Activation of the serine/threonine kinase Akt has been shown to be a critical component for growth factor and cytokine stimulation of cell survival. Although some of the immediate upstream activators of Akt have been defined, the roles of tyrosine kinases in the activation of Akt are not well delineated. Granulocyte colony-stimulating factor (G-CSF) regulates the proliferation, differentiation, and survival of neutrophilic granulocytes. G-CSF exerts its actions by stimulating several signaling cascades after binding its cell surface receptor. Both Jak (Janus) and Src families of tyrosine kinases are stimulated by incubation of cells with G-CSF. In this report, we show that G-CSF stimulation of cells leads to activation of Akt. The membrane-proximal 55 amino acids of the G-CSF receptor cytoplasmic domain are sufficient for mediating Akt activation. However, activation of Akt appears to be downregulated by the receptor's carboxy-terminal region of 98 amino acids, a region that has been shown to be truncated in some patients with acute myeloid leukemia associated with severe congenital neutropenia. Furthermore, we demonstrate that G-CSF–induced activation of Akt requires the activities of Src family kinases but can be clearly dissociated from G-CSF–stimulated activation of Stats (signal transducers and activators of transcripton) by the Jak kinases. Thus, cytokine activation of the Jak/Stat and other signaling cascades can be functionally separated.
Granulocyte colony-stimulating factor (G-CSF) plays a critical role in the regulation of the proliferation, differentiation, and survival of myeloid progenitor cells.1 G-CSF binds to a cell surface receptor that is a member of the cytokine receptor superfamily. Mutations in the G-CSF receptor leading to carboxy-terminal truncation have been reported in certain patients with acute myeloid leukemia who had a history of severe congenital neutropenia.2-4 Incubation of cells with G-CSF activates a variety of intracellular signaling cascades, including the Jak/Stat, Ras-Raf-MAP kinase, and Src family kinase pathways.1Activation of the Jak (Janus) tyrosine kinases permits the tyrosine phosphorylation of the Stat (signal transducers and activators of transcripton) transcription factors, which subsequently translocate to the nucleus, bind enhancer elements, and stimulate the transcription of cellular genes.5 Although expression of many of the Stat-dependent genes induced by G-CSF has not been well defined, evidence does indicate that this signaling pathway is essential for the biologic actions of this cytokine.6-8
Another signaling molecule that is activated by G-CSF is phosphatidylinositol (PI) 3-kinase.9 Recently, the protein serine/threonine kinase Akt (also known as PKB) has been identified as a downstream target of PI3-kinase.10 The N-terminal regulatory domain of Akt contains a pleckstrin homology domain (PH) that is important for Akt activation. A product of PI3-kinase, phophatidylinositol-3,4-bisphosphate (PI-3,4-P2), directly binds to the PH domain of Akt, leading to partial activation of Akt.11 Full activation of Akt also requires phosphorylation at threonine 308 and serine 473 by the recently identified protein kinases, PDK1 and PDK2.12
Evidence indicates that Akt plays a positive role in the regulation of cell survival. Overexpression of constitutively activated forms of Akt prevents apoptosis that occurs as a result of serum/growth factor deprivation,13 ultraviolet radiation,14 and loss of matrix attachment.15 In contrast, expression of dominant negative mutants of Akt accelerates cell death after cytokine withdrawal.16,17 Akt promotes cell survival by several mechanisms. Akt is responsible for phosphorylating BAD.18,19 Phosphorylation of BAD allows it to interact with the 14-3-3 protein family, thereby inhibiting its death function. Akt has also been shown to induce Bcl-2 expression20 and to inhibit the activity of glycogen synthase kinase-3, which appears to deliver a pro-apoptotic signal.21
Several studies have demonstrated that there is significant crosstalk between different signaling cascades that are activated by a given cytokine. For example, activation of Raf-MAP kinase signaling by both growth factors and interferons requires Jak2 or Jak1,22,23and expression of constitutively active forms of either of these kinases will activate MAP kinase in the absence of a ligand. Constitutively active Src family kinases have also been associated with stimulation of both the Jak/Stat pathway as well as activation of Raf-MAP kinase.24-26 In this study, we demonstrate that G-CSF stimulation of hematopoietic cells results in the activation of Akt and that this activation is regulated by distinct cytoplasmic regions of the G-CSF receptor. Surprisingly, our data also indicate that the Src family kinases, but not the Jaks, appear to play a major role in Akt activation mediated by G-CSF.
Materials and methods
Cells
Murine BAF3 and 32D cells, stably transfected with complementary DNAs (cDNAs) encoding either the wild type or the truncated forms of the human G-CSF receptor, have been described.27 Two individual clones for each G-CSF receptor form were used in all experiments. Cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, 2-mercaptoethanol (50 μM), gentamicin (50 μg/mL), and 10% WEHI-3B cell-conditioned media. COS-7 cells were maintained in DMEM medium containing 10% fetal calf serum and gentamicin (50 μg/mL). Blood was obtained from healthy volunteers after informed consent. Neutrophils were collected as the sedimented cell fraction after Ficoll-Isopaque centrifugation and further depleted of erythrocytes by hypotonic lysis
Reagents
Wortmannin was from Sigma (St Louis, MO). Ly294 002 was from Biomol Research Laboratories Inc (Plymouth Meeting, PA). PP1, genistein, herbimycin A, and bisindolylmaleimide were obtained from Calbiochem (San Diego, CA). Phospho-specific Akt and p42, p44 mitogen-activated protein kinase (MAPK) antibodies were purchased from New England Biolabs (Beverly, MA). Anti-Akt antibody for Western blotting was from Upstate Biotechnology Inc (Lake Placid, NY). Rabbit anti-Akt antiserum used for immunoprecipitation and kinase assays was raised against a synthetic peptide corresponding to the carboxy-terminal 15 amino acids of murine Akt. Anti-p42, p44 MAPK (Pan-Erk) antibody was from Transduction Laboratories (Lexington, KY). Antibody to JNK1 was obtained from Pharmingen (San Diego, CA). [γ-32P]ATP and ECL-Plus kit were purchased from Amersham (Piscataway, NJ).
Extract preparation and Western blotting
BAF3 cells were starved in the absence of serum and conditioned media for 6 hours, and they were subsequently stimulated with G-CSF (100 ng/mL) for the times indicated. Cells (107) were washed with ice-cold phosphate-buffered saline and resuspended in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 10 mM NaF, 0.5 mM dithiothreitol, 1% Triton X-100, 1 mM PMSF, and 1 mM vanadate). Lysates were cleared by centrifugation at 12 000g for 20 minutes at 4°C, and proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) prior to transfer to Immobilon membranes. The membranes were incubated with the appropriate antibodies. Western blots were developed using ECL-Plus kit.
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays (EMSAs) were performed as previously described using whole-cell extracts.28 The interferon-γ response region (GRR) probe (5′AGCATGTTTCAAGGATTTGAGATGTATTTCCCAGAAAAG3′) was end-labeled using polynucleotide kinase and [γ-32P] ATP, and it was used in all EMSAs.
Immunoprecipitations and Akt kinase assay
Whole-cell extracts were prepared as described above and incubated with rabbit anti-Akt antiserum for 2 hours at 4°C. Immunocomplexes were washed 3 times in lysis buffer, once in water, and once in kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, and 10 mM MnCl2 [pH 7.4]). Kinase reactions were carried out in 20 μL of kinase buffer containing 20 μM of unlabeled ATP and 370 kBq [γ-32P]ATP (222 TBq/mmol). Histone H2B (Boehringer, Indianapolis, IN) was used as an exogenous substrate at 100 μg/mL. After incubation at room temperature for 15 minutes, the reaction was terminated by adding 6 μL of Laemmli buffer. Samples were heated at 95°C for 5 minutes and separated by SDS-PAGE. The proteins were then transferred to Immobilon membranes followed by autoradiography.
JNK kinase assay
Whole-cell extracts were incubated with the anti-JNK antibody. Immunocomplexes were washed twice with lysis buffer and once with kinase buffer (20 mM HEPES [pH 7.5], 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM sodium fluoride, and 0.5 mM vanadate). JNK activity was determined by resuspension in 20 μL of kinase buffer containing 370 kBq [γ-32P]ATP, 20 μM unlabeled ATP, and 1 mg GST-ATF296 fusion protein as a substrate as previously described.29 After 15 minutes of incubation at room temperature, the reaction was terminated by addition of 6 μL of Laemmli buffer.
Expression vectors and transient transfection
The human wild-type G-CSF receptor was cloned in pLNCX expression vector as previously described.27 Murine Stat5a (with FLAG epitope in Prk vector) was a generous gift of J. Ihle.30Murine full-length Jak1 and Jak2 and kinase-deficient Jak1 (ATP binding site K to E) and Jak2 (K882 to E), all with FLAG tag cloned in Prk vector, were kindly provided by O. Silvennoinen.22Expression constructs of full-length Tyk2 and kinase-negative Tyk2 (ATP binding site K930 to I) were generous gifts of J. Krolewski.31 c-Src and Syk in pcDNA3 vector were gifts of S. Parsons and S. Gutkind, respectively. Transfection of cDNAs into COS-7 cells was performed by electroporation (1.6 kV, 95 μS, 2 pulses; Electro Square Porator, BTX Genetronics Inc, San Diego, CA). Twenty hours after transfection, cells were deprived of serum for 4 hours prior to treatment and preparation of cell extracts.
Results
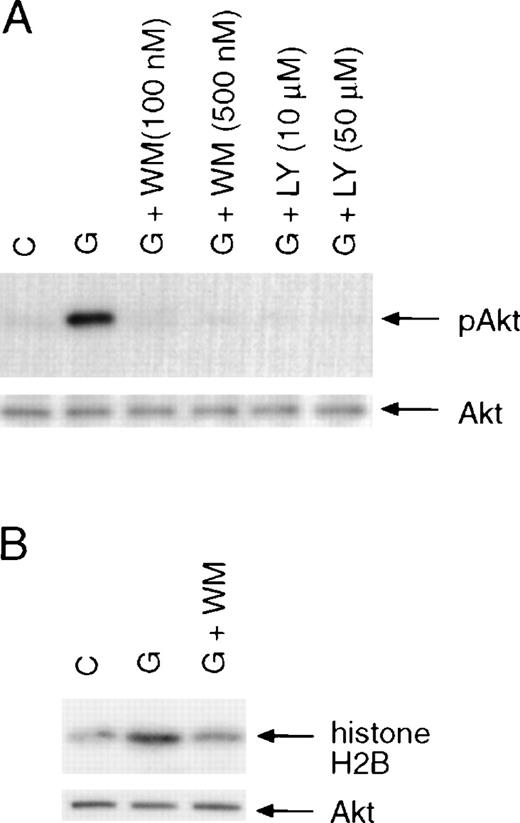
Many growth factors that support cell proliferation and survival have been shown to activate Akt kinase activity. Although PI3-kinase activity is enhanced in BAF3 cells treated with G-CSF,9activation of its downstream effector Akt has not been examined. To investigate the involvement of Akt in G-CSF–dependent signaling, we determined whether Akt became phosphorylated in response to G-CSF in murine Pro-B BAF3 cells that were transfected with the human G-CSF receptor.27 Cells were starved for 6 hours prior to stimulation with G-CSF for 10 minutes. Whole-cell lysates were prepared, resolved on SDS-PAGE, and transferred to Immobilon membranes. The membranes were incubated with a phospho-specific antibody that detects Akt only when phosphorylated at serine 473, which is required for full activation of Akt.32 As shown in Figure1A (upper panel), G-CSF treatment of BAF3 cells expressing the wild-type G-CSF receptor (BAF/WT) resulted in the phosphorylation of Akt at serine 473. Phosphorylation of Akt was blocked by pretreatment of cells with PI3-kinase inhibitors wortmannin or Ly294 002, consistent with Akt being the downstream target of PI3-kinase.11 33 To further demonstrate that Akt is activated by G-CSF stimulation, Akt was immunoprecipitated from whole-cell lysates, and the kinase activity of precipitated Akt was examined by in vitro kinase assays using histone H2B as a substrate. As shown in Figure 1B, G-CSF stimulation resulted in activation of Akt in BAF/WT cells, and the kinase activity of Akt was also blocked by wortmannin.
Activation of Akt by G-CSF treatment of BAF3 cells expressing the wild-type G-CSF receptor.
(A) Induction of Akt phosphorylation. Cells were incubated in serum-free medium for 6 hours and then stimulated with G-CSF (100 ng/mL) for 10 minutes with or without pretreatment for 15 minutes with wortmannin (WM) or Ly294 002 (LY). Akt phosphorylation was determined using a phospho-specific antibody that recognizes Akt only when phosphorylated on Serine 473 (upper panel). The membrane was reprobed with anti-Akt antibody (lower panel). (B) Activation of Akt kinase activity. Akt was immunoprecipitated from whole-cell extracts prepared from unstimulated or G-CSF–stimulated cells. The kinase activity of Akt was determined by in vitro kinase assay using histone H2B as a substrate (upper panel). The amounts of Akt kinase in each sample were determined by probing the membrane with anti-Akt antibody (lower panel).
Activation of Akt by G-CSF treatment of BAF3 cells expressing the wild-type G-CSF receptor.
(A) Induction of Akt phosphorylation. Cells were incubated in serum-free medium for 6 hours and then stimulated with G-CSF (100 ng/mL) for 10 minutes with or without pretreatment for 15 minutes with wortmannin (WM) or Ly294 002 (LY). Akt phosphorylation was determined using a phospho-specific antibody that recognizes Akt only when phosphorylated on Serine 473 (upper panel). The membrane was reprobed with anti-Akt antibody (lower panel). (B) Activation of Akt kinase activity. Akt was immunoprecipitated from whole-cell extracts prepared from unstimulated or G-CSF–stimulated cells. The kinase activity of Akt was determined by in vitro kinase assay using histone H2B as a substrate (upper panel). The amounts of Akt kinase in each sample were determined by probing the membrane with anti-Akt antibody (lower panel).
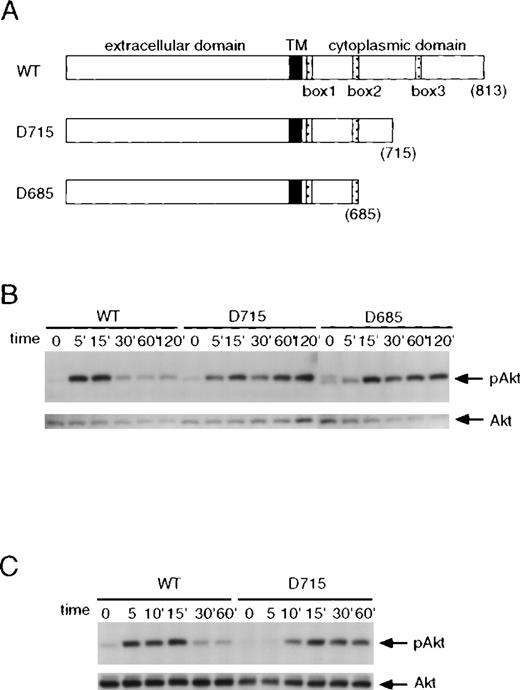
It has been shown previously that the membrane-proximal region of the G-CSF receptor is sufficient for activation of Jak2 and Stat5, whereas activation of MAP kinase and Stat3 requires additional regions distal to the membrane-proximal portion.8,27 34-36 We examined whether the carboxy-terminal region of the G-CSF receptor is also required for Akt activation. BAF3 cells transfected with different forms of the G-CSF receptor (Figure 2A) were stimulated with G-CSF for the indicated times, and whole-cell lysates were prepared and analyzed for Akt phosphorylation by Western blotting. In BAF/WT cells, G-CSF induced rapid Akt phosphorylation that peaked at 5 minutes before declining to near basal levels at 30 minutes of treatment (Figure 2B). A G-CSF–dependent Akt phosphorylation was also seen in cells that expressed the D715 (BAF/D715) or D685 (BAF/D685) mutants, notably at a rate that was significantly slower than that induced by the wild-type receptor, with maximal activation occurring at approximately 15 minutes of G-CSF treatment. Interestingly, Akt phosphorylation mediated by the 2 truncation mutants persisted for at least 2 hours without significant decay. Comparable results were obtained with myeloid 32D cells transfected with different forms of the G-CSF receptor (Figure 2C).
Kinetics of G-CSF–induced Akt phosphorylation in BAF3 cells expressing the different forms of the G-CSF receptor.
(A) Schematic diagram of the wild-type (WT) and truncated forms of the G-CSF receptor. Boxes B1, B2, and B3 denote subdomains conserved in several members of the cytokine receptor superfamily. The numbers in parentheses indicate amino acid positions; TM, transmembrane domain. (B) Akt phosphorylation induced by G-CSF in BAF3 cells expressing the different G-CSF receptor forms. Cells were left untreated or treated with G-CSF for the indicated times. Whole-cell extracts were immunoblotted with anti-phospho-Akt antibody (upper panel) and reprobed with anti-Akt antibody (lower panel). (C) G-CSF stimulated phosphorylation of Akt in myeloid 32D cells expressing the wild type or the D715 form of the receptor.
Kinetics of G-CSF–induced Akt phosphorylation in BAF3 cells expressing the different forms of the G-CSF receptor.
(A) Schematic diagram of the wild-type (WT) and truncated forms of the G-CSF receptor. Boxes B1, B2, and B3 denote subdomains conserved in several members of the cytokine receptor superfamily. The numbers in parentheses indicate amino acid positions; TM, transmembrane domain. (B) Akt phosphorylation induced by G-CSF in BAF3 cells expressing the different G-CSF receptor forms. Cells were left untreated or treated with G-CSF for the indicated times. Whole-cell extracts were immunoblotted with anti-phospho-Akt antibody (upper panel) and reprobed with anti-Akt antibody (lower panel). (C) G-CSF stimulated phosphorylation of Akt in myeloid 32D cells expressing the wild type or the D715 form of the receptor.
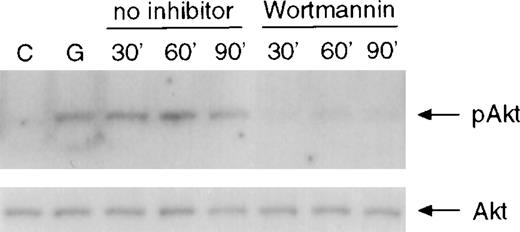
To determine whether prolonged activation of Akt is due to delayed Akt dephosphorylation or to sustained upstream signaling, BAF/D715 cells were treated with G-CSF for 10 minutes prior to addition of wortmannin to the culture to stop further activation of PI3-kinase. Whole-cell lysates were prepared at different times for the analysis of Akt phosphorylation. As shown in Figure 3, addition of wortmannin reduced Akt phosphorylation to basal levels within 30 minutes in BAF/D715 cells. Similar results were seen using the PI3-kinase inhibitor Ly294 002 (data not shown). Additional experiments with more time points demonstrated that wortmannin overrode Akt phosphorylation within only 10 minutes after its addition to cells previously exposed to G-CSF (data not shown). These results indicate that the prolonged activation of Akt seen in BAF/D715 cells was caused by continuous activation of PI3 kinase or a component upstream of PI3-kinase.
Inhibition of sustained activation of Akt by wortmannin.
BAF3 cells expressing the D715 receptor were unstimulated or stimulated with G-CSF for 10 minutes prior to addition of wortmannin (100 nM) to the cultures. Whole-cell extracts were prepared at the indicated times and used for analysis of Akt phosphorylation (upper panel). The same membrane was incubated with anti-Akt antibody (lower panel).
Inhibition of sustained activation of Akt by wortmannin.
BAF3 cells expressing the D715 receptor were unstimulated or stimulated with G-CSF for 10 minutes prior to addition of wortmannin (100 nM) to the cultures. Whole-cell extracts were prepared at the indicated times and used for analysis of Akt phosphorylation (upper panel). The same membrane was incubated with anti-Akt antibody (lower panel).
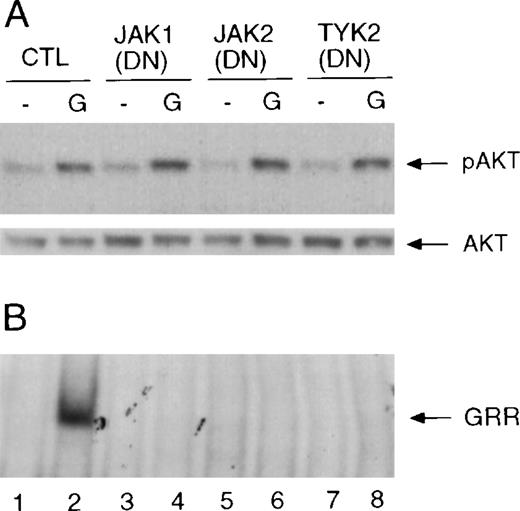
It has been shown that activation of Raf-MAP kinase activity by interferons requires expression of Jak1, and overexpression of Jak1, Jak2, or Tyk2 can activate MAP kinase in the absence of ligand.22,23 These observations suggest that one or more of the Jaks are necessary for the activation of signaling cascades that are distinct from the Jak/Stat pathway. G-CSF activates Jak1, Jak2, and Tyk2 in hematopoietic cells.37-39 To determine whether Jaks are involved in the activation of Akt by G-CSF, COS-7 cells were transiently transfected with cDNAs encoding the wild-type G-CSF receptor together with cDNAs encoding the dominant negative forms of Jak1, Jak2, or Tyk2. A Stat5a cDNA was also transfected to monitor G-CSF–induced Stat activation by EMSA using the GRR probe. The G-CSF receptor expressed in COS-7 cells activated Akt and Stat5 upon G-CSF stimulation (Figure 4B, lane 2). Expression of either of the dominant negative Jak proteins in COS-7 cells blocked G-CSF–induced activation of Stat5 (Figure 4B, lanes 3 to 8). However, these mutant Jak proteins had no effect on Akt phosphorylation induced by G-CSF (Figure 4A). Transfection of combinations of 2 kinase-inactive Jaks also did not influence Akt activation (data not shown). In BAF3 cells transiently transfected with the wild-type G-CSF receptor, coexpression of the kinase-deficient Jak1 also blocked G-CSF–induced Stat activation but did not affect the activation of Akt (data not shown).
Effects of dominant negative (DN) Jak mutants on the activation of Akt and Stat5.
(A) COS-7 cells were transfected with cDNAs encoding the wild-type G-CSF receptor and Stat5a only or were transfected together with cDNAs encoding the kinase inactive Jaks as indicated. Twenty hours after transfection, cells were starved for 4 hours prior to stimulation with G-CSF for 10 minutes. Whole-cell extracts were prepared and used for the analysis of Akt phosphorylation (upper panel). The membrane was reprobed with anti-Akt antibody (lower panel). (B) The same extracts were used for the analysis of Stat5a activation by EMSA using GRR probe. The complex that contains Stat5 is indicated with an arrow and labeled “GRR.”
Effects of dominant negative (DN) Jak mutants on the activation of Akt and Stat5.
(A) COS-7 cells were transfected with cDNAs encoding the wild-type G-CSF receptor and Stat5a only or were transfected together with cDNAs encoding the kinase inactive Jaks as indicated. Twenty hours after transfection, cells were starved for 4 hours prior to stimulation with G-CSF for 10 minutes. Whole-cell extracts were prepared and used for the analysis of Akt phosphorylation (upper panel). The membrane was reprobed with anti-Akt antibody (lower panel). (B) The same extracts were used for the analysis of Stat5a activation by EMSA using GRR probe. The complex that contains Stat5 is indicated with an arrow and labeled “GRR.”
The results presented in Figure 4 suggest that activated Jaks are not sufficient for G-CSF stimulation of Akt. To investigate whether other classes of kinases might play a role in this process, we treated BAF/WT cells with different kinase inhibitors prior to G-CSF stimulation. As shown in Figure 5A, G-CSF–induced activation of Akt was inhibited by genistein (GN; lane 5) and herbimycin (HB; lane 6), which are general tyrosine kinase inhibitors. These 2 inhibitors also blocked G-CSF–dependent activation of Stat proteins, as evidenced by a failure of G-CSF to induce Stat binding activities in EMSAs (Figure 5B). In contrast, PI3-kinase inhibitors wortmannin (WM; lane 3) and Ly294 002 (LY; lane 4) completely abolished G-CSF–induced activation of Akt but had no effect on Stat5 activation. Interestingly, a specific Src family kinase inhibitor, PP1,40 41 inhibited Akt phosphorylation by approximately 90% to 100% in several experiments (lane 7 and data not shown). However, PP1 exerted no effect on Stat activation by G-CSF (lane 7). Bisindolylmaleimide (BM; lane 8), a specific inhibitor of PKC kinase, and the protein synthesis inhibitor cycloheximide (CHX; lane 9) did not affect the activation of either Akt or Stats by G-CSF. In addition, we observed that Akt activation by G-CSF in primary neutrophils was also blocked by wortmannin or PP1 (Figure 5C).
Effects of different inhibitors on G-CSF–induced activation of Akt and Stats.
(A) BAF3 cells expressing the wild-type G-CSF receptor were unstimulated (lane 1) or stimulated with G-CSF for 10 minutes without (lane 2) or with preincubation with wortmannin (WM: 100 nM; lane 3), Ly294 002 (LY: 10 μM; lane 4), genistein (GN: 200 μM; lane 5), herbimycin A (HB: 1 μg/mL; lane 6), PP1 (10 μM; lane 7), bisindolylmaleimide (BM: 5 μM; lane 8) or cycloheximide (CHX: 30 μg/mL; lane 9). The preincubation times were 15 minutes except for herbimycin A (180 minutes). Whole-cell extracts were prepared and used for analysis of Akt phosphorylation by Western blotting. (B) The same extracts were used for the analysis of Stat5a activation by EMSA. (C) Peripheral blood neutrophils were left unstimulated or stimulated with G-CSF for 5 minutes following pretreatment with wortmannin or PP1 for 15 minutes as indicated. Whole-cell extracts were examined for Akt phosphorylation.
Effects of different inhibitors on G-CSF–induced activation of Akt and Stats.
(A) BAF3 cells expressing the wild-type G-CSF receptor were unstimulated (lane 1) or stimulated with G-CSF for 10 minutes without (lane 2) or with preincubation with wortmannin (WM: 100 nM; lane 3), Ly294 002 (LY: 10 μM; lane 4), genistein (GN: 200 μM; lane 5), herbimycin A (HB: 1 μg/mL; lane 6), PP1 (10 μM; lane 7), bisindolylmaleimide (BM: 5 μM; lane 8) or cycloheximide (CHX: 30 μg/mL; lane 9). The preincubation times were 15 minutes except for herbimycin A (180 minutes). Whole-cell extracts were prepared and used for analysis of Akt phosphorylation by Western blotting. (B) The same extracts were used for the analysis of Stat5a activation by EMSA. (C) Peripheral blood neutrophils were left unstimulated or stimulated with G-CSF for 5 minutes following pretreatment with wortmannin or PP1 for 15 minutes as indicated. Whole-cell extracts were examined for Akt phosphorylation.
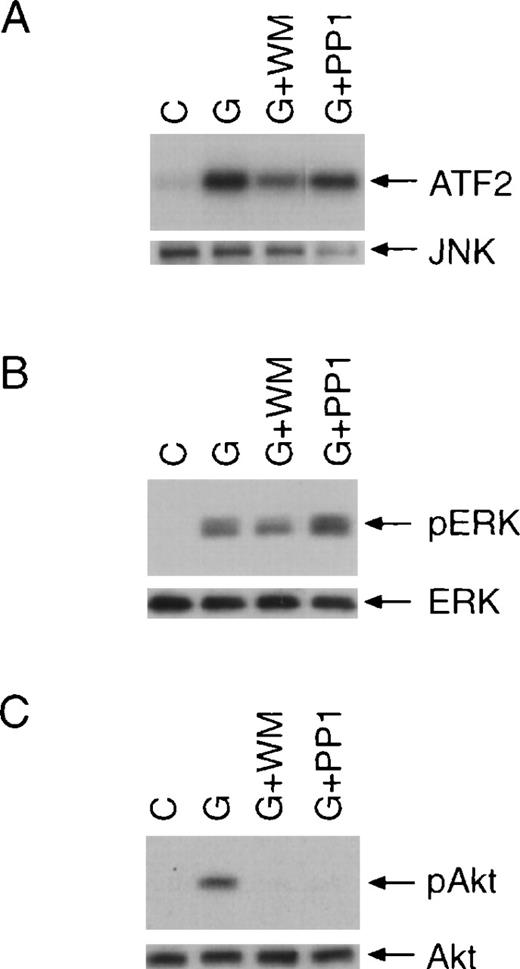
G-CSF has been shown to activate Ras-Raf-MAP kinase and JNK pathways.36 42 We further investigated whether these pathways are affected by Src family kinase inhibitor PP1. JNK kinase activity was determined by in vitro kinase assays using ATF2 as a substrate (Figure 6A). We determined the activation of p42, p44 MAPK by examining their phosphorylation at threonine 202 and tyrosine 204 (Figure 6B). Consistent with previous studies, incubation of cells with G-CSF activated p42, p44 MAPK, and JNK. Notably, preincubation of cells with PP1 had no effect on the activation of these kinases by G-CSF although, in the same experiment, activation of Akt was blocked by PP1 (Figure6C). Interestingly, wortmannin appeared to partially inhibit the activation of JNK and p42, p44 MAPK (Figure 6A and B), suggesting that a small fraction of G-CSF–activated JNK and p42, p44 MAPK might be regulated by a PI3-kinase–dependent mechanism.
PP1 has no effect on the activation of JNK and p42, p44 MAPK.
BAF/WT cells were stimulated with G-CSF for 10 minutes with or without pretreatment with wortmannin (WM) or PP1. (A) Whole-cell extracts were prepared, and JNK was immunoprecipitated with specific antiserum. The kinase activity of JNK was determined by in vitro kinase assay using ATF2 as a substrate (upper panel). The membrane was subsequently probed for JNK (lower panel). (B) The same cell extracts were also used in Western blot analysis for the determination of p42, p44 MAPK phosphorylation using a phospho-specific antibody (upper panel). Equal loading was confirmed by incubating the membrane with an anti p42, p44 MAPK antibody (lower panel). (C) Whole-cell extracts were also examined for Akt phosphorylation.
PP1 has no effect on the activation of JNK and p42, p44 MAPK.
BAF/WT cells were stimulated with G-CSF for 10 minutes with or without pretreatment with wortmannin (WM) or PP1. (A) Whole-cell extracts were prepared, and JNK was immunoprecipitated with specific antiserum. The kinase activity of JNK was determined by in vitro kinase assay using ATF2 as a substrate (upper panel). The membrane was subsequently probed for JNK (lower panel). (B) The same cell extracts were also used in Western blot analysis for the determination of p42, p44 MAPK phosphorylation using a phospho-specific antibody (upper panel). Equal loading was confirmed by incubating the membrane with an anti p42, p44 MAPK antibody (lower panel). (C) Whole-cell extracts were also examined for Akt phosphorylation.
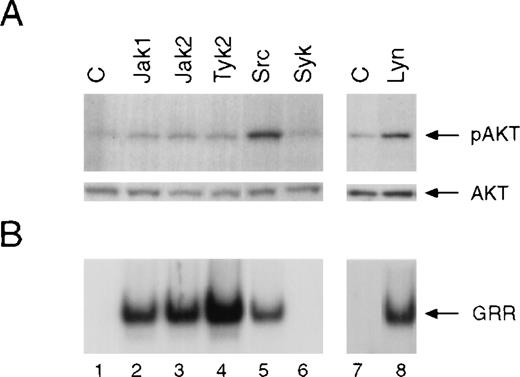
We then examined the effects of overexpression of different Jaks or Src family kinases on the activation of Akt. COS-7 cells were transiently transfected with cDNAs encoding Jak1, Jak2, Tyk2, c-Src, or Lyn. Under these conditions, these tyrosine kinases were constitutively active, which allowed us to examine their downstream targets in the absence of ligand-receptor interaction. Whole-cell lysates were prepared and analyzed for Akt phosphorylation by Western blotting and Stat activation by EMSA. Although overexpression of Jak1, Jak2, or Tyk2 protein leads to the activation of Stat5 binding activity (Figure7B), these Jaks did not noticeably stimulate phosphorylation of Akt (Figure 7A). In contrast, overexpression of c-Src or Lyn resulted in the activation of both Stat5 and Akt, which was inhibited by the Src family kinase inhibitor PP1 (data not shown). Notably, PP1 had no effect on Stat5 activation induced by overexpressed Jaks (data not shown).
Effect of overexpression of different Jaks, c-Src, or Lyn on the activation of Akt and Stat5.
COS-7 cells were transfected with either the Stat5a cDNA only (lanes 1 and 7) or together with cDNAs encoding the different Jaks (lane 2 to 4), c-Src (lane 5), Syk (lane 6), or Lyn (lane 8). Twenty hours after transfection, cells were serum-starved for 4 hours prior to preparation of whole-cell extracts. (A) Akt phosphorylation was determined by Western blotting using phospho-specific Akt antibody (upper panel). The blot was reprobed with anti-Akt antibody (lower panel). (B) Activation of Stat5a was measured by EMSA using GRR probe.
Effect of overexpression of different Jaks, c-Src, or Lyn on the activation of Akt and Stat5.
COS-7 cells were transfected with either the Stat5a cDNA only (lanes 1 and 7) or together with cDNAs encoding the different Jaks (lane 2 to 4), c-Src (lane 5), Syk (lane 6), or Lyn (lane 8). Twenty hours after transfection, cells were serum-starved for 4 hours prior to preparation of whole-cell extracts. (A) Akt phosphorylation was determined by Western blotting using phospho-specific Akt antibody (upper panel). The blot was reprobed with anti-Akt antibody (lower panel). (B) Activation of Stat5a was measured by EMSA using GRR probe.
Discussion
Cytokine stimulation of Stat transcription factors allows these proteins to translocate to the nucleus, bind to DNA, and activate a variety of immediate early genes.5 Treatment of cells with G-CSF results in the activation of several signaling cascades that include those regulated by Jaks, Ras-Raf-MAP kinase, Src family kinases, and PI3-kinase.9,36-38,43 Although these cascades are clearly distinct with regard to their downstream targets and their biologic roles in G-CSF–induced cell proliferation, differentiation, and survival, evidence suggests that they are functionally interdependent. For example, interferon activation of Raf-1 requires the expression of Jak1, and constitutively active Jaks activate Raf-1 and Erk2.22,23 It is also known that expression of constitutively activated Src family kinases leads to the activation of Jaks, Stats, and Raf-1.24-26
With respect to G-CSF receptor signaling, it is unclear whether Jaks also play a critical role in the activation of signaling cascades other than Stat pathway. We show in this study that activation of Akt by G-CSF is not inhibited by dominant negative forms of Jaks and that overexpression of Jak proteins in COS-7 cells leads to constitutive activation of Stat5 but has no effect on Akt activation. Together, these results indicate that activation of Jaks alone is not sufficient for G-CSF–induced phosphorylation of Akt. It should be noted that Jaks may also function as structural components independent of their role as tyrosine kinases.44 45 Our experiments do not eliminate such a role for the Jaks in G-CSF–induced activation of Akt.
The tyrosine kinase or kinases that are critically involved in the activation of Akt by G-CSF are not clear. The fact that PP1, a selective inhibitor of Src family tyrosine kinases,40,41prevents G-CSF–stimulated phosphorylation of Akt and that overexpression of c-Src or Lyn leads to Akt phosphorylation suggests that a member(s) of this family is intimately involved in this process. Notably, the Src homology 3 (SH3) domain of several Src family kinases, including Lyn, Fyn, and c-Src, has been shown to directly bind to p85 regulatory subunit of PI3-kinase, resulting in activation of PI3-kinase.46-48 In line with this, we consistently observed that PP1 inhibited the activity of PI3-kinase that was stimulated by G-CSF in cells expressing the wild-type G-CSF receptor (data not shown). Among the Src family members, Lyn and Hck have been shown to be activated by G-CSF treatment of hematopoietic cells.49,50 In addition to COS-7 cells, overexpression of Lyn in BAF3 cells also stimulates Akt phosphorylation (unpublished data), which was inhibited by PP1 treatment of the cells, suggesting that Lyn might be responsible for G-CSF–stimulated activation of Akt in hematopoietic cells. It is noteworthy that expression of Lyn has also been implicated as a requirement for G-CSF–stimulated cell growth.43
Although PP1 blocks G-CSF–stimulated activation of Akt, it has no significant effect on the activation of p42, p44 MAPK, JNK, and Stats (see Figures 5 and 6), indicating that activation of these signaling pathways does not require the Src family kinases. Together, these data appear to suggest that G-CSF–stimulated activation of the 2 major types of protein tyrosine kinases, ie, the Janus family and the Src family kinases, and their downstream signaling events can be functionally separated. It appears that Jaks may play a major role in G-CSF–induced activation of Stat signaling pathway, whereas activation of Akt by G-CSF is mainly mediated by Src family kinases, presumably by a PI3-kinase–dependent mechanism.
It is interesting that the carboxy-terminal region of the G-CSF receptor plays an important role in regulating G-CSF–stimulated phosphorylation of Akt in that truncation of this carboxy-terminus leads to delayed but sustained activation of Akt. It is unclear how the carboxy-terminal portion of the G-CSF receptor controls the rate at which Akt is activated. It is possible that truncation of this terminus of the G-CSF receptor may affect the rate of recruitment to the receptor's membrane-proximal region of certain key molecules required for activation of PI3-kinase/Akt signaling pathway. Alternatively, PI3-kinase/Akt could be activated independently by 2 functional domains of the G-CSF receptor, and the one located in the carboxy-terminal region could activate Akt at a rate faster than the one situated in the membrane-proximal region.
The mechanism by which the carboxy-terminus of the G-CSF receptor regulates the duration of Akt activation remains speculative. Prolonged activation of Akt induced by the G-CSF receptor mutants that lack the carboxy-terminus appears to be a result of continuous signaling that stimulates the phosphorylation of Akt rather than a result of defective dephosphorylation of activated Akt (see Figure 3). It has been shown recently that deletion of the G-CSF receptor carboxy-terminus causes delayed receptor internalization,51 52 which in theory could account for the prolonged activation of Akt. It is also possible that the wild-type G-CSF receptor might activate a phosphatase that would suppress the activation of PI3-kinase or upstream signaling molecules, resulting in the rapid downregulation of Akt activation. Dissection of the regulatory mechanisms by which the G-CSF–activated signaling cascades function will shed light on the biologic actions of G-CSF.
Akt has been implicated as a positive regulator of cell proliferation and survival.13-17 Recently, it was shown that Akt activation was significantly prolonged upon cytokine stimulation of bone marrow cells and neutrophils from mice lacking the SH2-containing inositol 5-phosphatase (SHIP), and these cells are more resistant to programmed cell death induced by cytokine withdrawal.53Moreover, SHIP-deficient mice exhibit dramatic chronic hyperplasia of myeloid cells and myeloid infiltration of various organs.53,54 Notably, certain acute myeloid leukemia patients who had a history of severe congenital neutropenia have been shown to express truncated G-CSF receptors.2-4 In fact, the D715 receptor was originally derived from one of these patients.55 BAF3 and 32D cells expressing the D715 receptor also displayed prolonged survival upon G-CSF removal from the culture medium.8 Whether the prolonged activation of Akt induced by the truncated G-CSF receptors could contribute to the development of leukemia needs further investigation.
Reprints:Andrew C. Larner, Cleveland Clinic Foundation, Lerner Research Institute, Department of Immunology, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: larnera@ccf.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal