prophylaxis. Recipients of transplants T-cell depleted by narrow-specificity antibodies had slightly more graft failure, less acute GVHD, more chronic GVHD but similar risks for relapse TRM and LFS. Recipients of T-cell–depleted transplants using other techniques had substantially higher risks for graft failure, less acute GVHD, similar risks for chronic GVHD, higher rates of relapse and TRM, and lower LFS than recipietns of non-T-cell–depleted transplants.
It is uncertain why there is an increased risk for chronic GVHD with transplants T-cell depleted by narrow-specificity antibodies. This effect occurred primarily in 2 to 3 antigen-mismatched– related and antigen-mismatched–unrelated transplants. Acute and chronic GVHD are related processes. Conceivably, the decreased reduction of T-lymphocytes delayed but did not prevent the development of GVHD, with manifestations appearing after day 100. Remaining NK cells or retained subclasses of T cells might contribute. Alternatively, this form of transplant might result in dysregulated immune reconstitution and, consequently, more GVHD. This increased chronic GVHD might explain, in part, the lower relapse rate with this versus other methods of T-cell depletion.
This study analyzed patients who underwent transplantation between 1982 and 1994. There was a significant change in outcome of T-cell–depleted and non-T-cell–depleted transplants over time. The risk for treatment failure in patients who underwent transplantation after 1989 was lower than in patients who underwent it from 1982 to 1988, but there was no significant interaction between year of transplantation and T-cell depletion effect. All transplant years were therefore analyzed simultaneously. There were no significant interactions between technique of T-cell depletion and disease. In other words, the relative efficacy of T-cell–depleted and non-T-cell–depleted transplants was the same throughout the study period and for all diseases.
The data analyzed have several limitations. First, recipients of T-cell–depleted transplants differed significantly from recipients of non-T-cell–depleted transplants in some patient, disease, and transplant characteristics. Recipients of T-cell–depleted transplants had more advanced disease and, most important, received grafts from donors with greater HLA disparities (Table 2), putting them at inherently higher risk for adverse outcomes. There were relatively few patients with fully haplo-disparate (3-antigen disparate) grafts in either group, but they were more frequent in the T-cell–depleted cohort. Although we adjusted for these differences in multivariate analysis, we might still have underestimated an effect of T-cell depletion, especially in the more HLA-disparate transplants.
Second, it is likely that techniques of T-cell depletion changed over time in ways not fully described by the categories in Table 1. Intensity of T-cell depletion was less in some recently described approaches.48-53 T-lymphocytes mediate not only GVHD but also engraftment and graft–versus–leukemia, and attempts have been made to engineer marrow to transplant predetermined T-lymphocyte doses or to add T-lymphocytes after transplantation.49,54Preliminary reports describe using high doses of peripheral CD34+ cells extensively depleted of T cells or T-cell–depleted transplants engineered to retain graft-facilitating cells.55-57Consideration of CD34+ cell dose, T-cell dose, and T-cell subset analysis is desirable, but detailed data were unavailable for this study. Approaches producing similar cell products were grouped to provide sufficient power for the analyses. Although comparison of techniques within each group showed no outcome difference, it is possible that we missed a particularly advantageous (or disadvantageous) approach applied in a small number of patients.
Finally, this was a nonrandomized study. Although we carefully considered potential confounding by a large number of variables, it is possible that some of the observed effects were caused by other unmeasured factors.
Transplants using donors other than HLA-identical siblings are increasingly performed. The optimal strategy for alternative donor transplantation is unknown. This study demonstrates that T-cell depletion reduced the risk for acute GVHD, the major cause of morbidity and mortality after transplantation. It is unique in comparing large groups of patients receiving T-cell–depleted with non-T-cell–depleted alternative donor transplants. The effects of T-cell depletion appeared similar regardless of disease, donor–recipient relationship, and donor–recipient HLA match. T-cell depletion using narrow-specificity antibodies resulted in LFS rates similar to those for non-T-cell depleted transplants using methotrexate and cyclosporine for GVHD prophylaxis. LFS rates after transplants that were T-cell depleted using other techniques were lower.
It appears, therefore, from this retrospective study that despite reduced acute GVHD, there was no advantage in LFS or survival rates with T-cell–depleted transplants over unmodified grafts with cyclosporine and methotrexate after transplantation. A prospective randomized study addressing this issue is in progress. Innovations to enhance engraftment and immune reconstitution may improve results of T-cell–depleted transplants and permit effective use of more thoroughly depleted grafts or use of broadly reactive antibodies for depletion. Further studies are warranted to assess novel strategies for graft engineering to enhance the results of allogeneic hematopoietic stem cell transplantation.
Bruno Speck died September 18, 1998.
Supported by Public Health Service grants P01-CA40053 and U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute of the United States Department of Health and Human Services and by grants from Alpha Therapeutic Corporation; Amgen; Anonymous; Basel Cancer League, Switzerland; Baxter Healthcare Corporation; Bayer Corporation; Berlex Laboratories; BioWhitakker; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Company; Cell Therapeutics; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies; Chiron Therapeutics; Ciba-Geigy Jubilaeums Foundation, Switzerland; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Free Academic Society, Basel, Switzerland; Genentech; Glaxo Wellcome Company; Human Genome Sciences; ICN Pharmaceuticals; Immunex Corporation; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg Jr and Helen C. Kleberg Foundation; Herbert H. Kohl Charities; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; NeXstar Pharmaceuticals; Samuel Roberts Noble Foundation; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer; Pharmacia and Upjohn; Principal Mutual Life Insurance Company; RGK Foundation; Rockwell Automation Allen Bradley Company; Roche Laboratories; SangStat Medical Corporation; Schering-Plough Oncology; Searle; SmithKline Beecham Pharmaceutical; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Foundation; Swiss National Fund for Research; SyStemix; United Resource Networks; and Wyeth-Ayerst Laboratories.
Reprints:Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal