Fanconi anemia (FA) is a genetic disorder characterized by bone marrow failure, congenital anomalies, and a predisposition to malignancy. FA cells demonstrate hypersensitivity to DNA cross-linking agents, such as mitomycin C (MMC). Mice with a targeted disruption of the FANCC gene (fancc −/− nullizygous mice) exhibit many of the characteristic features of FA and provide a valuable tool for testing novel therapeutic strategies. We have exploited the inherent hypersensitivity offancc −/− hematopoietic cells to assay for phenotypic correction following transfer of the FANCC complementary DNA (cDNA) into bone marrow cells. Murine fancc −/− bone marrow cells were transduced with the use of retrovirus carrying the humanfancc cDNA and injected into lethally irradiated recipients. Mitomycin C (MMC) dosing, known to induce pancytopenia, was used to challenge the transplanted animals. Phenotypic correction was determined by assessment of peripheral blood counts. Mice that received cells transduced with virus carrying the wild-type gene maintained normal blood counts following MMC administration. All nullizygous control animals receiving MMC exhibited pancytopenia shortly before death. Clonogenic assay and polymerase chain reaction analysis confirmed gene transfer of progenitor cells. These results indicate that selective pressure promotes in vivo enrichment offancc-transduced hematopoietic stem/progenitor cells. In addition, MMC resistance coupled with detection of the transgene in secondary recipients suggests transduction and phenotypic correction of long-term repopulating stem cells.
Fanconi anemia (FA) is a rare autosomal disorder characterized by developmental anomalies, bone marrow failure, and cancer predisposition.1 The hematologic manifestations of FA predominate, with the majority of patients developing aplasia, myelodysplasia, or acute leukemia,2 owing to defective repopulating hematopoietic stem cells. Stem cell reconstitution via histocompatible matched sibling donor bone marrow transplantation3 produces a hematologic cure. The potential of autologous stem cell harvest with subsequent gene transduction offers an attractive alternative to patients lacking a matched sibling donor.
We have used recombinant viral vectors to transfer fancccomplementary DNA (cDNA) to cells from FA patients. Phenotypic correction was demonstrated in vitro by resistance to mitomycin C (MMC), a DNA cross-linker known to induce cell death at nanomolar concentrations. Transduction of CD34+-enriched progenitors significantly improved clonogenic growth in both the absence and the presence of MMC.4 5 On the basis of these results, we hypothesized that gene-corrected FA stem/progenitor marrow cells maintain a selective growth advantage in vivo.
The fancc −/− knockout mouse provides a powerful tool with which to study the mechanism of FA and the potential benefit of novel therapeutic strategies in vivo.6,7 These mice appear hematologically intact; however, upon exposure to such agents as MMC, fancc −/− mice develop pancytopenia as a consequence of marrow aplasia,7,8 recapitulating the phenotype seen in FA patients. Furthermore, when bone marrow cells are assayed for clonogenic capacity7 or the ability to reconstitute themselves, they exhibit significantly reduced growth potential,9 suggesting that hematopoietic failure is caused by a primary defect of stem/progenitor cells. Here, using gene transfer, we demonstrate reconstitution of hematopoiesis infancc nullizygous mice.
Materials and methods
Animals
fancc heterozygous [B6.129Sv-fancc−/−, n = 3] mice were inbred by brother-sister mating [F = 5-6] to obtain homozygous null and wild-type offspring. All animals were maintained in the animal facility at University of North Carolina, Chapel Hill, NC, in accordance with Institutional Animal Care & Use Committee standards in hot-washed, micro-isolator cages supplied with unlimited mouse chow and water. Lethally irradiated mice were housed in autoclaved micro-isolator cages, given sterile food and water, and kept in a pathogen-free room. All mice used in these experiments were between 2 and 6 months of age.
Retroviral vector
The amphotropic, Murine Moloney Leukemia Virus-based, retroviral vector containing human FANCC cDNA was collected from supernatant of a stable producer cell line 52-19 as previously described.4 Briefly, 1 to 2 × 106producer 52-19 cells were plated in 10 cm dishes and grown to 75% confluence. Fresh medium was added, and supernatant containing virus was harvested 24 hours later. Viral supernatant, harvested from producer cell line #17,5carrying the Fanconi anemia group A (fanca) cDNA served as negative control. Mock transduction of wild-type cells served as a positive control.
Retroviral mediated bone marrow transduction
fancc−/− mice were euthanized by CO2asphyxiation, and bone marrow cells were harvested from both femurs and tibias. Red cells were depleted in ACK lysing buffer (Bio-Fluids, Rockville, MD). The bone marrow cells were then subjected to a 48-hour prestimulation period in Dulbecco's Modified Eagle Medium (GibcoBRL, Gaithersburg, MD), 20% fetal bovine serum (HyClone, Logan, UT), and penicillin/streptomycin, which was supplemented with rmIL-3 (25 ng/mL) (R&D Systems, Minneapolis, MN), rhIL-6 (50 ng/mL) (gift of Dr Robert Donahue, National Institutes of Health), and rmSCF (25 ng/mL) (gift from Genetics Institute, Cambridge, MA). The cells were pelleted and resuspended in viral supernatant with protamine sulfate (5 μg/mL) and cytokines for 24 hours. Transduction was repeated for an additional 24 hours before injection of cells into recipient animals.
Bone marrow transplantation
Recipient wild-type female mice received whole body γ-irradiation in a single dose of 10 Gy, by means of a 137Cs source 4 to 6 hours before transplantation.
Anesthetized recipient mice received injection of 1 × 106 transduced bone marrow cells through the retro-orbital plexus. At week 16 postinjection, MMC (CalBioChem, La Jolla, CA) was administered by means of intraperitoneal injection at a dose of 0.3 mg/kg weekly for 6 weeks.
Hematological assay
Peripheral blood (20 to 50 μL) was collected by means of tail venipuncture into microcapillary tubes precoated with ethylenediaminetetraacetic acid. The samples were analyzed at the UNC Animal Clinic Laboratory on an ABC Vet automated blood counter (ABX Hematology Inc, Garden Grove, CA).
DNA isolation and analysis
Peripheral blood (50 to 100 μL) was collected by means of tail venipuncture into heparin-treated microcapillary tubes, and mature red blood cells were depleted by suspension in ACK lysing buffer (Bio-Fluids, Rockville, MD). DNA from nucleated cells was isolated with the use of the QIAamp Blood Kit (Qiagen, Valencia, CA) and the manufacturer's protocol. DNA polymerase chain reaction (PCR) (Perkin-Elmer Cetus, Norwalk, CT) incorporating32P–deoxycytidine triphosphate was performed with the use of 100 ng DNA and primers specific for the vector (upstream primer 5′-ACAGATGGAA-TCGTCTTGGC; downstream primer 5′-CCTGTCTCTTGATCAGATCGG). Amplification conditions were as follows: 95°C for 2 minutes (1 cycle), 95°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes (35 cycles), followed by extension at 72°C for 8 minutes. Samples were electrophoresed on 5% polyacrylamide gels, and autoradiography was performed. Semiquantitative PCR, used to estimate the percentage of transduced cells within the graft, was based on comparison of band intensity, which was performed with the use of DNA isolated from a known vector copy-control (cell line 52-19).
In vitro clonogenic assays
Bone marrow cells were harvested from recipients; red cells were depleted as above; and 2 × 104 cells each were incubated in methylcellulose media (Methocult M3530-Stem Cell Technologies, Vancouver, BC, Canada) in both the presence and absence of 10 nM MMC (CalBioChem). The cultures were incubated with high humidity at 37°C and 5% CO2 for 15 days, and colonies were enumerated.
In vivo repopulation assays
Unfractionated bone marrow cells isolated from primary recipient mice were pooled, and 3 to 4 × 106 cells were injected into secondary lethally irradiated wild-type female mice following the same protocol described above. The secondary recipients received weekly doses of MMC (0.3mg/kg) for 7 weeks.
Statistical analyses
Two-tailed t tests were performed for comparison of mean colony numbers between corrected, wild-type, and null cells. Calculations were performed with statistical analysis software (Prism, GraphPad Software Inc.)
Results
Bone marrow cells were harvested from femurs and tibias of fancc −/− mice, and unfractionated cells were infected under conditions that promote efficient transduction.10,11 Supernatant containing recombinant retrovirus carrying either the human fancc cDNA4 or an irrelevant cDNA5 was used. Wild-type recipients were subjected to gamma-irradiation (10 Gy) and received either 1 × 106 unfractionated marrow cells transduced with rFANCC virus (n = 6), 1 × 106 cells transduced with rFANCA virus (n = 6), 1 × 106 mock infected wild-type (n = 3) cells, or no cells (n = 3). As expected, all animals not receiving cells died within 8 days of irradiation. Control animals receiving cells transduced with an irrelevent vector failed to engraft with the same efficiency as those transduced with fanccvirus, and only 50% survived the 12-week engraftment period. At 13 weeks posttransplant, peripheral blood counts were performed and found to be normal in all remaining mice, indicating that engraftment was satisfactory (Table).
Hematological protection against MMC exposure in FANCC-transduced (−/−) bone marrow
| Parameter . | Untreated (−/−) Mice* . | Recipients of FANCC-Transduced (−/−) Cells† . | Recipients of +/+ Cells‡ . | Secondary Recipients1-153 . | |||
|---|---|---|---|---|---|---|---|
| Before MMC . | After MMC . | Before MMC . | After MMC . | Before MMC . | After MMC . | After MMC . | |
| White cells (103/mm3) | 8.10 ± 0.10 | 4.05 ± 0.45 | 11.98 ± 1.81 | 8.42 ± 1.60 | 11.05 ± 1.05 | 7.97 ± 1.31 | 7.12 ± 0.98 |
| Red cells (106/mm3) | 9.08 ± 0.23 | 4.675 ± 1.18 | 6.64 ± 1.69 | 6.99 ± 1.77 | 6.94 ± 0.18 | 8.17 ± 0.25 | 5.96 ± 0.47 |
| Hemoglobin (g/dL) | 14.80 ± 0.00 | 7.95 ± 1.95 | 13.34 ± 1.00 | 13.72 ± 0.30 | 11.30 ± 0.29 | 13.03 ± 0.09 | 10.42 ± 0.85 |
| Hematocrit (%) | 44.40 ± 0.70 | 22.5 ± 5.70 | 38.34 ± 3.34 | 42.15 ± 0.84 | 30.93 ± 1.04 | 38.13 ± 0.47 | 30.80 ± 2.49 |
| Platelets (103/mm3) | 735.0 ± 45.0 | 289 ± 121.00 | 413.60 ± 42.59 | 661.75 ± 99.82 | 454.00 ± 41.31 | 859.33 ± 134.58 | 729.20 ± 131.65 |
| Parameter . | Untreated (−/−) Mice* . | Recipients of FANCC-Transduced (−/−) Cells† . | Recipients of +/+ Cells‡ . | Secondary Recipients1-153 . | |||
|---|---|---|---|---|---|---|---|
| Before MMC . | After MMC . | Before MMC . | After MMC . | Before MMC . | After MMC . | After MMC . | |
| White cells (103/mm3) | 8.10 ± 0.10 | 4.05 ± 0.45 | 11.98 ± 1.81 | 8.42 ± 1.60 | 11.05 ± 1.05 | 7.97 ± 1.31 | 7.12 ± 0.98 |
| Red cells (106/mm3) | 9.08 ± 0.23 | 4.675 ± 1.18 | 6.64 ± 1.69 | 6.99 ± 1.77 | 6.94 ± 0.18 | 8.17 ± 0.25 | 5.96 ± 0.47 |
| Hemoglobin (g/dL) | 14.80 ± 0.00 | 7.95 ± 1.95 | 13.34 ± 1.00 | 13.72 ± 0.30 | 11.30 ± 0.29 | 13.03 ± 0.09 | 10.42 ± 0.85 |
| Hematocrit (%) | 44.40 ± 0.70 | 22.5 ± 5.70 | 38.34 ± 3.34 | 42.15 ± 0.84 | 30.93 ± 1.04 | 38.13 ± 0.47 | 30.80 ± 2.49 |
| Platelets (103/mm3) | 735.0 ± 45.0 | 289 ± 121.00 | 413.60 ± 42.59 | 661.75 ± 99.82 | 454.00 ± 41.31 | 859.33 ± 134.58 | 729.20 ± 131.65 |
MMC indicates mitomycin C.
Negative control animals were subjected to MMC treatment only.
Transplanted animals were exposed to MMC (0.3 mg/kg for 6 weeks). Peripheral blood was obtained before and after MMC exposure. Results are expressed as the mean ± SE of all experimental animals tested (n = 5). Statistical analysis using the two-tailedt test was performed and demonstrated no significant difference between experimental animals and the wild-type controls. All −/− mice (n = 3) receiving FANCA-transduced cells and MMC died secondary to pancytopenia and marrow aplasia.
Positive control animals received +/+ cells and MMC treatment.
Secondary recipients received bone marrow cells harvested from the primary recipients of FANCC-transduced cells and MMC.
To test whether phenotypic correction of the defective fancc−/− bone marrow cells occurred, we challenged all mice with weekly intraperitoneal doses of MMC (0.3 mg/kg/wk for 6 weeks). This dosing regimen produces pancytopenia and bone marrow aplasia in untreated fancc −/− mice.8 Peripheral blood counts were performed to monitor the effectiveness of MMC. As expected, a marked decrease in all peripheral blood counts from nullizygous untreated mice was observed (Table). Bone marrow histology confirmed severe marrow hypocellularity (less than 5%). The 3 remaining animals that received cells transduced with irrelevant vector (negative control) demonstrated normalized peripheral blood counts before the MMC regimen; all of these animals, however, developed severe pancytopenia following MMC exposure and died. In marked contrast, all mice receiving FANCC-transduced cells demonstrated normal peripheral blood counts after completing the MMC regimen (Table). No evidence of leukemia or solid tumor was observed in any of the animals studied.
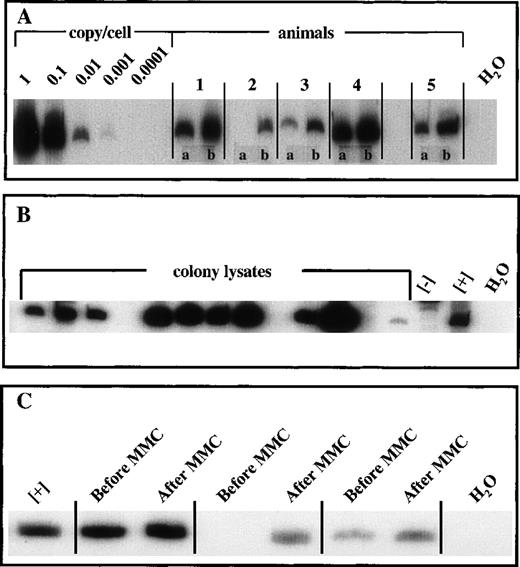
To verify gene transfer, we used DNA isolated from peripheral blood for semiquantitative PCR. Primers were designed to generate a PCR product spanning the vector and the transgene. We detected the transgene using samples from FANCC-transduced mice prior to MMC administration at levels averaging 0.01 vector genome copies/cell. Following MMC exposure, detection of the transgene increased roughly tenfold in all mice, to levels averaging 0.1 genome copies copies/cell (Figure 2A).
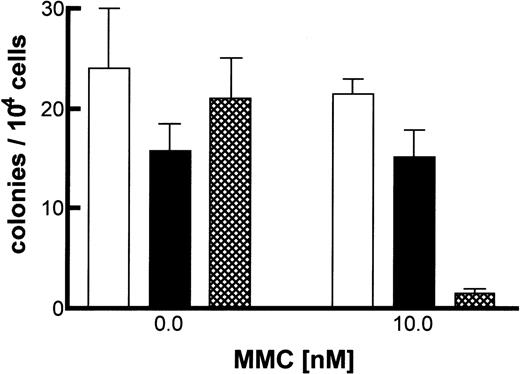
To demonstrate the transduction of primitive progenitor cells, we performed in vitro clonogenic assays. The animals were killed, and their marrow was harvested and plated in methylcellulose in both the absence and the presence of MMC. No significant colony growth of −/−cells is observed at 10 nM MMC, whereas clonogenic growth of +/+ cells is not impaired.12 Animals receiving either FANCC-transduced or wild-type (+/+) bone marrow produced similar clonogenic results at 10 nM MMC, (Figure 1). When wild-type or FANCC-corrected cells are compared with FANCC −/− cells, a significant difference in clonogenic capacity is observed (P = .0002, 2-tailed t test). To determine if phenotypic correction of progenitor cells correlated with vector transduction, PCR of individual colonies was performed. A positive vector-specific PCR product was detected in more than 70% of all colonies (in both the absence and presence of MMC) sampled (Figure 2B). Marrow was harvested from secondary recipients, and clonogenic assays were performed (data not shown). Results were comparable to those shown in Figure 1 for the primary recipients.
Clonogenic assay of FANCC gene-transduced bone marrow.
Marrow was harvested from primary recipients, and 2 × 104 cells were plated in methylcellulose in the presence or absence of 10 nM MMC. Duplicate cultures were established from each animal. Colonies were enumerated at day 15 and total number of colonies shown. (BFU, CFU-GM, and CFU-mix colonies represented 20%, 70%, and 10%, respectively.) Bone marrow from untreated knockout (−/−), +/+, and FANCC-transduced recipients is presented as the mean colony number ± SEM. □, wild-type; ▪, corrected; ▸, null.
Clonogenic assay of FANCC gene-transduced bone marrow.
Marrow was harvested from primary recipients, and 2 × 104 cells were plated in methylcellulose in the presence or absence of 10 nM MMC. Duplicate cultures were established from each animal. Colonies were enumerated at day 15 and total number of colonies shown. (BFU, CFU-GM, and CFU-mix colonies represented 20%, 70%, and 10%, respectively.) Bone marrow from untreated knockout (−/−), +/+, and FANCC-transduced recipients is presented as the mean colony number ± SEM. □, wild-type; ▪, corrected; ▸, null.
Vector-specific PCR.
(A) PCR was performed with the use of DNA isolated from peripheral blood samples taken from primary recipients before and after MMC administration. The standard curve was generated with the use of serial dilution of a known vector-copy cell line, 52-19. (B) Methylcellulose cultures were performed with the use of bone marrow isolated from animals receiving FANCC-transduced cells. Day-15 bone marrow colonies were enumerated and isolated for PCR analysis. (C) PCR was performed with the use of DNA isolated from peripheral blood samples taken from secondary recipients before and after MMC administration. Positive control DNA was derived from cell line 52-19 and negative control DNA was isolated from cell line 17.
Vector-specific PCR.
(A) PCR was performed with the use of DNA isolated from peripheral blood samples taken from primary recipients before and after MMC administration. The standard curve was generated with the use of serial dilution of a known vector-copy cell line, 52-19. (B) Methylcellulose cultures were performed with the use of bone marrow isolated from animals receiving FANCC-transduced cells. Day-15 bone marrow colonies were enumerated and isolated for PCR analysis. (C) PCR was performed with the use of DNA isolated from peripheral blood samples taken from secondary recipients before and after MMC administration. Positive control DNA was derived from cell line 52-19 and negative control DNA was isolated from cell line 17.
To determine if phenotypic correction had occurred at the level of the repopulating stem cell, marrow was harvested from the experimental animals and pooled for transfer to secondary recipients. Lethally irradiated wild-type mice were injected with 3 to 4 × 106 unfractionated bone marrow cells pooled from animals receiving FANCC-transduced cells. Thirteen weeks posttransplant, the secondary recipients received weekly doses of MMC (0.3 mg/kg, intraperitoneally) for 7 weeks. All 5 secondary recipients survived MMC with normal blood counts (Table).
DNA was isolated from peripheral blood samples taken before and after MMC, and vector-specific PCR was performed. Vector-specific PCR from peripheral blood DNA taken prior to MMC dosing detected the presence of the transgene at a level of 0.01 copies/cell or less. However, a tenfold to fiftyfold increase in the PCR signal of all secondary recipient animals was observed following MMC treatment (Figure2C). Normalization of peripheral blood counts (Table) in these secondary recipients is consistent with an expansion of stem cells incorporating the FANCC transgene.
Discussion
FA is a recessive inherited disorder characterized by a progressive pancytopenia due to marrow aplasia.13 Patients are diagnosed within the first decade of life, and without histocompatible allogeneic bone marrow transplant (BMT), they die as young adults. Patients undergoing BMT experience severe complications related to pretransplant conditioning regimens and have an elevated lifetime risk for developing malignancies.1 Alternative treatments for FA patients include autologous BMT using gene-transduced peripheral blood or bone marrow stem/progenitor cells.14Results of 4 Fanconi Anemia Group C patients receiving retroviral vector–transduced peripheral blood CD34+cells demonstrated gene-marked peripheral blood cells and increased clonogenic growth; however, no significant effect on peripheral blood counts were observed, implying that long-term repopulating cells were not transduced. Thus, for gene therapy in FA to be successful, transduction of the rare population of hematopoietic stem cells is required. Since FA constitutes a disorder of stem cell function, it is an excellent model for gene therapy of hematopoietic stem cells.
Several investigators14,15 have demonstrated defects within the hematopoietic stem/progenitor compartment in FA patients. The deficiency of functional FANC proteins in stem/progenitor cells is assumed to be involved in the pathophysiology in the hematologic manifestation of FA. It is experimentally difficult to study hematopoietic stem cell function in FA patients owing to the lack of a quantitative in vivo assay and the reduced numbers of cells available from aplastic donors. The availability of animal models with targeted disruptions of the FANCC gene6 7 provides a powerful tool with which to address basic questions related to stem cell function and an in vivo system to assess the utility of novel therapeutic strategies.
We previously developed an amphotropic retroviral vector carrying the cDNA for human FANCC.4 In this study, we use a murine model of FANCC deficiency7 to determine if our vector would transduce long-term repopulating cells (LTRC) and whether these corrected cells would restore normal hematopoietic function to aplastic animals. Our experimental strategy has taken into account the fact that stem/progenitor cells represent a rare subset of the marrow population16,17 (perhaps even rarer in FANCC null mice) and that stable transduction of murine marrow cells by an amphotropic retroviral vector is relatively inefficient.11,18 These limitations are directly analogous to the situation found in aplastic FA patients, who have fewer cells to target with a vector known to have a relatively low efficiency of transduction for human cells.11 Because of these built-in restrictions, we ensured that transduction of a stem/progenitor cell followed by expression of the transgene would be an exceedingly rare event. We then asked whether the few corrected cells would restore and maintain normal hematopoiesis in aplastic (lethally irradiated) animals. Animals with irradiation-induced marrow failure were used in these experiments in order to more closely mimic the FA patient. We previously reported similar results using nonablated FANCC−/− animals as recipients.19
We believe this is the first report of phenotypic correction of a stem cell disorder using retroviral gene transfer. Gene-corrected LTRCs fromFANCC −/− mice will reconstitute aplastic recipients and maintain normal peripheral blood counts following exposure to MMC. It was significant to note that the dose of MMC chosen (0.3 mg/kg, chronic administration) is sufficient to cause marrow failure without affecting other organ systems.8 In order to authenticate phenotypic correction of progenitor cells, we performed in vitro assays using marrow harvested from the primary recipients and demonstrated improved clonogenic growth. We further demonstrated phenotypic correction of an LTRC population by performing reconstitution assays into secondary recipients. Control animals, which received cells transduced with an irrelevant cDNA, all died, strongly suggesting that the FANCC gene product is required for radio-protection, stable engraftment of transplanted marrow, and maintenance of peripheral blood counts upon exposure to genotoxic agents.
The biochemical function of the FA proteins is currently the subject of debate.20,21 Our data support the hypothesis that FANCC plays a key role in maintenance and expansion of the stem cell population. For these experiments, in vivo administration of a low-dose genotoxic stressor (MMC) enforced expansion and allowed selection of the corrected cells; however, preliminary experiments suggest that gene-corrected FANCC −/− marrow cells are able to compete favorably in this model system without drug selection.19 This hypothesis is supported by the observation of somatic reversion with hematopoietic reconstitution in an FA patient.22 Further studies are needed to confirm this proposition.
This study is relevant for the treatment of FA patients who do not have an HLA-matched donor for bone marrow transplant. Gene therapy shows great promise for sustained and permanent correction of FA; however, the potential of this approach has yet to be demonstrated, owing to limitations in transduction efficiency and inability to confirm successful gene transfer into repopulating stem cells.23This report demonstrates that the efficiency of transduction is not a limiting factor in gene therapy for FA. The presence of a few corrected LTRCs is sufficient to restore normal hematopoiesis to aplastic animals. Gene-correction of stem/progenitor cells will remain a rare event, and therefore both corrected and uncorrected cells will be transferred into the patient. Here we set out to determine if gene-corrected stem/progenitor cells would exhibit preferential growth and expansion in vivo. Repopulation by gene-corrected cells was enhanced by low-dose administration of a genotoxic stressor (MMC). This produced a reduction of the uncorrected mutant cells while simultaneously enforcing expansion of gene-corrected stem/progenitor cells. Normalization of blood counts upon exposure to MMC and enhancement of vector signal detection show that this in vivo selection strategy is able to influence the repopulation kinetics in favor of the few gene-corrected stem cells. The observation that a few gene-corrected stem/progenitor cells are able to restore normal hematopoiesis in a background of defective marrow cells suggests that sustained correction of FA patients is achievable. This study used MMC in order to demonstrate phenotypic correction (maintenance of normal peripheral blood counts) and to apply an exogenous selective pressure. Concerns of toxicity associated with the use of such agents in FA patients24-26 may limit the usefulness of this approach. Whether gene-transduced stem cells from aplastic FA patients will outcompete defective cells without the systemic administration of marrow toxic drug is currently under investigation.
Supported by National Institutes of Health grant #HL048347, Leukemia Society of America grant LSA# 6229-97, and American Cancer Society grant #RPG-98-246-01-LBC.
Reprints:Christopher E. Walsh, UNC Gene Therapy Center, Room 7101, Thurston Building, CB#7352, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: cwalsh@med.unc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal