To the editor:
Horizontal transfer of DNA and the “genometastasis hypothesis”
In a recent issue of Blood, Holmgren et al1 raised the question of whether DNA can be transferred from one cell to another via the phagocytosis of apoptotic bodies. Their data show conclusively that DNA can be rescued and reused from apoptotic bodies by somatic cells. Their results have considerable relevance to the possible role in metastasis of tumor DNA that is circulating in the plasma.2
Circulating tumor DNA in the plasma of cancer patients has been detected by the polymerase chain reaction with primers specific for different mutations in the K-ras oncogene.3However, it remains to be determined whether mutant K-ras DNA in the plasma represents extracellular DNA released from a tumor, DNA released from necrotic or apoptotic cells, or DNA released as a result of the lysis of fragile, circulating cancer cells. Mutant K-rasDNA can be detected in plasma even when it is undetectable in the cellular fraction of blood. This finding suggests that circulating cancer cells might not be responsible for the presence of such DNA.3 Apoptosis or cellular necrosis would be expected to yield detectable amounts of extracellular DNA. However, it is also possible that detectable extracellular DNA might have been shed by viable tumor cells.4 It is commonly assumed that extracellular DNA in the plasma of normal individuals is susceptible to digestion by DNases and it has been proposed, moreover, that the elevated levels of circulating DNA in the plasma of cancer patients might be due to the presence of circulating inhibitors of DNases in these patients.5 Whatever the explanation for the presence of the DNA, it is now clear that oncogenes can circulate in the plasma fraction of the blood. We must now ask whether this phenomenon might have important implications in cancer patients.
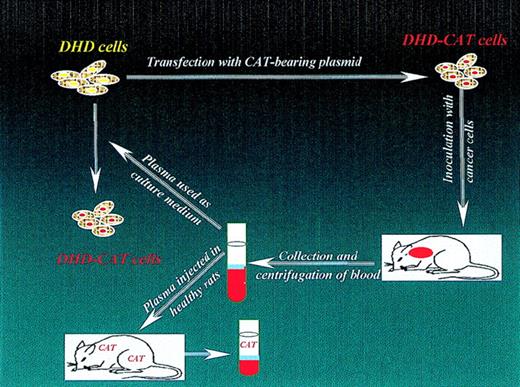
Using a rat model, we have demonstrated the presence of tumor DNA in plasma using cancer cells (DHD/K12-PROb cells; abbreviated as DHD cells) with a genome-associated tag that have been stably transfected with pCDNA3.1CAT (Invitrogen, Groningen, Netherlands). This expression plasmid includes a bacterial gene for chloramphenicol acetyl transferase (CAT), as well as a neomycin-resistance gene.2 6
We demonstrated that native DHD cells (lacking the CAT tag sequences) could be transfected with circulating DNA simply as a result of incubation with plasma from rats that had been rendered cancerous by injection of DHD-CAT cells several weeks previously. We found that non-tagged tumor cells (DHD) became genomically tagged cells (DHD-CAT) when they were cultured for a week in a medium that contained 10% (v/v) plasma from rats with cancer (Figure1).2 Also, when we inoculated healthy rats intraperitoneally with plasma from tumor-bearing rats, the marker gene for CAT was found in extracts of the lungs of all tested animals some weeks later (Figure 1).2 These results are supported in part by those of Pulciani et al,7 who demonstrated the presence of dominant oncogenes in tumor cells as a result of transmission of the malignant phenotype from tumor cells to normal cells via transfection with purified genomic DNA.
Schematic representation of CAT-transfection experiments.
DHD cells were converted to tagged cells (DHD-CAT cells) in two ways: as a result of direct transfection or as a result of culture in medium supplemented with plasma from rats with DHD-CAT cancerous tumors. Reprinted with permission from Histol Histopathol. 1999;14:1089.
Schematic representation of CAT-transfection experiments.
DHD cells were converted to tagged cells (DHD-CAT cells) in two ways: as a result of direct transfection or as a result of culture in medium supplemented with plasma from rats with DHD-CAT cancerous tumors. Reprinted with permission from Histol Histopathol. 1999;14:1089.
Moreover, 1 of the 11 surviving cultures showed neomycin-resistance2; thus, the genome of some of the cultured cells not only had incorporated a neomycin-resistance gene but also had expressed it. Recent experiments have shown that by modifying the protocol used for the addition of geneticin to cultures (adding it just after removing the plasma from medium, instead of waiting for several days), all cultures have expressed the neomycin-resistance gene (unpublished data).
Considering the available experimental and clinical evidence, we suggested the following hypothesis. Metastasis might occur via transfection of susceptible cells located in distant target organs with dominant oncogenes that are derived from the primary tumor and are circulating in the plasma. We tentatively proposed the termgenometastasis to describe this putative phenomenon.2
Holmgren et al1 demonstrated that genomic DNA from apoptotic bodies is transferred to the nuclear compartment of phagocytosing cells and that this transferred DNA is stable over time. Their findings might be closely related to our observations and might provide an explanation for the way in which tumor DNA that is circulating in the plasma might be transferred to cultured cells.
It is possible that apoptotic bodies, derived from tumor cells and circulating in the plasma, might be taken up by phagocytosing cells and that this phenomenon might be associated with the dissemination of cancer. The horizontal transfer of DNA might occur between cancer cells and other somatic cells, and such transfer might provide a putative mechanism for genometastasis.
The evidence in support of the “genometastasis hypothesis” is very provocative and suggests that further investigations are warranted.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal