Human CD100, the first semaphorin identified in the immune system, is a transmembrane protein involved in T-cell activation. In the present study, we showed that activation of peripheral blood or tonsillar B lymphocytes induced the expression of CD100 in CD38+CD138− cell populations, including in CD148+ subpopulations, thus expressing a memory B-cell–like phenotype. Using an in vitro enzymatic assay, we found that protein tyrosine phosphatase (PTP) activities were immunoprecipitated with CD100 in these cell populations, which were isolated by cell sorting, as well as in most B-cell lines representing various stages of B-cell differentiation. Immunodepletion and Western blotting experiments demonstrated that CD45 was the PTP associated with CD100 in cell lines displaying pre-B, activated B, and pre-plasma cell phenotypes. CD45 also accounted for PTP activity immunoprecipitated with CD100 in CD38+CD138− cells sorted after activation of peripheral blood or tonsillar B lymphocytes. In contrast, no CD100-CD45 association was observed in plasma cell lines corresponding to the terminal B-cell differentiation stage. CD148, the other transmembrane PTP known to be implicated in lymphocyte signaling pathways, was either only partly involved in the CD100-associated PTP activity or not expressed in plasma cell lines, indicating the association of CD100 with another main PTP. Our data show that CD100 is differentially expressed and can functionally associate with distinct PTPs in B cells depending on their activation and maturation state. They also provide evidence for a switch in the CD100-associated PTP at terminal stage of B-cell differentiation.
CD100 was defined in our laboratory as a 150 kDa transmembrane homodimeric protein expressed by various human cells of the immune system, such as thymic T-cell clones, T and B lymphocytes, natural killer (NK) cells, monocytes, and neutrophils, but not by eosinophils or immature hematopoietic precursors.1-3 Evidence for CD100 as a T-cell activation molecule was provided by our studies showing that its triggering with monoclonal antibodies (mAbs) delivers a proliferative signal to T cells in the presence of submitogenic concentrations of anti-CD3 or anti-CD2 antibodies.2,4 Moreover, we demonstrated that CD100 is associated with CD45, a cell surface protein tyrosine phosphatase (PTP) considered a key molecule in the T-cell receptor (TCR) activation process, and that CD100-associated CD45 PTP activity increases upon T-cell activation.5 In addition, CD100 was found to be associated with a serine kinase activity in T and NK cells.6 The molecular characterization of CD100 revealed that it is a member of the semaphorin family.7 Semaphorins are transmembrane and secreted proteins involved in axon guidance during nervous system development.8,9 They are structurally defined by a conserved 500-amino acid extracellular domain with 16 cysteines (sema domain). The human CD100, like its murine homologue M-SemaG,10 belongs to the group of transmembrane semaphorins containing an immunoglobulin-like domain in its extracellular part. The cytoplasmic tail, which has no significant homology with that of other semaphorins, encodes a site for tyrosine phosphorylation and multiple sites for serine/threonine phosphorylation.8 So far, no CD100 ligands have been described. CD100 can be cleaved from the cell surface to release a soluble semaphorin.5 From all of these data, CD100 appeared as the first semaphorin identified in the immune system.7, 11
However, functional or signaling roles of CD100, such as those reported for T cells, were not investigated in other types of leukocytes. The only available data were reported on the effects of CD100 transfectants on B lymphocytes, mimicking a signal delivered by CD100-bearing cells through interaction with a counter-receptor: CD100 induced B lymphocytes to aggregate and improved their viability in vitro.7 In that study, CD100 was found to be expressed in activated B lymphocytes from the germinal center of secondary lymphoid follicules, where B-cell expansion and differentiation occur.12,13 A more recent study on malignant lymph nodes from various B-cell non-Hodgkin lymphomas (NHL) indicated that CD100 was generally not expressed in follicular NHL (only 3 positive cases out of 40), whereas it was detected in 5 out of 5 cases of high-grade, small, noncleaved B-cell NHL.14 Although suggesting that CD100 may have a physiological role in the processes of germinal center formation and B-cell differentiation,7 14 these data also raise the question of a differential expression of CD100 during B-cell maturation. Furthermore, the signaling and functional events triggered by CD100 itself on the surface of B cells remain to be established.
Signal transduction cascades driven by tyrosine phosphorylation are known to regulate important cellular mechanisms, such as proliferation, differentiation, migration, or cell death. The degree of tyrosine phosporylation is tightly controlled by the concerted activities of protein tyrosine kinases and PTPs.15,16 The leukocyte PTP CD45 positively regulates signaling during not only TCR activation, as mentioned above, but also during B-cell antigen receptor (BCR) engagement. CD45 acts by dephosphorylation of specific tyrosine kinases, allowing their initial activation. Until now, CD45 was considered to be the only transmembrane PTP involved in lymphocyte signal transduction.15 Recently, it appeared that the CD148 transmembrane PTP,17,18 corresponding to the previously described PTP HTP-η/DEP-1, is also involved in lymphocyte signal transduction.19 If the association of CD100 with CD45 on T cells is also taken into account, this also may be the case on B cells.
To study the signaling and functional roles of CD100 in B cells, we first quantified the density of CD100 on the surface of peripheral blood B lymphocytes and B-cell lines, which are blocked at different B-cell maturation stages, and we then investigated the association of CD100 with transmembrane PTPs. CD100 was found to be induced by activation of B lymphocytes and to associate with different PTP activities during B-cell maturation stages. CD45 was identified as the CD100-associated PTP in activated B lymphocytes displaying a memory cell–like phenotype and in cell lines with phenotypes of pre-B, activated B, and pre-plasma cells, but not in plasma cell lines corresponding to the terminal differentiation stage. CD148, which was expressed in some but not all plasma cell lines, did not appear to be the main CD100-associated PTP, suggesting the association with a third enzyme at plasma cell stage.
Materials and methods
Fourteen human B-cell lines with phenotypes corresponding to various B-cell differentiation stages were obtained at the American Type Culture Collection (Rockville, MD), unless otherwise stated: Nalm-6 and Reh-6, 2 pre-B–cell lines established from common acute lymphoblastic leukemia; Ramos, Daudi, and Raji, 3 lymphoblastoid cell lines established from Burkitt Lymphoma (BL); DZ, Sanchez (both isolated in our laboratory), and JY, 3 cell lines of activated B lymphocytes obtained by Epstein-Barr virus (EBV)–transformation of normal B lymphocytes; Eskol, a pre-plasma cell line, established from hairy cell leukemia20 provided by Dr E. F. Srour (University of Indiana, Indianapolis, IN); and 5 plasma cell lines established from multiple myeloma: LP-1, producing IgA, provided by Professor J. C. Brouet (Hopital Saint-Louis, Paris, France); U-266, producing IgE λ; RPMI-8226 (λ light chain); and XG-1(IgA κ) and XG-2 (IgG λ), both provided by Dr B. Klein (Unit 291 INSERM, Montpellier, France). XG-1 was established from peripheral blood after secondary plasma cell leukemia, and XG-2 from pleural effusion after relapse.21
Most cell lines were cultured in RPMI-1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, and antibiotics. This medium was supplemented with 50 μmol/L β-mercapto-ethanol and 100 U/mL of interleukin (IL)–6 for XG-1 and XG-2 cell culture. LP-1 cells were grown in Iscove's modified Dulbecco's medium containing FCS and antibiotics. Cells were maintained at 37°C in an incubator containing 5% CO2at densities between 105 and 106cells/mL by 2 to 3 subcultures a week. All experiments were performed by 48 hours after subculture at 2 × 105 cells/mL.
After isolating peripheral blood and tonsillar B lymphocytes, we performed activation and cell sorting. Peripheral blood and tonsil samples were obtained from healthy donors after informed consent according to institutional recommendations on biomedical ethics. Mononuclear cells were isolated by Ficoll-Paque density gradient centrifugation. Peripheral blood mononuclear cells were depleted of T cells by E rosette assays with sheep red blood cells treated with amino-ethyl-thiouronium bromide. Peripheral blood E cells contained E+ cells (fewer than 6% CD2+ cells) and monocytes, as determined by cell surface staining (see below). Tonsillar mononuclear cells were purified by 2 successive E rosette assays and 2 successive depletions of adherent cells. More than 95% of tonsillar nonadherent E− cells were CD19+. Both cell types were cultured in RPMI-1640 medium supplemented with 10% FCS, and activation of B lymphocytes was performed with the use of 300-19 cells transfected with the CD154 complementary DNA in the presence of Staphylococcus aureus(SAC) (1/5000; Pansorbin, Calbiochem, Meudon, France) and 10 ng/ml IL-4.22 After 7 days of activation, cells were harvested, and the different populations were analyzed for size, granulosity, and phenotypic markers with the use of an ELITE cytometer (Coulter, Miami, FL). Cell sorting was then performed according to 3 parameters: size, granulosity, and CD138 negativity. Recovery rate, rather than high purity, was privileged. Under these conditions, sorted cell suspensions contained more than 85% cells of interest. The sorted cells were recultured for 1 hour before all experiments.
BD16 (anti-CD100), BJ45, and O509 (anti-CD45), BB27 (anti-CD101), and O275 (anti-CD2) mAbs were produced in our laboratory as described previously.2,4 Anti-CD148 (143-41 and A3) and anti-CD22 (5T108) mAbs were obtained through exchanges of the Sixth International Workshop on White Cell Differentiation Antigens23; 143-41 mAb was also provided by Dr R. Vilella (Hospital Clinic, Barcelona, Spain). Other mAbs used in this study (CD2, CD3, CD4, CD8, CD14, CD56, CD19, CD38, and CD138) were purchased from commercial sources. Polyclonal anti–SHP-1 antibody was purchased from Santa Cruz Biotechnology (Lake Placid, NY).
The expression of various cell surface molecules was studied either classically by indirect immunofluorescence with specific mAbs and flow cytometry analysis according to techniques previously described,24 or by quantitative determination of antigens with the use of the DAKO QIFIKIT (DAKO A/S, Glostrup, Danemark). The QIFIKIT procedure allows the quantification of antigen density as follows: cell samples were washed with phosphate-buffered saline (PBS) and incubated for 30 minutes at 4°C in the dark with the corresponding mAbs and with negative control-isotype–matched mAb at saturating concentrations. Cells were then washed and incubated for 30 minutes at 4°C in the dark with the F(ab′)2 fragment of fluorescein isothiocyanate–conjugated goat anti-mouse antibody provided by the QIFIKIT. After washings, cell samples were immediately analyzed with a flow cytometer, EPICS (Coulter, Miami, FL). Set-up beads and calibration beads provided by the QIFIKIT were treated in parallel to the cell samples and were used to establish the optimal voltage amplification and draw a calibration curve, respectively. The mean fluorescence intensity was then determined for each population of the calibration beads and for the positive peak of cell samples. From these data, the numbers of primary mAb-binding sites per cell were calculated. They correspond to the mean numbers of accessible antigenic sites per cell and are expressed in sites per cell. For the quantitative determination of antigens expressed by various peripheral blood lymphocyte subsets, experiments were performed on 100 μL of total blood with primary mAbs (specific for each subset) conjugated with different fluorochromes, and the analysis was done as previously described after hemolysis of red blood cells.25 In order to evaluate the density of antigens, cell surface was calculated by measuring cell size with a Coulter counter.
An in vitro PTP-activity assay, based on a colorimetric enzymatic reaction using a specific substrate,5 was performed from cell lysates immunoprecipitated with appropriate mAbs in 96-well microtiter plates (Immunoplate Maxisorb, Nunc, Denmark). For immunoprecipitation, wells were first coated with purified mAbs (10 μg in PBS) overnight, washed extensively, and saturated with 1% bovine serum albumin in PBS for at least 3 hours. Then, 100 μL of cell lysates (5 × 106 cells in all experiments unless otherwise stated) were added to each well for 4-hour incubation. Cell lysis was performed with 1% Brij58 in lysis buffer (20 mmol/L Tris-pH 7.5, 150 mmol/L NaCl, 1 mmol/L paramethyl sulfonyl fluoride (PMSF), 2 μg/mL aprotinin, 2 μg/mL leupeptin). All these steps were carried out at 4°C. After immunoprecipitation, wells were washed 4 times with 20 mmol/L Tris-pH 7.5, 150 mmol/L NaCl, 1 mmol/L PMSF, and 0.1% Brij58. Then, 50 μL of the colorless buffer containing the substrate para-nitrophenyl phosphate (pNPP) was added to each well (1 mg/mL pNPP, 20 mmol/L Tris-pH 7.5, 150 mmol/L NaCl, 20 mmol/L ethylenediaminetetraacetic acid [EDTA], 5 mg/mL di-thiothreitol, and 0.05% Brij58). Microplates were incubated for 40 hours at 37°C with 90% humidity in the dark, and the absorbance was determined at 405 nm.
We performed immunodepletion experiments, as follows: depletion of antigens with corresponding mAbs was performed on cell lysates (5 × 106 cells/100 μL) in all experiments (unless otherwise stated) in 4 cycles (1 to 2 μg of mAb for each cycle). Immunodepletion with an irrelevant-isotype–matched antibody was done simultaneously as a control of depletion. Immunodepletion of SHP-1 was generally used as a control of depletion because SHP-1 is a PTP involved in the regulation of BCR activation through interaction of its SH2 domains with the ITIM motif of activated substrates, receptor FcRγIIB, and B-cell coreceptor CD2215 and because CD100 cannot bind SHP-1, owing to the absence of ITIM motif in its intracellular domain.7 In some experiments, CD101 immunodepletion was also used as a control of depletion because this cell surface antigen26 was not expressed on the B cells studied. All steps of the experiments were carried out at 4°C. Cell lysates, obtained as described above, were incubated with the appropriate antibodies for 40 minutes. Protein A–coupled Sepharose CL-4B beads (Pharmacia, Uppsala, Sweden) were then added for 30 minutes under constant agitation. After centrifugation, supernatants (cell lysates) were subjected to a second cycle of depletion according to the same procedure. The third and fourth cycles were performed with the appropriate antibodies, which were previously adsorbed to protein A–Sepharose beads. After 40 minutes under agitation, the beads were eliminated and cell lysates were incubated with protein A–Sepharose for 30 minutes. Immunodepleted lysates were recovered by centrifugation and assayed for PTP activity.
For Western blot analysis, cells were lysed with 1% Brij58, 50 mmol/L Tris-pH 7.4, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 2 μg/mL aprotinin, and 2 μg/mL leupeptin for 30 minutes at 4°C. Cell lysates were precleared with protein A—Sepharose for 1 hour, then immunoprecipitated for 2 hours with 10 μg of the appropriate mAbs and protein A–Sepharose beads under agitation. After washings, immunoprecipitates were eluted from beads by boiling in Laemmli buffer for 3 minutes. Samples were electrophoresed by sodium dodecyl sulfate 7% polyacrylamide gel electrophoresis (SDS-PAGE), and separated proteins were electrotransferred overnight at 4°C on PVDF membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were saturated with 3% nonfat dried milk in PBS containing 0.2% Tween 20, then incubated for 1 hour with antibodies recognizing the molecule of interest at adequate dilution in blocking conditions. After washings, membranes were incubated with goat anti-mouse horseradish peroxidase complex to reveal the immunoblotted proteins. Membranes were washed again and developed with the use of an enhanced chemoluminescence system (Amersham Pharmacia Biotech, Rainham, UK).
Results
CD100 was previously shown to be expressed on the surface of most hematopoietic cells, including lymphocytes.1-3 The levels of CD100 expression were first quantified in resting peripheral blood B lymphocytes and compared with those of different subsets of T and NK lymphocytes (Table1). While 4000 to 9000 CD100 antigenic sites per cell were found in CD56+, CD8+, or CD4+ subsets, approximately 1000 CD100 sites per cell were detected in CD19+ cells. Therefore, the number of CD100 sites per cell was weak on resting peripheral blood B lymphocytes and much lower than on resting T and NK lymphocytes.
Quantified expression of CD100 on resting peripheral blood lymphocyte subsets from healthy donors
| Donor No. . | Lymphocyte Subset . | |||
|---|---|---|---|---|
| CD19+ . | CD3+CD4+ . | CD3+CD8+ . | CD56+ . | |
| 1 | 1300 | 9300 | 5900 | 6900 |
| 2 | 1100 | 6400 | 6200 | 3900 |
| 3 | 800 | 7600 | 6700 | 5900 |
| Donor No. . | Lymphocyte Subset . | |||
|---|---|---|---|---|
| CD19+ . | CD3+CD4+ . | CD3+CD8+ . | CD56+ . | |
| 1 | 1300 | 9300 | 5900 | 6900 |
| 2 | 1100 | 6400 | 6200 | 3900 |
| 3 | 800 | 7600 | 6700 | 5900 |
CD100 expression was quantified by means of the QIFIKIT procedure with the BD16 mAb and flow cytometry analysis, as described in “Materials and Methods.” Results are expressed as the mean numbers of accessible CD100 antigenic sites per cell.
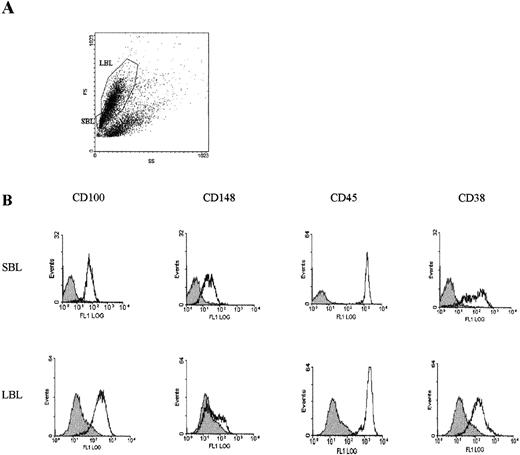
Further, peripheral blood B lymphocytes were activated in vitro with CD154 presented by transfected cells in the presence of SAC and IL-4 for 7 days. Cells were then analyzed for their size, granulosity, and expression of surface markers (Figure 1). Populations of granular cells were observed (Figure 1A). These populations were negative for CD100 and CD38, and in addition, they included dead cells, as shown by propidium iodine staining (data not shown). More interestingly, 2 main populations of nongranular cells were clearly defined: a population of large B lymphocytes (LBL) and a population of small B lymphocytes (SBL). Both LBL and SBL populations were positive for CD100, CD45, and CD38 (Figure 1B), but negative for CD138 (data not shown). In each of these 2 populations, a subpopulation also expressed the CD148 PTP (Figure 1B): a large subpopulation of SBL cells but only a small subpopulation of LBL cells were CD148+. The possibility of a contamination of the LBL population with SBL cells was excluded since CD148 positivity remained unchanged by the use of different gates for analysis of the cytometric profiles shown in Figure 1A.
Induction of CD38+CD138−cell populations by activation of peripheral blood B lymphocytes.
E cells were activated for 7 days with CD154-transfected 300-19 cells in the presence of SAC (1/5000) and 10 ng/mL IL-4. (A) Analysis of cell size and granulosity with the use of a cytometer according to forward scatter/side scatter (FS/SS). We could clearly define 2 populations of nongranular small B lymphocytes (SBL) and large B lymphocytes (LBL); cell populations with high granulosity included cells positive for propidium iodine staining. (B) Expression of surface markers in LBL and SBL populations identified with the use of indirect immunofluorescence with specific mAbs and flow cytometry analysis. Dark histograms represent the fluorescence obtained with negative control isotype–matched mAbs. Top panels: SBL population. Bottom panels: LBL population. All experimental procedures are described in “Materials and Methods.” Both LBL and SBL populations were negative for CD138 (not shown).
Induction of CD38+CD138−cell populations by activation of peripheral blood B lymphocytes.
E cells were activated for 7 days with CD154-transfected 300-19 cells in the presence of SAC (1/5000) and 10 ng/mL IL-4. (A) Analysis of cell size and granulosity with the use of a cytometer according to forward scatter/side scatter (FS/SS). We could clearly define 2 populations of nongranular small B lymphocytes (SBL) and large B lymphocytes (LBL); cell populations with high granulosity included cells positive for propidium iodine staining. (B) Expression of surface markers in LBL and SBL populations identified with the use of indirect immunofluorescence with specific mAbs and flow cytometry analysis. Dark histograms represent the fluorescence obtained with negative control isotype–matched mAbs. Top panels: SBL population. Bottom panels: LBL population. All experimental procedures are described in “Materials and Methods.” Both LBL and SBL populations were negative for CD138 (not shown).
Quantification experiments showed that CD100 was expressed at a high level on LBL cells (more than 10 000 sites/cell) and that several thousand CD148 sites per cell were found in a subpopulation. The number of CD100 (or CD148) sites per cell was lower in SBL cells (Table2). This was due to a difference in CD100 density but not to the different cell size, as evaluated by measuring the cell surfaces (data not shown). These results demonstrated that activation of peripheral blood B lymphocytes induced CD100 expression in cell populations with a CD38+CD138−phenotype, in which subsets of cells coexpressed CD148. Note that no CD100 induction was observed when IL-4 was replaced by IL-10, or in the absence of SAC.
Quantified expression of CD100, CD148, and CD45 in CD38+CD138− SBL and LBL populations of activated peripheral blood B lymphocytes
| . | LBL . | SBL . |
|---|---|---|
| CD100 | 11 600 | 3 600 |
| (4 700-19 600) | (2 200-5 500) | |
| CD148 | 6 800* | 1 700* |
| (1 700-12 000) | (500-2 900) | |
| CD45 | 115 000 | 85 400 |
| (65 000-170 000) | (53 000-120 000) |
| . | LBL . | SBL . |
|---|---|---|
| CD100 | 11 600 | 3 600 |
| (4 700-19 600) | (2 200-5 500) | |
| CD148 | 6 800* | 1 700* |
| (1 700-12 000) | (500-2 900) | |
| CD45 | 115 000 | 85 400 |
| (65 000-170 000) | (53 000-120 000) |
LBL indicates large B lymphocytes; SBL, small B lymphocytes. LBL and SBL populations were defined according to Figure 1. The number of antigenic sites per cell was determined by means of the QIFIKIT procedure and flow cytometry analysis described in “Materials and Methods,” with BD16, 143-41, and O509 mAbs specific for CD100, CD148, and CD45, respectively. Results are expressed as the mean (range) from at least five experiments performed with different donors.
Only one subpopulation expressed CD148.
Finally, activation of purified tonsillar B lymphocytes (97% of CD19+ and 86% CD23+ cells after activation), under the same experimental conditions as for peripheral blood cells, also induced CD100 expression in a population of CD38+CD138− large cells: in 2 independant experiments with different donors that gave the same results, 87% of cells were CD100+, with similar fluorescence intensity as in peripheral blood cells (data not shown).
To confirm that CD100 was differentially expressed during B-cell activation and maturation, CD100 expression was quantified in 14 B-cell lines which are characteristic of various B-cell differentiation stages (Table 3). The number of CD100 antigenic sites per cell was low (2000) in pre-B cell lines and was higher in 2 of 3 BL lymphoblastoid cell lines. The highest levels (more than 10 000 sites/cell) were found in cell lines derived from EBV-transformed normal B lymphocytes, corresponding to the phenotype of activated B lymphocytes, and in a pre-plasma cell line and some plasma cell lines that express more mature phenotypes. Note also that CD45 expression was high in most cell lines, but low or hardly detectable levels in 4 out of 5 plasma cell lines (Table 3). We concluded that CD100 was expressed at different levels on B-cell lines, depending on their activation and differentiation phenotypes.
Quantified expression of CD100, CD45, and CD148 and their associated PTP activities in B-cell lines at various stages of differentiation
| Cell Line . | CD100 . | CD45 . | CD148 . | |||
|---|---|---|---|---|---|---|
| Sites/Cell . | PTP . | Sites/Cell . | PTP . | Sites/Cell . | PTP . | |
| Pre-B | ||||||
| Nalm-6 | 2 000 | nt | 1 500 | nt | nt | nt |
| Reh-6 | 2 300 | 0.09 | 31 000 | 0.51 | nt | nt |
| Lymphoblastoid | ||||||
| Daudi | 7 100 | nt | 160 200 | nt | 2 300 | nt |
| Raji | 1 000 | 0.15 | 24 900 | 1.50 | 0 | nt |
| Ramos | 3 800 | 0.15 | 65 000 | 1.03 | nt | nt |
| EBV-activated | ||||||
| DZ | 21 800 | 0.13 | 41 900 | 0.68 | nt | nt |
| JY | 10 800 | 0.05 | 20 800 | 0.40 | nt | nt |
| Sanchez | 12 500 | NS | 42 500 | 0.30 | 1 000 | 0.20 |
| Pre-plasma | ||||||
| Eskol | 15 000 | 0.12 | 87 000 | 1.90 | 5 200 | 0.38 |
| Plasma | ||||||
| LP-1 | 19 800 | 0.12 | 0 | NS | 0 | nt |
| RPMI-8226 | 4 100 | NS | 3 700 | 0.05 | 0 | NS |
| U-266 | 7 300 | 0.54 | 200 | NS | 4 800 | 0.63 |
| XG-1 | 8 900 | 0.26 | 85 500 | 1.10 | 12 500 | 1.24 |
| XG-2 | 34 300 | NS | 300 | nt | 0 | nt |
| Cell Line . | CD100 . | CD45 . | CD148 . | |||
|---|---|---|---|---|---|---|
| Sites/Cell . | PTP . | Sites/Cell . | PTP . | Sites/Cell . | PTP . | |
| Pre-B | ||||||
| Nalm-6 | 2 000 | nt | 1 500 | nt | nt | nt |
| Reh-6 | 2 300 | 0.09 | 31 000 | 0.51 | nt | nt |
| Lymphoblastoid | ||||||
| Daudi | 7 100 | nt | 160 200 | nt | 2 300 | nt |
| Raji | 1 000 | 0.15 | 24 900 | 1.50 | 0 | nt |
| Ramos | 3 800 | 0.15 | 65 000 | 1.03 | nt | nt |
| EBV-activated | ||||||
| DZ | 21 800 | 0.13 | 41 900 | 0.68 | nt | nt |
| JY | 10 800 | 0.05 | 20 800 | 0.40 | nt | nt |
| Sanchez | 12 500 | NS | 42 500 | 0.30 | 1 000 | 0.20 |
| Pre-plasma | ||||||
| Eskol | 15 000 | 0.12 | 87 000 | 1.90 | 5 200 | 0.38 |
| Plasma | ||||||
| LP-1 | 19 800 | 0.12 | 0 | NS | 0 | nt |
| RPMI-8226 | 4 100 | NS | 3 700 | 0.05 | 0 | NS |
| U-266 | 7 300 | 0.54 | 200 | NS | 4 800 | 0.63 |
| XG-1 | 8 900 | 0.26 | 85 500 | 1.10 | 12 500 | 1.24 |
| XG-2 | 34 300 | NS | 300 | nt | 0 | nt |
PTP indicates protein tyrosine phosphatase. The numbers of antigenic sites per cell were quantified by means of the QIFIKIT procedure and flow cytometry analysis with BD16, O509, and 143-41 mAbs, respectively. All cell lines were negative for CD101 expression, as evaluated with the BB27 mAb. PTP activity was assayed on cell lysates after immunoprecipitation with the specific mAbs BD16, BJ45 (anti-CD45), a mixture (1:1) of 143-41 and A3 (anti-CD148), and BB27. All experimental procedures are described in “Materials and Methods.” PTP activity is expressed as the absorbance at 405 nm. CD101 negative control values ranged from 0.00 to 0.04 (mean = 0.02).
NS, not significant (<.05); nt, not tested.
CD100 was previously reported to be associated with PTP activity in human T cells.5 To test the possibility of such an association in B cells, PTP activity was assayed in CD100 immunoprecipitates obtained from 12 B-cell lines. The enzymatic activity of the transmembrane PTP CD45 was measured simultaneously as a positive control. CD101 immunoprecipitates were also assayed in parallel as negative controls, since the cell surface molecule CD101 was not expressed in these cell lines.26 As shown in Table 3, PTP activity was detected in CD100 immunoprecipitates over CD101 negative control values in most cell lines, with 3 exceptions: 1 BL cell line (Sanchez), and 2 plasma cell lines (RPMI-8226 and XG-2). The levels of PTP activity associated with CD100 depended on each cell line regardless of its differentiation phenotype and did not seem to be related to the number of CD100 sites per cell.
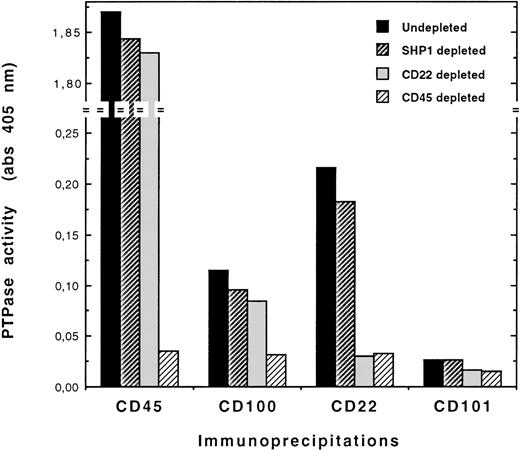
To identify the PTP associated with CD100, we tested the hypothesis of an association with CD45, because most cell lines with phenotypes ranging from pre-B to pre-plasma cell stages expressed high numbers of CD45 sites per cell and displayed high levels of CD45 PTP activity (Table 3). To this end, CD45 immunodepletion experiments were performed on cell lysates before immunoprecipitations and PTP assays. As exemplified with the Eskol cell line in Figure2, CD45 depletions induced nearly a complete loss of PTP activity in CD100 immunoprecipitates and CD45 immunoprecipitates, as compared with the values obtained with negative control depletions (SHP-1; see “Materials and Methods”). As expected, these depletion control values were reduced compared with the basal levels of PTP activity found in both CD100 and CD45 immunoprecipitates from undepleted lysates, probably owing to protein loss and dilution during the 4 cycles of depletion. Because CD22 is a cell surface molecule known to associate with CD45,15 CD22 immunodepletions were also performed in parallel (Figure 2). PTP activity was detected in CD22 immunoprecipitates. As expected, this activity was completely abolished upon CD22 or CD45 depletions, whereas PTP activity measured in CD100 immunoprecipitates was not greatly modified by CD22 depletion. Thus, the data obtained with pre-plasma Eskol cell line clearly showed that (1) PTP activity immunoprecipitated with CD100 resulted from CD100-CD45 association and (2) this association did not seem to involve the CD22 molecule. Similar data (not shown) were obtained with a number of other cell lines, such as Reh-6 (pre-B), DZ (EBV-activated normal lymphocytes), or Ramos (lymphoblastoid). Therefore, CD100 was associated with CD45 PTP activity in most B-cell lines with phenotypes ranging from pre-B to pre-plasma cell stages, including the phenotype of activated B lymphocytes.
CD100-bound PTP activity is due to association of CD100 with CD45 PTP in Eskol cells.
Cell lysates were CD45-immunodepleted through the use of 4 cycles of depletion with O509 mAb. Negative control depletions consisted of SHP-1 immunodepletions (see “Materials and Methods”). Experiments of CD22 immunodepletion were done in parallel. Then, depleted lysates were immunoprecipitated with BJ45 (anti-CD45), BD16 (anti-CD100), or an anti–CD22 mAb, and the immunoprecipitates were assayed for PTP activity. Immunoprecipitates from undepleted lysates were also assayed to determine the basal levels of PTP activity. Negative control values of PTP activity were obtained by using depleted and undepleted lysates that were immunoprecipitated with an anti–CD101 mAb (BB27); this procedure was based on the observation that this cell surface molecule was not expressed on Eskol cells. All procedures are detailed in “Materials and Methods.”
CD100-bound PTP activity is due to association of CD100 with CD45 PTP in Eskol cells.
Cell lysates were CD45-immunodepleted through the use of 4 cycles of depletion with O509 mAb. Negative control depletions consisted of SHP-1 immunodepletions (see “Materials and Methods”). Experiments of CD22 immunodepletion were done in parallel. Then, depleted lysates were immunoprecipitated with BJ45 (anti-CD45), BD16 (anti-CD100), or an anti–CD22 mAb, and the immunoprecipitates were assayed for PTP activity. Immunoprecipitates from undepleted lysates were also assayed to determine the basal levels of PTP activity. Negative control values of PTP activity were obtained by using depleted and undepleted lysates that were immunoprecipitated with an anti–CD101 mAb (BB27); this procedure was based on the observation that this cell surface molecule was not expressed on Eskol cells. All procedures are detailed in “Materials and Methods.”
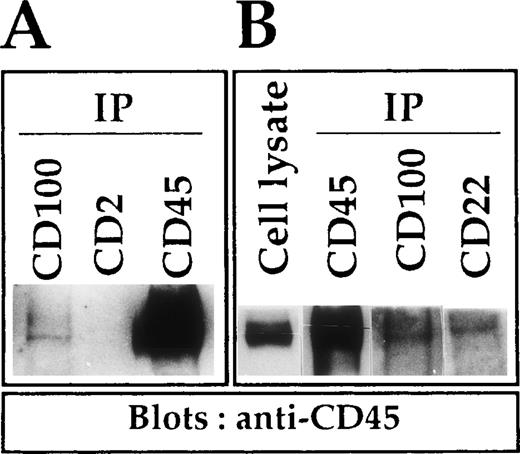
To confirm the physical association between CD100 and CD45 molecules, Western blotting experiments using an anti-CD45 mAb were performed on CD100 immunoprecipitates from cell lines with pre-B to pre-plasma cell phenotype. Results obtained with pre-plasma Eskol cells showed clearly that CD45 coprecipitated with CD100 (Figure3). The intensities of the bands were similar to those detected with CD22 immunoprecipitates, although weaker than the intensities of the bands observed with CD45 immunoprecipitates (positive controls). The different negative controls used (CD2 immunoprecipitates or blotting of CD100 immunoprecipitates with an anti–SHP-1 antibody) remained repeatedly negative, even after prolonged exposure of the blots (Figure 3 and not shown). Similar results were observed with other cell lines, such as Reh-6 (pre-B), Raji (lymphoblastoid), or JY (EBV-activated normal lymphocytes). These results demonstrated the molecular interaction between CD100 and CD45 in cell lines with pre-B to pre-plasma cell phenotypes.
Co-immunoprecipitation of CD45 with CD100 in Eskol cells.
Cells lysates were immunoprecipitated with different mAbs: BD16 (anti-CD100), an anti-CD2 (O275) as a negative control, and an anti-CD22 (a molecule known to associate with CD45) and an anti-CD45 (O509) as positive controls. Immunoprecipitates (IP) and cell lysate were subjected to SDS-PAGE, and proteins were transferred on PDVF membrane and blotted with a mixture of anti-CD45 mAbs (O509 and BJ45). Immunoblotted proteins were revealed with goat anti-mouse horseradish peroxidase complex and a system of enhanced chemoluminescence. For detailed experimental procedures, see “Materials and Methods.” We show 2 distinct Western blots: (A) Western blot exposed for 1 minute. (B) Western blot in which cell lysate and CD45 IP were exposed for 5 seconds while CD100 and CD22 IP were exposed for 30 seconds.
Co-immunoprecipitation of CD45 with CD100 in Eskol cells.
Cells lysates were immunoprecipitated with different mAbs: BD16 (anti-CD100), an anti-CD2 (O275) as a negative control, and an anti-CD22 (a molecule known to associate with CD45) and an anti-CD45 (O509) as positive controls. Immunoprecipitates (IP) and cell lysate were subjected to SDS-PAGE, and proteins were transferred on PDVF membrane and blotted with a mixture of anti-CD45 mAbs (O509 and BJ45). Immunoblotted proteins were revealed with goat anti-mouse horseradish peroxidase complex and a system of enhanced chemoluminescence. For detailed experimental procedures, see “Materials and Methods.” We show 2 distinct Western blots: (A) Western blot exposed for 1 minute. (B) Western blot in which cell lysate and CD45 IP were exposed for 5 seconds while CD100 and CD22 IP were exposed for 30 seconds.
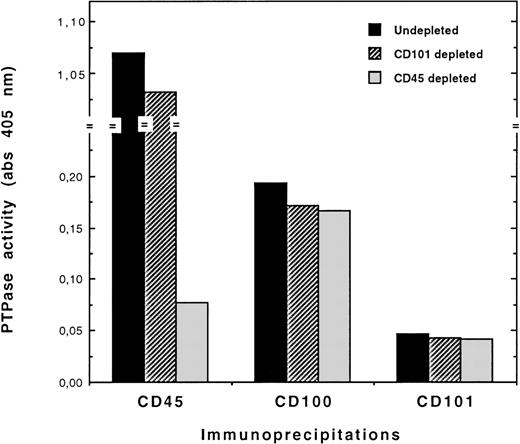
The association of CD100 with CD45 PTP activity was then investigated in the XG-1 plasma cell line that expressed a high number of CD45 sites per cell (Table 3). Results showed clearly that the levels of PTP activity immunoprecipitated with CD100 remained unchanged after CD45 depletion compared with depletion control values (Figure4) and that no CD100-CD45 coprecipitation could be visualized in Western blotting experiments (data not shown). In addition, 2 plasma cell lines (U-266 and LP-1) also displayed CD100-associated PTP activity, despite no or hardly detectable CD45 expression (Table 3). Consequently, CD100 was not associated with the CD45 PTP in plasma cell lines corresponding to the terminal stage of differentiation.
CD100-bound PTP activity is not due to association of CD100 with CD45 PTP in XG-1 cells.
Cell lysates were CD45-immunodepleted before CD100 or CD45 immunoprecipitations and PTP assays, as described in Figure 2 and detailed in “Materials and Methods.” Immunodepletions of CD101 (not expressed on XG-1 cells) with the BB27 mAb were used as negative control depletions. Basal levels of PTP activity were measured in immunoprecipitates from undepleted lysates. CD101 immunoprecipitates from depleted and undepleted lysates were also assayed for PTP activity to determine the negative control values.
CD100-bound PTP activity is not due to association of CD100 with CD45 PTP in XG-1 cells.
Cell lysates were CD45-immunodepleted before CD100 or CD45 immunoprecipitations and PTP assays, as described in Figure 2 and detailed in “Materials and Methods.” Immunodepletions of CD101 (not expressed on XG-1 cells) with the BB27 mAb were used as negative control depletions. Basal levels of PTP activity were measured in immunoprecipitates from undepleted lysates. CD101 immunoprecipitates from depleted and undepleted lysates were also assayed for PTP activity to determine the negative control values.
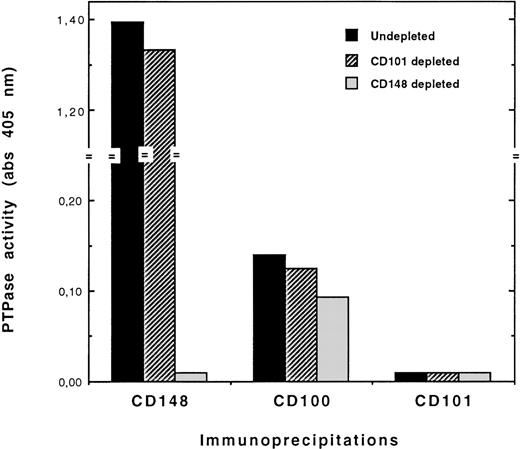
This indicated the association of CD100 with another PTP. The possible association between CD100 and CD148, a recently identified cell surface molecule with PTP activity,17-19 was therefore examined. We first investigated CD148 expression and PTP activity in various B-cell lines. We found that CD148 was expressed in some but not all of the B-cell lines tested and that there was a good relationship between the number of CD148 antigenic sites per cell and the levels of PTP activity immunoprecipitated with CD148 (Table 3). No CD148 expression was detected on LP-1 cells, indicating that CD148 was not the CD100-associated PTP in this cell line. In contrast, elevated numbers of CD148 sites per cell were found on XG-1 and U-266 cells (12 500 and 4800, respectively). We further investigated the effects of CD148 immunodepletion on the PTP activity immunoprecipitated with CD100 in these 2 cell lines. In both cases, CD148 depletion reduced by 20% to 25% the PTP activity measured in CD100 immunoprecipitates, while this activity was completely abolished in CD148 immunoprecipitates, as exemplified for XG-1 cells in Figure 5. Although the effect of CD148 depletion was rather weak, it was reproducibly observed in repeated experiments. Thus, CD148 could participate only partly in the CD100-associated PTP activity in XG-1 and U-266 cells. These data provided evidence for the association of CD100 with at least another major PTP, yet unidentified, in plasma cells.
Effect of CD148 immunodepletion on CD100-associated PTP activity in XG-1 cells.
CD148 immunodepletions with a mixture (1:1) of 2 anti-CD148 mAbs (143-41 and A3) and CD101 immunodepletions (negative control depletions) were performed on cell lysates. Depleted and undepleted lysates were immunoprecipitated with either BD16 (anti-CD100), BB27 (anti-CD101), or a mixture (1:1) of the 2 anti-CD148 mAbs, and the immunoprecipitates were then assayed for PTP activity. All experimental procedures are described in “Materials and Methods.”
Effect of CD148 immunodepletion on CD100-associated PTP activity in XG-1 cells.
CD148 immunodepletions with a mixture (1:1) of 2 anti-CD148 mAbs (143-41 and A3) and CD101 immunodepletions (negative control depletions) were performed on cell lysates. Depleted and undepleted lysates were immunoprecipitated with either BD16 (anti-CD100), BB27 (anti-CD101), or a mixture (1:1) of the 2 anti-CD148 mAbs, and the immunoprecipitates were then assayed for PTP activity. All experimental procedures are described in “Materials and Methods.”
In order to know whether CD100 was also associated with PTP activity in peripheral blood B lymphocytes, resting cells were activated as described above, and the CD100+ positive cells were isolated through cell sorting. Activated B lymphocytes were negatively selected for CD138 and granulosity to avoid triggering of CD100 or CD38. The sorted cells corresponded to CD100+CD38+CD138− cells from LBL and SBL populations. PTP activity was then assayed in CD100, CD148, and CD45 immunoprecipitates. Results of the experiments performed with 9 different donors are shown in Table 4. PTP activity was consistently found at various levels in CD148 and CD45 immunoprecipitates. PTP activity immunoprecipitated with CD100 was detected in 6 out of 9 donors (donors 3 to 8) although at rather low levels, but not in donors 1, 2, and 9.
Phosphatase activities in sorted CD100+CD38+CD138− cells induced by activation of peripheral blood B lymphocytes
| Donor No. . | Immunoprecipitates . | ||
|---|---|---|---|
| CD100 . | CD45 . | CD148 . | |
| 1 | NS | 0.21 | 0.07 |
| 2 | NS | 0.28 | 0.54 |
| 3 | 0.11 | 0.66 | 0.36 |
| 4 | 0.11 | 1.38 | 0.76 |
| 5 | 0.20 | 0.40 | ND |
| 6 | 0.10 | 1.02 | ND |
| 7 | 0.17 | ND | ND |
| 8 | 0.07 | 1.14 | ND |
| 9 | NS | 0.40 | 0.55 |
| Donor No. . | Immunoprecipitates . | ||
|---|---|---|---|
| CD100 . | CD45 . | CD148 . | |
| 1 | NS | 0.21 | 0.07 |
| 2 | NS | 0.28 | 0.54 |
| 3 | 0.11 | 0.66 | 0.36 |
| 4 | 0.11 | 1.38 | 0.76 |
| 5 | 0.20 | 0.40 | ND |
| 6 | 0.10 | 1.02 | ND |
| 7 | 0.17 | ND | ND |
| 8 | 0.07 | 1.14 | ND |
| 9 | NS | 0.40 | 0.55 |
Nongranular and CD138− cells were isolated with the use of cell sorting from populations of activated B lymphocytes. Sorted cells corresponded to CD100+CD38+CD138− cells from small B lymphocyte and large B lymphocyte populations, as defined in Figure 1.
PTP activity was assayed in CD100, CD45, and CD148 immunoprecipitates (2 × 106 cells/100 μL of lysates) and is expressed as the absorbance at 405 nm. All experimental procedures are described in “Materials and Methods.”
NS, not significant value (absorbance < 0.05); ND, not determined.
CD45 and CD148 immunodepletion experiments were carried out to characterize the PTP activity immunoprecipitated with CD100 in the sorted CD38+CD138− cells. CD45 depletion was found to reduce PTP activity in CD100 immunoprecipitates to levels corresponding to negative control values (CD101 immunoprecipitates), as shown by the example of donor 8 in Table 5. In contrast, CD148 depletion had no effect, with the exception of 1 donor for which a decrease of 30% was detected (data not shown). In addition, CD45 depletion reduced CD100-immunoprecipitated PTP activity to negative control levels in CD100+CD38+CD138− cells sorted after activation of purified tonsillar B lymphocytes, under the same experimental conditions of activation and cell sorting as for peripheral blood cells. Results of 1 of 2 similar experiments are shown in Table 5. We concluded that CD100-associated PTP activity resulted mainly from the association between CD100 and CD45 in CD38+CD138− cells induced by activation of peripheral blood as well as tonsillar B lymphocytes.
Effect of CD45 immunodepletion on PTP activity immunoprecipitated with CD100 in CD38+CD138− cells sorted from activated peripheral blood and tonsillar B lymphocytes
| . | Immunoprecipitates . | ||
|---|---|---|---|
| CD101 . | CD100 . | CD45 . | |
| Peripheral blood cells | |||
| No depletion | NS | 0.07 | 1.14 |
| Control depletion (CD101) | ND | 0.06 | 0.67 |
| CD45 depletion | ND | NS | 0.13 |
| Tonsillar cells | |||
| No depletion | NS | 0.08 | 0.52 |
| Control depletion (CD101) | ND | 0.07 | 0.36 |
| CD45 depletion | ND | NS | 0.08 |
| . | Immunoprecipitates . | ||
|---|---|---|---|
| CD101 . | CD100 . | CD45 . | |
| Peripheral blood cells | |||
| No depletion | NS | 0.07 | 1.14 |
| Control depletion (CD101) | ND | 0.06 | 0.67 |
| CD45 depletion | ND | NS | 0.13 |
| Tonsillar cells | |||
| No depletion | NS | 0.08 | 0.52 |
| Control depletion (CD101) | ND | 0.07 | 0.36 |
| CD45 depletion | ND | NS | 0.08 |
Nongranular CD138− cells were isolated with the use of cell sorting from populations induced by activation of peripheral blood (donor 8) or tonsillar B lymphocytes, as defined in Figure 1. Sorted cells were CD100+CD38+CD138−. PTP activity was assayed on CD101 (negative control value), CD100, and CD45 immunoprecipitates obtained from depleted or undepleted cell lysates (2 × 106 peripheral blood cells and 1.5 × 106 tonsillar cells per 100 μL of lysates). All procedures are described in “Materials and Methods.” Results are expressed as in Tables 3 and 4.
NS, not significant; ND, not determined.
Discussion
In the present study, we show that the human leukocyte semaphorin CD100 is differentially expressed and can functionally associate with distinct PTPs in B cells, depending on their activation and differentiation stage.
The induction of CD100 during T-cell activation has been previously reported.2-5 Our quantification experiments demonstrate for the first time that CD100, which was weakly expressed on resting peripheral blood B lymphocytes (1000 sites/cell) and weaker than on resting T lymphocytes and NK cells (several thousand sites per cell), was induced at a high density (more than 10 000 sites per cell) upon activation in a population of large B lymphocytes positive for CD38 but negative for CD138. CD100 was also induced but at a lesser extent in a population of SBLs displaying the same CD38+CD138− phenotype. Furthermore, in each of these cell populations, a subpopulation coexpressed CD148, which was recently shown to clearly identify the memory B-cell phenotype.27 Thus, these results indicated that CD100 was induced during activation and maturation of circulating B lymphocytes toward a memory B-cell phenotype. Interestingly, CD100 induction was also observed in a population of activated tonsillar B lymphocytes exhibiting the CD38+CD138− phenotype. That CD100 was differentially expressed during B-cell maturation was strengthened by our results on B-cell lines: the number of CD100 sites per cell was low (2000) in pre-B cell lines in agreement with the previous observation that CD100 was weakly represented on immature hematopoietic precursors,2 3 but was high (more than 10 000 sites/cell) in cell lines with a phenotype of activated lymphocytes and with more mature phenotypes (pre-plasma and some plasma cell lines).
Our data suggested a role for CD100 in cell surface signaling events involved in B-cell activation and differentiation. Therefore, we investigated whether CD100 was associated with transmembrane PTPs known to be implicated in these processes. CD100 was found to be associated with PTP activity in activated peripheral blood B lymphocytes from most donors as well as in most B-cell lines. We clearly identified CD45 as the CD100-associated PTP in cell lines, with phenotypes ranging from pre-B to pre-plasma cell stages. We also found that CD100 was associated with CD45 PTP activity in CD38+CD138− cell populations of activated peripheral blood and tonsillar B lymphocytes, as previously reported for activated T cells.5 In contrast, CD100 was not associated with CD45 in plasma cell lines representing the terminal stage of differentiation, including 1 expressing CD45 at a high level (XG-1). Consequently, at least another PTP was involved in the high CD100-associated PTP activity observed in several plasma cell lines. We further examined the possible association of CD100 with CD148, a transmembrane PTP previously reported to be expressed by some but not all B-cell lines.19 While poorly or not expressed on BL and EBV-transformed cell lines, CD148 was present at a rather elevated level on a plasma cell line and 2 of 5 plasma cell lines. In these latter, however, CD148 did not seem to play a crucial role in CD100-associated PTP activity. This indicated the involvement of another PTP in plasma cells, as evidenced by the observation that a plasma cell line (LP-1) showing a high level of CD100-immunoprecipitated PTP activity did not express either CD148 or CD45.
Although we cannot exclude the possibility that some results on B-cell lines may be due to intrinsic properties that were not directly related to the differentiation state, our data suggest a switch in the PTP associated with CD100 at the terminal stage of B-cell differentiation. The following model can be proposed: CD100 is weakly expressed in pre-B cells and resting B lymphocytes and is induced at high levels upon B-cell activation. Until this stage, CD100 is associated with the CD45 PTP. This is still the case when B lymphocytes are committed to differentiate toward either memory cells or pre-plasma cells. However, at terminal differentiation into plasma cells, a switch would occur in the CD100-associated PTP from CD45 to another, yet unidentified PTP. In certain differentiating B-cell subsets that express CD148 at elevated levels, the CD100-associated PTP might also switch from CD45 to CD148. Finally, in some terminally differentiated cells, CD100 could be no longer associated with a PTP (as illustrated by RPMI-8226 or XG-2 cells).
According to this model, the selective interactions of CD100 with several PTPs might be critical for B-cell activation and differentiation signaling. The association of CD100 with CD45 might have a permissive effect on the positive signals delivered by CD45 during B-cell activation and proliferation. In support of this hypothesis, serine phosphorylation of CD45 is known to enhance its PTP activity,28 and it has been recently reported that CD100 can associate with serine kinase activity in T cells.6However, it cannot be excluded that CD100 might negatively regulate the CD45 PTP activity. In this view, CD100, which was shown to exist as a dimeric form,2 might induce CD45 dimerization, known to inhibit its PTP activity.29 Therefore, it would be of interest to determine whether CD100 triggering results in stimulation or inhibition of B-cell proliferation. Additional questions remain to be elucidated regarding the association of CD100 with CD45. For instance, we do not know whether CD100 is actually a receptor (for a ligand presented by other cells) that would be involved in the positive or negative regulation of CD45 activity or is instead a substrate for CD45 since CD100 has a tyrosine residue in its intracellular domain. Neither do we know whether CD100 interacts with CD45 directly or through other molecules.
After switching, the association of CD100 with the unidentified PTP activity might be involved in such processes as termination of proliferation signals or initiation of terminal differentiation signals leading to the formation of plasma cells. The characterization of this CD100-associated PTP activity will determine whether it corresponds to a novel transmembrane PTP or to a previously described enzyme. At present, the possibility that this PTP activity corresponds to SHP-1, known to deliver a negative signal during B-cell activation, can be excluded since CD100 does not contain an ITIM motif and thus cannot bind SHP-1.7 15 Furthermore, the molecular basis for CD100-associated PTP switching also remains to be investigated. Among several hypotheses, it can be assumed that the unidentified PTP, either induced or activated from a preexisting inactive form, could have a better affinity for CD100 than CD45. Alternatively, structural changes of CD100 might be involved.
In other respects, our observations that CD148 was expressed and showed a functional enzymatic activity in particular subpopulationsof differentiating B cells (activated lymphocytes with a memory cell-like phenotype) or in B-cell lines representing terminal differentiation stages (pre-plasma and plasma cell lines) seem of interest. Indeed, although the exact role of CD148 in B-cell activation and differentiation has not been described so far, it was recently reported that CD148 negatively regulates T-cell activation.30
Finally, by showing the selective and functional interactions of CD100 with several PTPs in B cells, the present work enlarges the field of CD100 partners: CD100 associates with not only protein kinases31 but also phosphatases in B cells, as in T cells.5,6 Thus, CD100 appears to be the only transmembrane semaphorin clearly behaving as a cell surface receptor. No similar data are available regarding transmembrane semaphorins of the nervous system although Sema VIb, whose function is unknown, could bind c-src.32 Concerning soluble semaphorins whose function in the nervous system is known, they all act as ligands for specific receptors on target cells.33-35 CD100 may also have effects through binding to a counter-receptor at B-cell surface, as reported elsewhere using CD100-transfected cells.7 In this view, a soluble form of CD100 can be cleaved from T-cell surface,5and interestingly, the first evidence for a semaphorin receptor in the immune system has been recently provided.36 Therefore, CD100 turns out to be a discrete semaphorin.
Acknowledgments
We are indebted to Dr Ramon Vilella for his generous gift of monoclonal antibody, Dr Bernard Klein and Professor Jean-Claude Brouet for kindly providing cell lines, and Professor Stuart F. Schlossman for CD154-transfected cells and helpful discussions. We gratefully acknowledge Dr Jean-Pierre Roy for preparing monoclonal antibodies and Dominique Charue for flow cytometry analysis.
Supported by INSERM, Sidaction, the Association pour la Recherche sur le Cancer and the University of Paris 12.
C.B. and S.D. contributed equally to this work.
Submitted April 26, 1999; accepted September 22, 1999.
Reprints:Christian Billard, INSERM Unit 448, Faculté de Médecine, 8 rue du général Sarrail, F-94010 Créteil (cedex), France.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal