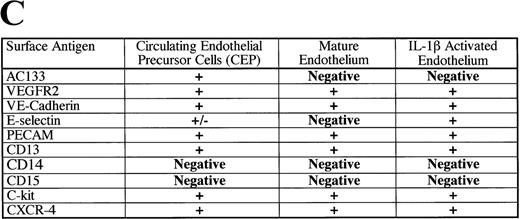
Emerging data suggest that a subset of circulating human CD34+ cells have phenotypic features of endothelial cells. Whether these cells are sloughed mature endothelial cells or functional circulating endothelial precursors (CEPs) is not known. Using monoclonal antibodies (MoAbs) to the extracellular domain of the human vascular endothelial receptor-2 (VEGFR-2), we have shown that 1.2 ± 0.3% of CD34+ cells isolated from fetal liver (FL), 2 ± 0.5% from mobilized peripheral blood, and 1.4 ± 0.5% from cord blood were VEGFR-2+. In addition, most CD34+VEGFR-2+ cells express hematopoietic stem cell marker AC133. Because mature endothelial cells do not express AC133, coexpression of VEGFR-2 and AC133 on CD34+ cells phenotypically identifies a unique population of CEPs. CD34+VEGFR-2+ cells express endothelial-specific markers, including VE-cadherin and E-selectin. Also, virtually all CD34+VEGFR-2+ cells express the chemokine receptor CXCR4 and migrate in response to stromal-derived factor (SDF)-1 or VEGF. To quantitate the plating efficiency of CD34+ cells that give rise to endothelial colonies, CD34+ cells derived from FL were incubated with VEGF and fibroblast growth factor (FGF)-2. Subsequent isolation and plating of nonadherent FL-derived VEGFR-2+ cells with VEGF and FGF-2 resulted in differentiation of AC133+VEGFR-2+ cells into adherent AC133−VEGFR-2+Ac-LDL+(acetylated low-density lipoprotein) colonies (plating efficiency of 3%). In an in vivo human model, we have found that the neo-intima formed on the surface of left ventricular assist devices is colonized with AC133+VEGFR-2+ cells. These data suggest that circulating CD34+ cells expressing VEGFR-2 and AC133 constitute a phenotypically and functionally distinct population of circulating endothelial cells that may play a role in neo-angiogenesis.
Wound healing and tumor growth require active endothelial proliferation, a process referred to as neo-angiogenesis. Neo-angiogenesis involves the recruitment of endothelial cells to the site of injury or to the tumor vascular bed. Two possible sources of endothelialization are (1) endothelial migration and sprouting from preexisting endothelial cells or (2) recruitment of endothelial precursor cells from the circulation. There is ample evidence for the first scenario. However, the existence of circulating endothelial precursor (CEP) cells in adult humans has only recently been suggested and is under intensive scrutiny.
Many studies have demonstrated the presence of mature circulating endothelial cells in the peripheral circulation.1-6 Mature endothelial cells may appear in the circulation randomly by shedding from the vascular wall. Trauma induced by surgery or increased intravascular turbulence may also result in the introduction of endothelial cells to the peripheral circulation. Patients with sickle cell crisis have been shown to have increased numbers of activated circulating endothelial cells.5 Although circulating endothelial cells have been suspected to have the capacity to colonize vascular grafts,1,4,6 7 the contribution of these cells to postnatal angiogenesis or vasculogenesis is not known.
Endothelial precursor cells have properties similar to those of embryonic angioblasts, which can be defined as migratory endothelial cells with the capacity to circulate, proliferate, and differentiate into mature endothelial cells, but which have not yet acquired characteristic mature endothelial markers and have not yet formed a lumen.8-10 Although there is plethora of evidence for the existence of angioblasts during embryonic development, the existence of angioblast-like endothelial precursor cells in adult circulation has been hampered by the absence of specific phenotypic markers and functional assays to define this unique cell population.
Both endothelial precursor cells and mature endothelial cells may express similar endothelial-specific markers, including vascular endothelial growth factor receptor-2 (VEGFR-2),11Tie-1,12,13 Tie-2,14 and vascular endothelial (VE)-cadherin.15 16 Therefore, it may be impractical to use these markers to differentiate between the 2 populations. Identification of the differences between endothelial precursor cells and circulating mature endothelial cells is further complicated by the fact that hematopoietic stem and progenitor cells express markers similar to those of endothelial cells, such as VEGFR-1 (Flt-1), CD34, platelet endothelial cell adhesion molecule (PECAM), Tie-1, Tie-2, and von Willebrand's factor (vWF), and they also have the capacity to incorporate acetylated low-density lipoprotein (Ac-LDL).
One study has shown that CD34+VEGFR-2+ cells can be detected in the peripheral circulation.17 However, in this report a polyclonal antibody to the intracellular domain of VEGFR-2 was used to identify viable Ac-LDL+CD34+ endothelial progenitor cells. This may have resulted in the co-isolation and transplantation of contaminating hematopoietic cells as well as endothelial cells. We have shown that allogeneic sex-mismatched bone marrow transplantation results in the transfer of endothelial cells to recipient dogs.2 Replacement of the aorta of the recipient dogs months after transplantation with impervious Dacron grafts resulted in graft endothelialization arising exclusively from the transplanted bone marrow. In humans, similar evidence for endothelial precursor cells originates from patients implanted with a left ventricular assist device (LVAD). We demonstrated colonization of the flow surface of the titanium housing of LVADs with CD34+endothelial-like cells 6 months after the devices were removed.1 However, none of these studies has conclusively demonstrated the existence of a phenotypically and functionally distinct population of CEPs.
Endothelial precursor cells may share similar characteristics with hematopoietic stem cells. CD34+ hematopoietic stem cells can be detected at low numbers in bone marrow, fetal liver, and umbilical cord blood. Although the number of circulating hematopoietic stem cells in the peripheral circulation is very low, cytokines such as granulocyte colony-stimulating factor (G-CSF) promote mobilization of stem cells from bone marrow to the peripheral blood (PB). In addition, hematopoietic stem and progenitor cells express unique surface markers, such as CD34 and the newly discovered early hematopoietic stem cell marker AC133. Expression of CD34 and AC133 diminish with maturation and differentiation.18 19
AC133 is a novel 120-kd glycosylated polypeptide that contains 5-transmembrane domains with an extracellular N-terminus and a cytoplasmic C-residue.18,19 The function of AC133, which does not share homology with any previously described hematopoietic cell surface antigen, is not known. However, isolation of a subpopulation of CD34+ cells using monoclonal antibody (MoAb) to human AC133 has resulted in the identification of functional CD34+ population of hematopoietic stem cells. Human-derived AC133+ cells can repopulate sheep bone marrow19 and, therefore, can be considered pluripotent hematopoietic stem cells. Expression of AC133 is rapidly downregulated as hematopoietic progenitors and stem cells differentiate into more mature postmitotic cells. In fact, virtually all mature hematopoietic cells, including mature myeloid, megakaryocytes, erythroid, and lymphoid cells and terminally differentiated hematopoietic cells, fail to express AC133.18 19 Therefore, subsets of CD34+ cells that express AC133 are truly a phenotypic and functional marker of an immature population of hematopoietic stem and progenitor cells.
In search of unique endothelial markers to facilitate the isolation and characterization of endothelial precursor cells, we have found that AC133 is expressed on subset of CEPs but not on mature differentiated endothelial cells. To examine the possibility that the endothelial-specific marker VEGFR-2 may be expressed on subsets of circulating AC133+ cells, we have used a combination of high affinity to the extracellular domain of VEGFR-2 to identify CEPs. In this paper, we demonstrate that a small subset of CD34+cells derived from different hematopoietic sources express AC133 and VEGFR-2. In addition, these cells express endothelial-specific antigens, including E-selectin and VE-cadherin. Almost all circulating VEGFR-2+ cells express the chemokine receptor CXCR-4 and migrate in response to the CXCR-4 ligand, stromal-derived factor (SDF-1), as well as VEGF. Incubation of nonadherent CD34+cells coexpressing AC133 and VEGFR-2 cells with VEGF, fibroblast growth factor (FGF)-2, and collagen results in their proliferation and differentiation into adherent AC133−VEGFR-2+ mature endothelial cells. In a relevant in vivo model, we demonstrate that the neo-intimal surface of LVADs is also colonized with large numbers of AC133+VEGFR-2+cells. Taken together, these results suggest that circulating CD34+ cells expressing VEGFR-2 and AC133 comprise a functional population of CEPs cells that may play a role in postnatal angiogenesis or vasculogenesis.
Materials and methods
Flow cytometry studies
CD34+ cells were isolated from cord blood (CB), G-CSF–mobilized PB, and human fetal liver (FL) using standard immunomagnetic techniques (MACS; Miltenyi Biotech, Auburn, CA). Permission for obtaining the samples was obtained from the Institutional Review Board.
For identification of VEGFR-2+ cells, CD34+cells were incubated with 1.5 μL of FITC-labeled high-affinity, nonneutralizing MoAbs to VEGFR-2 (clones 4.13 and 6.64; ImClone Systems, New York, NY) and a phycoerythrin (PE; red fluorescence)-labeled anti-CD34 antibody (Becton Dickinson, San Jose, CA) for 20 minutes. The number of positive cells was compared to immunoglobulin G isotype control (FITC; Immunotech, Marceille, France) and determined using Coulter Elite flow cytometer (COULTER, Hialeah, FL). Nonviable cells identified by propidium iodide staining and, also, contaminating monocytes marked by expression of CD15 and CD14 were excluded.
In addition, different combinations of endothelial (EC)-specific markers (PE-labeled), along with FITC-conjugated MoAb directed to VEGFR-2, were used in dual-color flow cytometry for quantification of CEP phenotype. EC-specific markers included E-selectin (BioSource International, Camarillo, CA, VE-cadherin (BV9 clone; ImClone Systems), and less specific but common markers that are expressed on endothelial cells, including PECAM (Becton Dickinson), vascular cell adhesion molecule (VCAM)-1 (BioSource International, Camarillo, CA), intercellular adhesion molecule (ICAM)-1 (Immunotech), CD13 (Immunotech), and CXCR-4 (clone 12G5; Pharmingen, San Diego, CA), the chemokine receptor for SDF-1.
Migration studies
Migration of CD34+VEGFR-2+ cells was studied by a modified in vitro transmigration assay. An enriched population of CD34+ cells derived from mobilized PB at 105 cells per well (containing 2 × 103CD34+VEGFR-2+ cells) was placed on the upper chamber of a 5-micron–diameter bare Costar transwell plate (Modified Boyden Chamber, Costar Corp, Cambridge, MA). Immediately, 200 ng/mL of SDF-1 (Pepro Tech, Rocky Hill, NJ) were added to the lower chamber; after 3 hours of incubation, the number of CD34+VEGFR-2+ cells migrating to the lower chamber were quantified by 2-color flow cytometry. AC133 staining was done with a PE-conjugated antibody (Miltenyi Biotech) as described above. Human umbilical vein endothelial cells (HUVEC), used as a control for the presence of AC133, were isolated as described previously.22
To test the ability of CD34+VEGFR-2+ cells to migrate in response to VEGF, 105 CD34+ cells were placed in the upper chamber of a 5-micron Costar transwell plate, and immediately VEGF165 (Pepro Tech) 100 ng/mL was added to the lower chamber. CD34+VEGFR-2+ cells migrating to the lower chamber were quantified with 2-color flow cytometry.
Endothelial colony assay
Freshly isolated CD34+ cells were analyzed for the presence of AC133+VEGFR-2+ cells. Subsequently, 106 CD34+ cells were incubated in EC media containing medium 199 (M199; GIBCO-BRL, Gaithersburg, MD) supplemented with 20% fetal bovine serum (HY CLONE, UT), FGF-2 5 ng/mL (human recombinant fibroblast growth factor-basic; SIGMA, St. Louis, MO), and heparin 5 units/mL. After 3 days, nonadherent cells were transferred to collagen-coated (1 μg/mL) plastic dishes and grown in the same media for 2 weeks. This incubation resulted in attachment and proliferation of CD34+ cells expressing VEGFR-2 into monolayers of mature endothelial cells. Cells were stained for endothelial-specific antigens, including vWF, VE-cadherin and, after activation with interleukin (IL)-1β (10 units/mL), for E-selectin expression. AC133 staining of the newly formed endothelium was done using the protocol as described above.
CD34+ cells were purified from human FL (obtained at 14 to 16 weeks of gestation) using Miltenyi isolation technique. After isolation, CD34+ cells were analyzed for the presence of VEGFR-2 and AC133 expression and incubated with the above EC media containing FGF-2 and VEGF for 3 days.
Consequently, 3 × 106 nonadherent cells were incubated with Biotin-labeled anti-VEGFR-2 (1 μg/mL clone 4.13; ImClone Systems) and, after 30 minutes of mixing, washed and reincubated with 160 μg of Strepavidin Dynabeads M-280 (DYNAL A.S., Oslo, Norway) for 45 minutes on bidirectional mixer at 4°C. Rosetted cells were isolated using magnetic DYNAL MPC and, after removal of the supernatant, the cells were washed and incubated with EC media for 18 hours to allow for detachment of the beads. A total of 100 cells released from the beads were plated on collagen-coated dishes in EC-specific media using different dilutions: 1:2, 1:10, 1:20, 1:50, 1:100, and 1:200. After 10 to 14 days, the number of EC colonies were quantified and analyzed using Dil-Ac-LDL incorporation. Dil-Ac-LDL (1 μg/mL, Human Dil Acetyl-LDL; PerImmune, Inc, Rockville, MD) was added to the media for 4 hours, and EC-specific metabolic labeling was assessed by visualization under Nikon-Diaphot microscope with mercury lamp 20 × phase objective.
Identification of VEGFR-2+ cells from LVAD neo-intima
Mononuclear cells from LVAD neo-intimal surfaces were obtained as previously described.1 Briefly, after explantation of LVADs, the neo-intima covering the textured surface was removed, washed, and digested with 0.1% collagenase. Mononuclear cells were recovered and washed, and the number of CD34+VEGFR-2+ cells and AC133+VEGFR-2+ cells was determined by dual-color flow cytometry. A total of 6 LVADs were processed and extracted cells analyzed for the presence of AC133+VEGFR+ cells. Depending on the clinical circumstances, the LVADs were explanted at different times, ranging from 28 days to 6 months.
Statistical analysis
Data are expressed as mean ± SEM of at least 3 independent experiments. To detect differences between migrating and nonmigrating cells, the t test for paired samples was applied. A Pvalue of <.05 was considered statistically significant.
Results
MoAbs to the VEGFR-2 identify a small subpopulation of circulating CD34+ endothelial cells
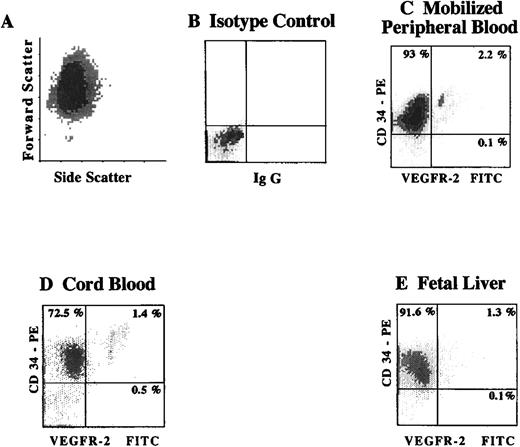
To quantify the number of CD34+VEGFR-2+cells within different hematopoietic sites, CD34+ cells isolated from cytokine-mobilized PB, CB, and FL were analyzed for the presence of CD34+ VEGFR-2+ cells by 2-color flow cytometry. Using FITC-labeled MoAbs to the extracellular domain of VEGFR-2, we found that 2.0 ± 0.5% (n = 5) of CD34+cells derived from G-CSF mobilized PB, and 1.4 ± 0.5% (n = 3) of CB-derived CD34+ cells, and 1.2 ± 0.3% of CD34+ isolated from FL (n = 3) were VEGFR-2+(Figure 1). Similar analysis of the frequency of CD34+VEGFR-2+cells in nonmobilized PB showed a very small number of these cells (data not shown). These results demonstrate the presence of small but distinctive population of VEGFR-2+ cells within CD34+ hematopoietic cells.
Expression of VEGFR-2 on CD34+ cells from different hematopoietic sources identifies a small subpopulation of circulating endothelial cells.
Viable CD34+ cells, as shown by the typical fluorscence in forward and side scatter (A), were isolated from cytokine-mobilized PB (C), CB (D), and FL (E). Subsequently, the number of CD34+VEGFR-2+ cells was analyzed by 2-color flow cytometry using a combination of FITC-labeled MoAbs to the extracellular domain of VEGFR-2 (clones 4.13 and 6.64) and PE-labeled MoAb directed to CD34. X-axis represents log fluorescence intensity. Percentage of positive cells was compared to isotype control (B).
Expression of VEGFR-2 on CD34+ cells from different hematopoietic sources identifies a small subpopulation of circulating endothelial cells.
Viable CD34+ cells, as shown by the typical fluorscence in forward and side scatter (A), were isolated from cytokine-mobilized PB (C), CB (D), and FL (E). Subsequently, the number of CD34+VEGFR-2+ cells was analyzed by 2-color flow cytometry using a combination of FITC-labeled MoAbs to the extracellular domain of VEGFR-2 (clones 4.13 and 6.64) and PE-labeled MoAb directed to CD34. X-axis represents log fluorescence intensity. Percentage of positive cells was compared to isotype control (B).
AC133 is expressed on CEPs but not on mature endothelial cells.
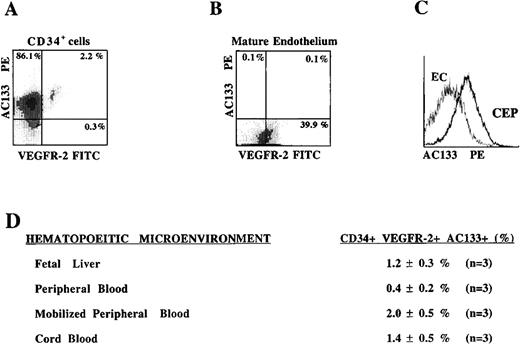
VEGFR-2 is expected to be expressed on both mature circulating endothelial cells and CEPs. Therefore, the CD34+VEGFR-2+ cells identified in Figure 1 may represent a heterogeneous population of mature and immature endothelial cells. In this respect, we have searched for a specific marker that is expressed on CEP but not on mature endothelial cells. We have found that the most CD34+VEGFR-2+ cells express the newly discovered hematopoietic stem cell marker AC133, which is also present on immature hematopoietic cells but is absent on mature endothelial or differentiated hematopoietic cells.
CD34+ cells isolated from different hematopoietic sources were analyzed by 2-color flow cytometry using PE-conjugated MoAb to AC133 and FITC-conjugated MoAb to VEGFR-2. Almost all of the VEGFR-2+ cells derived from mobilized PB that expressed VEGFR-2 also coexpressed AC133 (Figure 2A). However, although mature early-passage HUVECs expressed VEGFR-2, they failed to express AC133 (Figure 2B, 2C). These data suggest that CD34+ cells coexpressing VEGFR-2 and AC133 may represent a phenotypically distinct population of CEPs. The frequency of CD34+ cells expressing VEGFR-2 and AC133 in PB was only 0.4 ± 0.2% of the total CD34+ population (0.002% of total mononuclear cells). However, with G-CSF mobilization there was an increase in the number of AC133+VEGFR-2+ CEPs to 2.0 ± 0.5% of the CD34+ cells (0.02% of total mobilized mononuclear cells). CB- and FL-derived CD34+cells contained 1.4 ± 0.5% and 1.2 ± 0.3% AC133+VEGFR-2+ cells, respectively. The number of AC133−CD34+VEGFR-2+ cells, which most likely represent mature circulating endothelial cells, composed a very small percentage of the circulating population in mobilized blood. These data demonstrate the existence of low levels but persistent numbers of circulating AC133+VEGFR-2+ cells in various hematopoietic sites.
AC133, an early hematopoietic stem cell marker is expressed on VEGFR-2+ CEPs but not on mature endothelial cells.
CD34+ cells derived from mobilized peripheral blood (A) and HUVEC monolayers were analyzed for the expression of AC133. Only the circulating CD34+ VEGFR-2+ cells express AC133, but not mature adherent HUVECs (B, C). CD34+ cells coexpressing VEGFR-2 and AC133 were present in different hematopoietic sources (D), with the highest percentage found in cytokine-mobilized peripheral blood (2 ± 0.5%) and the lowest in unmobilized peripheral blood (0.4 ± 0.2%). X-axis represents log fluorescence intensity.
AC133, an early hematopoietic stem cell marker is expressed on VEGFR-2+ CEPs but not on mature endothelial cells.
CD34+ cells derived from mobilized peripheral blood (A) and HUVEC monolayers were analyzed for the expression of AC133. Only the circulating CD34+ VEGFR-2+ cells express AC133, but not mature adherent HUVECs (B, C). CD34+ cells coexpressing VEGFR-2 and AC133 were present in different hematopoietic sources (D), with the highest percentage found in cytokine-mobilized peripheral blood (2 ± 0.5%) and the lowest in unmobilized peripheral blood (0.4 ± 0.2%). X-axis represents log fluorescence intensity.
Phenotypic characteristics of putative circulating CEPs.
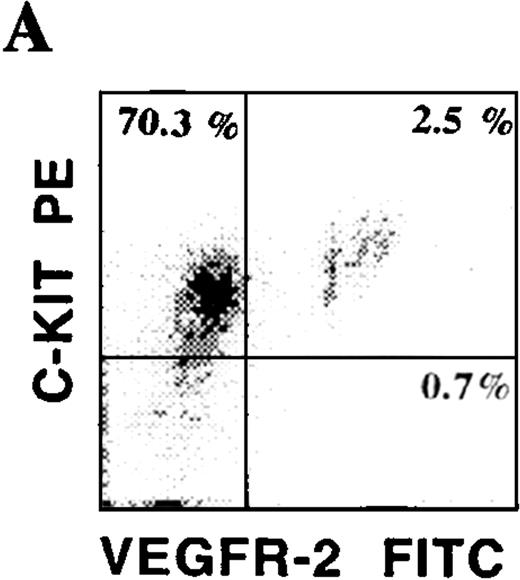
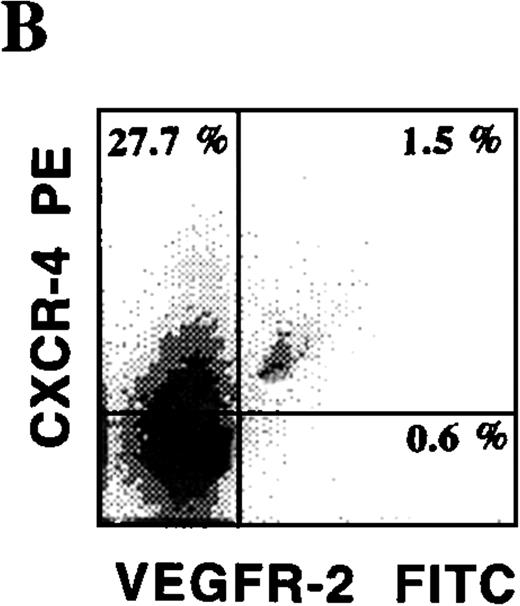
CD34+ cells isolated from various hematopoietic microenvironments were analyzed for the presence of other hematopoietic and endothelial markers. Using dual-color flow cytometry, we showed that human CD34+VEGFR-2+ cells express endothelial-specific markers, including E-selectin and VE-cadherin (Figure 3C). In addition, they also coexpressed common hematopoietic and endothelial markers, including C-kit (Figure 3A), PECAM, and CD13. CD34+VEGFR-2+ cells also expressed CXCR-4, the chemokine receptor for SDF-1 (Figure 3B). Among all of these markers, AC133 was the only specific marker that was expressed on CD34+ VEGFR-2+ cells but was absent on both resting and activated mature HUVECs. VEGFR-2+ cells did not express myelomonocytic markers, including CD15 and CD14.
Phenotypic characterization of circulating endothelial cells and CEPs.
Circulating VEGFR-2− endothelial cells express common hematopoietic and endothelial markers, such as C-kit (A), CD13, and PECAM as well as endothelial-specific markers, including E-selectin and VE-cadherin (C). The chemokine receptor for SDF-1, CXCR-4, is expressed on almost all of the CD34+VEGFR-2+ cells (B). VEGFR-2+ cells do not express myelomonocytic specific markers, including CD15 and CD14.
Phenotypic characterization of circulating endothelial cells and CEPs.
Circulating VEGFR-2− endothelial cells express common hematopoietic and endothelial markers, such as C-kit (A), CD13, and PECAM as well as endothelial-specific markers, including E-selectin and VE-cadherin (C). The chemokine receptor for SDF-1, CXCR-4, is expressed on almost all of the CD34+VEGFR-2+ cells (B). VEGFR-2+ cells do not express myelomonocytic specific markers, including CD15 and CD14.
SDF-1 and VEGF induce migration of CD34+VEGFR-2+cells.
Although mature endothelial cells grow in an anchorage-dependent manner, their capacity to respond to chemotactic factors can be assessed using a Boyden chamber transmigration assay. In these studies, adherent populations of mature endothelial cells are removed by collagenase digestion, and their capacity for migration is examined in collagen-coated Boyden chambers.
A distinctive feature of freshly isolated CD34+VEGFR-2+ cells was their lack of capacity to adhere to extracellular matrix at the time of isolation. Therefore, their capacity to respond to chemotactic factors could readily be examined by a simple transmigration assay designed for nonadherent cells. Among the known chemotactic factors, SDF-1 has been shown to induce migration of CD34+ hematopoietic stem cells. Given that CD34+VEGFR-2+ cells express CXCR-4, we explored the possibility that SDF-1 may also induce migration of nonadherent putative CEPs. In response to SDF-1, 45% ± 2.5% of CD34+VEGFR-2+ migrated to the lower chamber within 3 hours. In addition, an important aspect of angiogenic properties of VEGF is its capacity to induce endothelial cell migration.20 21 VEGF induced migration of 41% ± 3% of CD34+VEGFR-2+ cells added to the upper chamber, compared to 7.1 ± 1.2% migrated cells in control group (Figure 4).
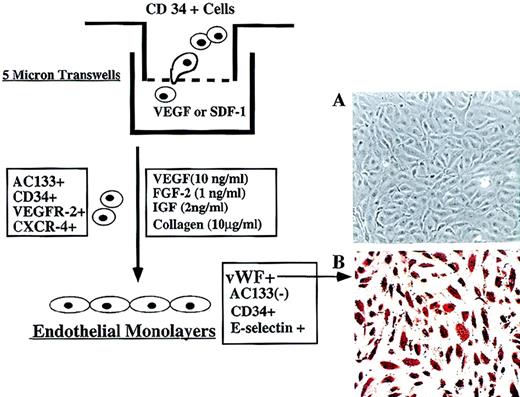
Migration and differentiation of CEPs.
(A) Freshly isolated CD34+ cells at 105 per well containing 2 × 103CD34+VEGFR-2+ cells were placed in the upper chamber of 5-μ Costar transwell plates. Immediately, SDF-1 (200 ng/mL) or VEGF (100 ng/mL) was added in the lower chamber and, after 3 hours, migrated cells were quantified for the presence of CD34+VEGFR-2+ endothelial colonies. As a control, serum and cytokine-free media were used in the lower chamber in a separate experiment. (B) Incubation of migrated CD34+ cells with VEGF and FGF-2 on collagen-coated plastic dishes for 2 weeks resulted in differentiation of CD34+VEGFR-2+ cells into adherent endothelial cell monolayers. Adherent endothelial colonies expressed endothelial-specific markers E-selectin, VE-cadherin, and vWF but did not express AC133 (magnification 200x). E-selectin expression was induced by IL-1β stimulation.
Migration and differentiation of CEPs.
(A) Freshly isolated CD34+ cells at 105 per well containing 2 × 103CD34+VEGFR-2+ cells were placed in the upper chamber of 5-μ Costar transwell plates. Immediately, SDF-1 (200 ng/mL) or VEGF (100 ng/mL) was added in the lower chamber and, after 3 hours, migrated cells were quantified for the presence of CD34+VEGFR-2+ endothelial colonies. As a control, serum and cytokine-free media were used in the lower chamber in a separate experiment. (B) Incubation of migrated CD34+ cells with VEGF and FGF-2 on collagen-coated plastic dishes for 2 weeks resulted in differentiation of CD34+VEGFR-2+ cells into adherent endothelial cell monolayers. Adherent endothelial colonies expressed endothelial-specific markers E-selectin, VE-cadherin, and vWF but did not express AC133 (magnification 200x). E-selectin expression was induced by IL-1β stimulation.
Incubation of freshly isolated, nonadherent CD34+VEGFR-2+ in medium containing VEGF and FGF-2 in collagen-coated plastic dishes for 2 weeks resulted in differentiation of CD34+VEGFR-2+ cells into adherent monolayers of endothelial cells. The newly formed endothelial colonies, which were vWF+ (Figure4A, B) and VE-cadherin+, did not express AC133, thus supporting the notion that circulating AC133+VEGFR-2+cells may be considered endothelial cells with CEP potential.
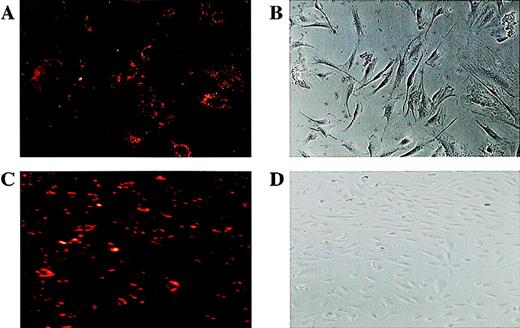
Plating efficiency of CD34+ cells to form endothelial cell colonies.
A highly pure population of CD34+ cells was isolated from human FL cells and maintained in VEGF (10 ng/mL) and FGF-2 (2 ng/mL). VEGFR-2+ cells were isolated using Biotin-labeled anti-VEGFR-2 MoAb and Streptavidin Dynal magnetic beads. Isolated cells were incubated in EC-specific media (containing FGF-2+ and VEGF) in limiting dilutions approximating 1 single cell per well, and after 10-14 days endothelial colonies were quantified using Dil-Ac-LDL labeling. Typical Dil-Ac-LDL± endothelial cells (A) within an adherent endothelial colony (A,B; phase contrast microscopy 350 × ) are shown. Maintaining of the endothelial colonies in the FGF-2 and VEGF resulted in the proliferation of characteristic endothelial cobblestone colonies (D), where most of cells were Dil-Ac-LDL+ (C,D; 200 × ).
Plating efficiency of CD34+ cells to form endothelial cell colonies.
A highly pure population of CD34+ cells was isolated from human FL cells and maintained in VEGF (10 ng/mL) and FGF-2 (2 ng/mL). VEGFR-2+ cells were isolated using Biotin-labeled anti-VEGFR-2 MoAb and Streptavidin Dynal magnetic beads. Isolated cells were incubated in EC-specific media (containing FGF-2+ and VEGF) in limiting dilutions approximating 1 single cell per well, and after 10-14 days endothelial colonies were quantified using Dil-Ac-LDL labeling. Typical Dil-Ac-LDL± endothelial cells (A) within an adherent endothelial colony (A,B; phase contrast microscopy 350 × ) are shown. Maintaining of the endothelial colonies in the FGF-2 and VEGF resulted in the proliferation of characteristic endothelial cobblestone colonies (D), where most of cells were Dil-Ac-LDL+ (C,D; 200 × ).
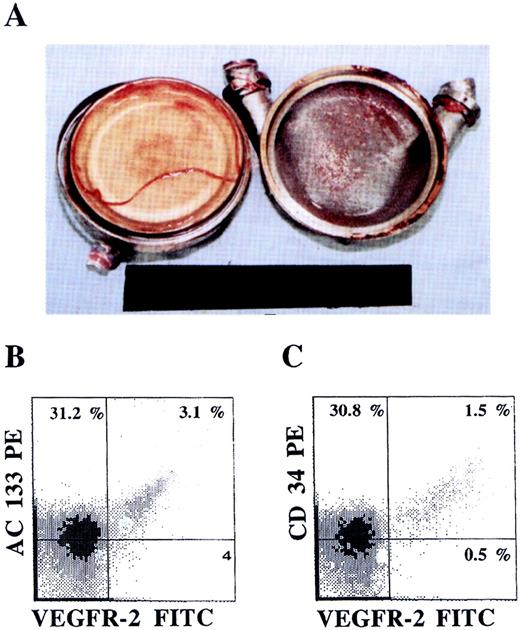
Colonization of LVADs with AC133+VEGFR-2+ cells.
An LVAD opened after explantation (A) 28 days after placement revealed formation of neo-intima on both the sintered titanium housing (right) and the polyurethane diaphragm (left) surfaces. The phenotype of the mononuclear cells derived from the neo-intima formed, analyzed by dual-color flow cytometry, demonstrated the presence of AC133+VEGFR+ (B) as well as CD34+VEGFR-2+ (C) endothelial precursor cells.
Colonization of LVADs with AC133+VEGFR-2+ cells.
An LVAD opened after explantation (A) 28 days after placement revealed formation of neo-intima on both the sintered titanium housing (right) and the polyurethane diaphragm (left) surfaces. The phenotype of the mononuclear cells derived from the neo-intima formed, analyzed by dual-color flow cytometry, demonstrated the presence of AC133+VEGFR+ (B) as well as CD34+VEGFR-2+ (C) endothelial precursor cells.
To quantitate the plating efficiency of VEGFR-2+ cells to form adherent endothelial cell colonies, freshly isolated CD34+ cells from human FL were maintained in VEGF and FGF-2 for 48 hours to remove the adherent mature cells. Subsequently, nonadherent VEGFR-2+ cells were isolated by magnetic beads and plated in limiting dilutions approximating 1 single cell per well. After 10 to 14 days, adherent endothelial cell colonies were identified by Dil-Ac-LDL labeling (Figure 5). The number of endothelial cell colonies varies with the extent of dilution, being the highest in 1:2 dilution, where 24 colonies were detected. On average, of the 500 VEGFR+ cells plated, 15 Dil-Ac-LDL+ colonies could be detected, giving rise to a plating efficiency of 3%. Given that higher dilution resulted in higher numbers of EC colonies, this suggests that accessory cells may facilitate differentiation of VEGFR-2+ cells to endothelial colonies.
LVAD neo-intimal surfaces are colonized with AC133+VEGFR-2+cells.
We have previously shown that surfaces of LVADs in contact with circulating blood are colonized with CD34+ cells with high proliferative capacity, giving rise to a biologically nonthrombogenic neo-intima. Close scrutiny of the LVAD surfaces shows that 4 ± 1% of the mononuclear cells express AC133+ and VEGFR-2+ cells, suggesting that circulating CEPs have the capacity to colonize neo-intimal surfaces (Figure 6). The presence of AC133+VEGFR-2+ cells detected on LVADs was more prominent in formed neo-intima explanted early after operation (28 days).
Discussion
Isolation, identification, and characterization of CEP cells have been hampered by the absence of (1) specific endothelial markers to differentiate between CEPs and contaminating sloughed endothelial cells and hematopoietic progenitor/stem cells and (2) functional assays to differentiate between mature endothelial cells and CEPs. In this report, we have found that AC133, an early hematopoietic stem cell marker, is expressed on a large subset of CEPs but not on the mature endothelium. The percentage of CD34+ cells expressing AC133+ and VEFGR2+ cells is only 2% of circulating CD34+ cells. This figure is much less than the percentage of CD34+ VEGFR-2+ cells previously reported by other groups.17 Using a polyclonal antibody to the intracellular domain of VEGFR-2, another group showed that 27% of CD34+ cells were also VEGFR-2+. However, we have used a specific MoAb to the extracellular domain of VEGFR-2, allowing a more accurate estimation of the circulating CD34+VEGFR-2+ cells. In addition, because AC133 is expressed on putative CEPs, the remaining circulating AC133− but CD34+VEGFR-2+cells may represent a more mature differentiated population of endothelial cells.
We have also demonstrated that CD34+ cells coexpressing AC133 and CD34 are functionally nonadherent endothelial cells that have the capacity to migrate and differentiate into mature adherent endothelial cells. VEGF and FGF-2 are 2 growth factors that have been shown to promote the growth of angioblasts. Incubation of CD34+–derived VEGFR-2+ cells with VEGF and FGF-2 in the presence of collagen results in differentiation of nonadherent AC133+VEGFR-2+ into mature AC133−VEGFR-2+ cells. Adherent, mature endothelial cells grow in a typical cobblestone manner and express endothelial cell markers, including VE-cadherin; they also up-regulate E-selectin upon activation with IL-1β. Formation of mature endothelium from CEPs requires at least a 2-week period, in contrast to the growth pattern of circulating mature endothelial cells, which attach and proliferate immediately after placement in culture medium.
Many studies have shown that endothelial cells can be detected in the peripheral circulation as a result of trauma or pathologic states. However, these studies have not provided data as to what percent of the circulating endothelial cells reported are indeed unique endothelial precursor cells that may contribute to postnatal angiogenesis. Based on our results, cytokine-induced mobilization of CD34+ cells results in the circulation of VEGFR-2+ cells, which have the capacity to migrate and differentiate into adherent endothelial colonies. The percentage of CEPs may be modulated by pathophysiologic processes, such as tumor neoangiogenesis and wound healing. On the other hand, vascular injury due to trauma or increased shear stress may induce nonspecific detachment of mature endothelial cells into circulation.
Vascular implants such as LVADs provide a useful model to study the contribution of CEPs to neo-intima formation. The textured surfaces of these devices are colonized by a nonthrombogenic cellular neo-intima that is formed within the first few weeks of implantation. Detection of CD34+VEGFR-2+ and AC133+VEGFR-2+ cells in the LVAD neo-intima demonstrates that successful angiogenesis in vivo may be achieved by mobilization and recruitment of CEP to the sites of vascular injury such as LVAD surfaces.
Selective homing and recruitment of CEPs into the tumor vascular bed or sites of vascular injury may be mediated through interaction with specific chemokines and adhesion molecules. CXCR-4, the natural receptor for the chemokine SDF-1, is expressed on endothelial cells.23,24 However, although SDF-1 induces calcium fluxes in mature endothelial cells, its exact function in the regulation of angiogenesis remains unknown. CXCR-4 knock-out mice are not viable and have multiple defects, including angiogenesis, suggesting that this receptor may play a role in vascular remodeling and, possibly, trafficking of CEPs.25 26 We demonstrate that almost all nonadherent CD34+VEGFR-2+ cells express functional CXCR-4. SDF-1 induced migration of CD34+VEGFR-2+ cells that, in the presence of VEGF, FGF-2, and collagen, differentiate into adherent endothelial monolayers. These data suggest that SDF-1 released by tumor cells or injured tissue may play a key role in chemoattraction of CEPs. Migration of CEPs in response to VEGF underscores the physiologic role of this factor in supporting various aspects of angiogenesis and also suggest a mechanism for attracting circulating CEPs to the sites of vascular injury or tumor vascular beds.
During embryonic development, angioblasts and primitive hematopoietic stem cells originate from a common stem cell or hemangioblast.27,28 Several studies have shown that hemangioblasts express common endothelial markers, including VEGFR-2.29 Therefore, it is possible that subsets of CD34+VEGFR-2+ cells may have hemangioblast potential and, under appropriate cytokine stimulation, may also have the capacity to differentiate into hematopoietic stem cells.
In summary, our data provide evidence for a distinct population of circulating human CD34+ cells that coexpress AC133 and VEGFR-2 and that have the capacity to migrate and differentiate into adherent mature endothelial cells, supporting the existence of CEPs with the potential to contribute to postnatal angiogenesis. Quantification of these cells in the peripheral circulation may provide useful information regarding the contribution of CEPs in different disease states. Our ability to isolate pure populations of these cells will facilitate further characterization and examination of their potential to contribute to neo-angiogenesis in wound healing and tumor growth.
S.R. supported by the American Heart Association Grant-In-Aid; NHLBI grants R01-HL-58707 and R01-HL-61849; the Dorothy Rodbell Foundation for Sarcoma Research; and the Rich Foundation. M.P. supported by a grant from the National Childhood Cancer Foundation. M.A.S.M. supported by NHLBI grant R01-HL-61401 and the GAR Reichman Fund of the Cancer Center support grant CA-08748.
Reprints:Shahin Rafii, Weill Medical College of Cornell University, Hematology-Oncology Division, 1300 York Ave, Room C-606, New York, NY 10021; e-mail:srafii@mail.med.cornell.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal