Abstract
Chronic myeloid leukemia (CML) is a clonal, multilineage myeloproliferative disorder characterized by the Philadelphia chromosome (Ph) and a marked expansion of myeloid cells. Previous studies have indicated that the telomere length in blood cells may indicate their replicative history. However, the large variation in telomere length between individuals complicates the use of this parameter in CML and other hematologic disorders. To circumvent this problem, we compared the telomere length in peripheral blood or bone marrow cells with purified normal (Ph−) T lymphocytes from the same CML patient using fluorescence in situ hybridization and flow cytometry. Overall telomere fluorescence was significantly reduced in Ph+ cells from patients with CML compared to blood leukocytes from normal individuals (P < 0.001) or normal (Ph−) T lymphocytes from the same individuals (n = 51, P < 0.001). Cells from patients in accelerated phase or blast phase (AP/BP) showed significantly shorter average telomere length than cells from patients in chronic phase (CP,P = 0.02) or cytogenetic remission (CR,P = 0.03). Patients in CP who subsequently developed BP within 2 years had significantly shorter telomeres than those who did not develop BP for at least 2 years (P < 0.05). Accelerated replication-dependent telomere shortening in Ph+ versus Ph− leukocytes supports previous evidence that Ph+ stem cells cycle more actively than their counterparts in normal individuals. Our data further suggest that telomere shortening may serve as a surrogate marker of disease progression in patients with CP CML, supporting a mechanistic link between CML stem cell turnover, genetic instability, and malignant evolution in this disease. (Blood. 2000;95:1883-1890)
Chronic myeloid leukemia (CML) is a malignant disorder that originates in a pluripotent hematopoietic stem cell1(HSC) that acquires a Philadelphia (Ph) chromosome2,3encoding the BCR-ABL oncogenic fusion protein.4,5 In chronic phase (CP), increased numbers of Ph+ myeloid, erythroid, and megakaryocytic progenitor cells are found both in the hyperplastic bone marrow and in the peripheral blood of patients with highly elevated white blood cell counts. Despite convincing evidence that CML can originate in a lymphohematopoietic precursor cell, Ph+ cells typically outnumber the progeny of normal cells only in the myeloid lineages. Some Ph+ B-lineage cells are found in a proportion of patients and circulating T cells are predominantly Ph−.1,6,7 At diagnosis the stem cell compartment of most patients with CML is still dominated by normal stem cells as assayed both by long-term culture-initiating cell assays (LTC-IC)8,9 and their transplantation into irradiated immunodeficient mice.10-12 Nevertheless, at later stages of myelopoiesis, the marrow and blood of CP CML patients is usually dominated by Ph+ cells of all lineages. Although the mechanisms underlying these stage-specific population dynamics are not fully understood, the turnover of all types of Ph+ stem and progenitor cells is known to be increased,13-15 whereas the self-renewal ability of at least the more primitive Ph+cells appears to be decreased.9,16 After a CP of variable duration, transformation to an acute leukemia with myeloid, B-lymphoid, or occasionally T-lymphoid features occurs.17-20 Although the Ph chromosome is commonly the only cytogenetic abnormality detected in CP patients, cells with additional chromosomal abnormalities are usually present at blast phase (BP).
In human somatic cells, chromosomes terminate in several kilobases of repetitive TTAGGG sequences and associated proteins. Telomere repeats in such cells are successively lost with repeated cell division; hence, with age, this process leads to genetic instability and cell senescence.21-28 Telomerase is required to maintain the length of telomere repeats in the germ line,29,30 and variable levels of telomerase activity can also be detected in normal hematopoietic progenitor cells, activated T cells, germinal center B lymphocytes, as well as in the majority of human cancers (for reviews, see Shay and Bacchetti,31 Weng et al,32 and de Lange33). Recently, studies of telomerase knock-out mice have highlighted the functional importance of telomeres and telomerase in the biology of multicellular organisms.29,34,35 The finding that overexpression of hTERT in telomerase-negative human somatic cells results in the extension of the in vitro life span of otherwise normal epithelial cells and fibroblasts further supports a linkage between telomere shortening and replicative senescence.36-38
The level of telomerase found in normal HSCs appears to be too low to prevent telomere shortening with replication and age.39-42In addition, we have shown the proliferative capacity of HSCs at different stages of ontogeny to be correlated with the overall telomere length of the corresponding hematopoietic tissue.39,43-45Telomere shortening appears to be accelerated in donor-derived HSCs regenerated in recipients of allogeneic bone marrow transplants (BMT).46 47 Taken together, these studies suggest that telomere length measurements may be useful indications of stem cell turnover in vitro and in vivo.
Recent studies in CML have suggested that telomere length shortens with progression from CP to BP and that telomerase is up-regulated in association with the acquisition of additional cytogenetic aberrations.40,48-50 It has also been reported that longer telomeres may be a feature of patients with a more favorable prognosis and likelihood to respond to treatment with interferon-α.50 However, the interpretation of leukocyte telomere length measurements in such studies is complicated by the large variation in telomere length between individuals51and the heterogeneity of nucleated cells in the peripheral blood. One approach to resolving these problems is to assess telomere length in defined populations of malignant and nonmalignant cells obtained from the same individual. This has now been made possible by the development of a method for performing fluorescence in situ hybridization (FISH) with assessment of the cells by flow cytometry (flow-FISH) to yield telomere length measurements for defined populations.52 In the present study, this methodology was used to measure telomere length in Ph+ and Ph− cells from peripheral blood and bone marrow taken from patients with CML. The findings were correlated with disease stage and Ph+ status of the hematopoietic progenitor cell compartment and also compared to age-adjusted telomere lengths for cells from a large number of normal adults.
Materials and methods
CML patient characteristics
In total, 174 samples of previously frozen peripheral blood or bone marrow mononuclear cells, fresh peripheral blood leukocytes (PBL), or in vitro expanded T lymphocytes from 59 patients were analyzed. The samples were obtained from patients in CP (n = 96), in accelerated phase (AP) or BP (n = 15), and in cytogenetic remission (CR) after autologous BMT or treatment with interferon-α (CR, n = 12). Patients undergoing allogeneic BMT were excluded from the study. The median age of the patients at the time the sample was taken was 41 years (range, 19-76 years).
Cells
Heparinized blood and bone marrow samples were obtained from normal donors or as part of the routine assessment of patients with Ph+/BCR-ABL+ CML. When fresh cells were analyzed directly, red blood cells were lysed by incubating the pelleted cells with ammonium chloride solution (StemCell Technologies, Vancouver, BC) in a 1:4 ratio for 10 minutes on ice and the nucleated cells were then recovered by centrifugation. Cells that were obtained from cryopreserved material had been first centrifuged on Ficoll-Hypaque density centrifugation (< 1.077g/mL, Pharmacia Biotech AB, Uppsala, Sweden) to isolate the low-density fraction and then frozen in 10% dimethyl sulfoxide (DMSO) and 90% fetal calf serum (FCS; StemCell Technologies). After thawing, cells were slowly resuspended by adding Iscove's modified Dulbecco's medium (IMDM; StemCell Technologies) supplemented with 20% FCS and 100 μg/mL deoxyribonuclease (DNAse, Sigma Chemical, St. Louis, Mo). Dead cells were removed by another Ficoll-Hypaque centrifugation step. Recovered cells were washed twice in IMDM/2% FCS.
FACS analysis
Cell populations were incubated for 10 minutes at 4°C with Hanks' balanced salt solution (HBSS, StemCell Technologies) containing 5% human serum prior to addition of antihuman CD3-fluorescein isothiocyante (FITC) 1:50 (Becton Dickinson, San Jose, Calif). Cells were incubated at 4°C for another 30 minutes and finally washed twice with HBSS/2% FCS; 1 μg/mL propidium iodide (PI, Sigma) was added to the second wash to allow discrimination of dead (PI+) cells before analysis. Acquisition and analysis of the cells were performed on a FACSort using LYSIS II software (Becton Dickinson).
Isolation of CD3+ cells
The CD3+ cells were either first separated from myeloid cells by immunomagnetic negative selection on a column (StemSep, StemCell Technologies) or were directly expanded from either low-density or whole blood leukocyte preparations without their pre-enrichment. Monoclonal antibodies against CD36, CD34, IgE, CD66b, and CD66e were added to the standard StemSep T-cell enrichment cocktail to improve the purity of negatively selected CD3+ cells. After the depletion step, the CD3+-enriched cells were cultured in RPMI 1640 medium (GIBCO, Grand Island, NY) containing 10% human serum and 1.0 μg/mL phytohemagglutinin (PHA, Murex Diagnostics, Schaffausen, Switzerland) and 100 U/mL recombinant human interleukin-2 (rhIL-2; Roche, Nutley, NJ). In some cases, 106/mL irradiated allogeneic low-density normal blood cells were added as feeders as well. After 10 to 15 days of incubation, these cultures typically yielded sufficient numbers of T cells for analysis of telomere length.
Flow cytometric detection of telomere FISH (flow-FISH)
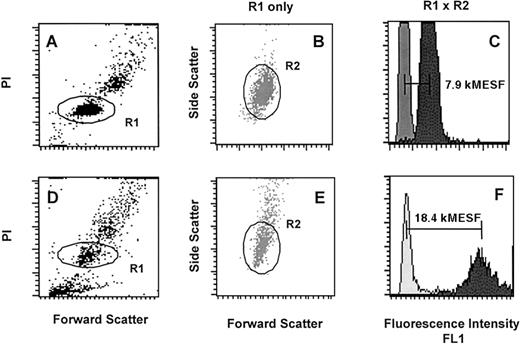
Telomere length measurements of PBL and selectively expanded T lymphocytes were performed by flow-FISH as previously described.52 Analysis of PBL (Figure1A-C) and T lymphocytes (Figure 1D-F) of 1 individual CML patient is shown. Daily shifts in the linearity of the flow cytometer and fluctuations in the laser intensity and alignment were compensated using FITC-labeled fluorescent beads (Quantum-24 Premixed; Flow Cytometry Standards, San Juan, Puerto Rico). Green fluorescence (FL1) was measured on a linear scale and results were expressed in molecular equivalents of soluble fluorochrome units (kMESF).53 After gating on single diploid cells (R1, see Figure 1A and D), specific telomere fluorescence was calculated by subtracting the mean fluorescence of the background control (no probe, see Figure 1C and F, light gray) from the mean fluorescence obtained from cells hybridized with the telomere probe (see Figure 1C and F, dark gray, difference indicated by horizontal bars). In previous studies, we have shown that telomere fluorescence of PBL and T lymphocytes is directly proportional to the mean size of terminal restriction fragments measured by Southern blot analysis.54
Flow-FISH analysis of PBL and T lymphocytes from a patient with CML.
Nucleated cells after lysis of red blood cells (A-C) as well as cultured T lymphocytes (D-E) were analyzed following hybridization with or without FITC-(C3TA2)3 PNA (respectively dark gray and light gray histograms in C and F). The cells were gated on region 1 (R1) on the basis of propidium iodide (PI) fluorescence and forward light scatter (FSC) as is shown in A and D. An additional region 2 (R2) was selected within R1 from FSC versus side scatter (SCC) dot plot histograms as is shown in B and E. Note that the telomere fluorescence signal of lymphocytes (F, dark gray) is almost 2 times higher and more heterogeneous than that of the total blood leukocytes (C, dark gray) reflecting longer telomere length and a more diverse replicative history.52 54
Flow-FISH analysis of PBL and T lymphocytes from a patient with CML.
Nucleated cells after lysis of red blood cells (A-C) as well as cultured T lymphocytes (D-E) were analyzed following hybridization with or without FITC-(C3TA2)3 PNA (respectively dark gray and light gray histograms in C and F). The cells were gated on region 1 (R1) on the basis of propidium iodide (PI) fluorescence and forward light scatter (FSC) as is shown in A and D. An additional region 2 (R2) was selected within R1 from FSC versus side scatter (SCC) dot plot histograms as is shown in B and E. Note that the telomere fluorescence signal of lymphocytes (F, dark gray) is almost 2 times higher and more heterogeneous than that of the total blood leukocytes (C, dark gray) reflecting longer telomere length and a more diverse replicative history.52 54
To analyze the day-to-day variation in flow-FISH results, aliquots of cells derived from a primary non-Hodgkin's lymphoma were analyzed in each experiment over the 1-year period of the studies described in this paper. In 48 experiments performed over the last 2 years (in part reported already in Rufer et al52), the mean (± SD) fluorescence value was 12.1 ± 1.7 k(kilo) MESF with a C.V. of 14%.
LTC-IC assay
Cells were seeded onto pre-established irradiated murine fibroblasts genetically engineered to produce human IL-3 (10 ng/mL), human granulocyte colony-stimulating factor (G-CSF; 130 ng/mL), and human Steel factor (10 ng/mL) in long-term culture medium (Myelocult, StemCell Technologies) supplemented with freshly dissolved 10−6 mol/L hydrocortisone sodium hemisuccinate (Sigma). These cultures were then maintained in a humidified atmosphere of 37°C, 5% CO2 in air for 6 weeks with weekly exchanges of half the medium and removal of half the nonadherent cells as described.55 At the end of 6 weeks, the nonadherent cells were combined with the trypsinized adherent cells and assayed for their content of granulopoietic, erythroid, and multilineage colony-forming cells in methylcellulose medium (H4330, StemCell Technologies) supplemented with 50 ng/mL Steel factor, 20 ng/mL IL-3 (Novartis, Basel, Switzerland), 20 ng/mL IL-6 (Cangene, Mississauga, ON, Canada), 20 ng/mL granulocyte-macrophage colony-stimulating factor (Novartis), 20 ng/mL G-CSF (StemCell Technologies) and 3 U/mL erythropoietin (StemCell Technologies). These cultures were then incubated for 12 to 16 days at 37°C at the end of which colonies were scored in situ using standard criteria.55
Cytogenetic analysis
Colonies generated from 6-week-old LTC-IC assays and in vitro expanded CD3+ T cells were genotyped as Ph+ or Ph− by cytogenetic analysis of Giemsa-banded metaphases.56
Statistical analysis
Fluorescence values in kMESF units for different cell types, donor age, disease stage (CP, AP/BP), Ph status of the patients' LTC-IC, disease duration since diagnosis, and the remaining duration of CP from the time point the sample was taken were the parameters used for statistical analysis of the data. Controls were taken from a previous study performed in this center on 301 normal individuals.52Age adjustments were made based on the linear regression analysis for total PBL values obtained from a subpopulation of this cohort, aged 16 to 80 years (n = 147). Telomere data of different patient groups consisted of generally mixed cross-sectional and serial measurements. To counteract any weighting effect of using multiple specimens from single patients in the analyses and to identify within-patient, across-patient, and between-group levels of variation, multilevel maximum likelihood analyses were carried out using the MLn program of the University of London (MLn, Multilevel Models Project, University of London, 1998). Differences of paired data within patients were also analyzed by the MLn method.
Results
Initial comparison of telomere fluorescence measurements in blood or marrow cells from patients with CML and healthy controls
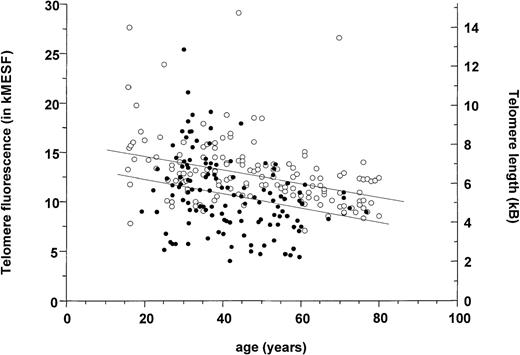
Flow-FISH measurements of telomere fluorescence were obtained on 123 unseparated or low-density blood or marrow samples from 59 patients (solid circles, Figure 2). Considerable variation between values even when plotted as a function of the patient's age is seen. Also shown in Figure 2 for comparison are results for total fresh blood leukocytes taken from a study on a large series of normal individuals assessed concurrently in our center using the same methodology.52 Much of the variation seen in telomere values at any given age in normal individuals appears to be genetically determined as indicated by an analysis of twins in this previous study. Nevertheless, as summarized in Table1, the average age-adjusted telomere length determined for CML cells (11.0 ± 0.3 kMESF) proved to be significantly (P < .001) lower than the corresponding value for normal cells from age-adjusted donors (13.2 ± 0.3 kMESF). In CML, a number of factors are expected to exacerbate the normal variation in telomere length. These include effects due to the stage of the disease as well as other factors including the proportion of normal cells in the sample. Indeed (normal) T lymphocytes may have been slightly enriched in some of the CML samples due to a greater loss of (Ph+) myeloid cells on thawing. Accordingly, a comparison was made between paired telomere length measurements for CML cells and purified normal T cells from the same patient.
Loss of telomere fluorescence in total blood leukocytes from patients with CML (closed circles) measured by flow-FISH.
Data for control individuals (open circles) is taken from a cohort reported on previously.52 The specific telomere fluorescence was analyzed after gating on diploid cells as shown in Figure 1A. Note the heterogeneity in telomere fluorescence values in CML cells as well as in controls, the significantly lower average telomere length found in CML samples as shown by linear regression analysis (P < .001), and the overall decline in telomere fluorescence in both populations.
Loss of telomere fluorescence in total blood leukocytes from patients with CML (closed circles) measured by flow-FISH.
Data for control individuals (open circles) is taken from a cohort reported on previously.52 The specific telomere fluorescence was analyzed after gating on diploid cells as shown in Figure 1A. Note the heterogeneity in telomere fluorescence values in CML cells as well as in controls, the significantly lower average telomere length found in CML samples as shown by linear regression analysis (P < .001), and the overall decline in telomere fluorescence in both populations.
Statistical subgroup analysis
| Telomere fluorescence in kMESF . | N . | p . | Estimated mean . | SE . | Range . | Estimated mean shift from normal . | SE . | P Value . |
|---|---|---|---|---|---|---|---|---|
| PBLs of patients with CML | ||||||||
| total | 123 | 59 | 11.0 | 0.3 | 5.0-25.4 | −2.1 | 0.3 | P < .001 |
| in CP | 96 | 53 | 11.3 | 0.4 | 4.4-25.4 | −1.9 | 0.4 | P < .001 |
| in AP/BC | 15 | 12 | 9.5 | 0.7 | 5.1-13.9 | −4.1 | 0.8 | P < .001 |
| in CR | 12 | 8 | 11.4 | 0.6 | 7.9-15.7 | −1.8 | 0.5 | P = .016 |
| All clinical stages | ||||||||
| PBLs vs | 51 | 42 | 10.3 | 0.4 | 5.4-17.1 | P < .001 | ||
| Ph− T lymphocytes | 51 | 42 | 15.0 | 0.4 | 8.0-20.7 | |||
| CP samples with | ||||||||
| rdCP <2 y vs | 27 | 15 | 10.9 | 0.8 | 5.1-21.1 | P = .046 | ||
| rdCP ≥2 y | 49 | 11 | 12.6 | 0.5 | 5.9-25.4 | |||
| Samples with | ||||||||
| Ph+LTC-ICs* vs | 15 | 12 | 11.2 | 1.1 | ||||
| Ph−LTC-ICs | 16 | 11 | 12.6 | 1.0 |
| Telomere fluorescence in kMESF . | N . | p . | Estimated mean . | SE . | Range . | Estimated mean shift from normal . | SE . | P Value . |
|---|---|---|---|---|---|---|---|---|
| PBLs of patients with CML | ||||||||
| total | 123 | 59 | 11.0 | 0.3 | 5.0-25.4 | −2.1 | 0.3 | P < .001 |
| in CP | 96 | 53 | 11.3 | 0.4 | 4.4-25.4 | −1.9 | 0.4 | P < .001 |
| in AP/BC | 15 | 12 | 9.5 | 0.7 | 5.1-13.9 | −4.1 | 0.8 | P < .001 |
| in CR | 12 | 8 | 11.4 | 0.6 | 7.9-15.7 | −1.8 | 0.5 | P = .016 |
| All clinical stages | ||||||||
| PBLs vs | 51 | 42 | 10.3 | 0.4 | 5.4-17.1 | P < .001 | ||
| Ph− T lymphocytes | 51 | 42 | 15.0 | 0.4 | 8.0-20.7 | |||
| CP samples with | ||||||||
| rdCP <2 y vs | 27 | 15 | 10.9 | 0.8 | 5.1-21.1 | P = .046 | ||
| rdCP ≥2 y | 49 | 11 | 12.6 | 0.5 | 5.9-25.4 | |||
| Samples with | ||||||||
| Ph+LTC-ICs* vs | 15 | 12 | 11.2 | 1.1 | ||||
| Ph−LTC-ICs | 16 | 11 | 12.6 | 1.0 |
Samples with Ph+ LTC-IC were defined as those in which ≥4 colonies from the LTC-IC assays were analyzed cytogenetically of which ≥50% were Ph+. All remaining samples analyzed were considered Ph−.
AP/BC indicates accelerated phase/blast phase; CML, chronic myeloid leukemia; CP, chronic phase; CR, cytogenic remission; LTC-IC, long-term culture-initiating assays; kMESF, kilo molecular equivalents of soluble fluorochrome units; N, number of samples analyzed in each group; p, number of patients; PBL, peripheral blood leukocytes; rdCP, remaining duration of CP; comparison of mixed populations of cross-sectional and sequential samples was performed as indicated in “Materials and methods.”
Comparison of paired telomere fluorescence in CML cells and normal T lymphocytes from the same patients
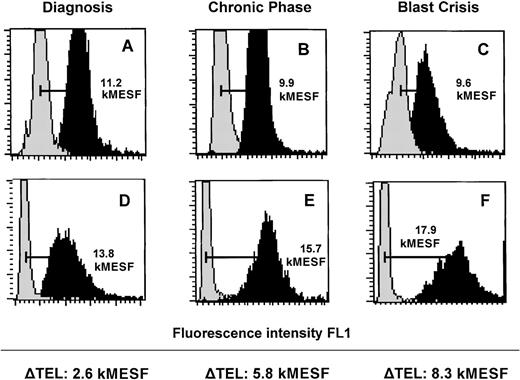
In healthy individuals, granulocytes can be readily separated from lymphocytes on the basis of their different light scattering properties.However, in CML samples, such a separation is hampered due to an abundance of immature myeloid cells (see Figure 1B). Therefore, to obtain a suitably enriched population of normal cells for use as an internal control, we selectively enriched CD3+ T lymphocytes and expanded these in vitro using rhIL-2 and PHA as described in “Materials and Methods.” Using this approach, we obtained CD3+ populations of > 90% purity (after 8-10 days) that were cytogenetically Ph− in all 28 cases analyzed. These T lymphocytes were analyzed by flow-FISH when > 106 cells had been generated in culture. Examples of flow-FISH analysis of paired unseparated (mostly myeloid) cells and expanded normal T cells from the peripheral blood or bone marrow of representative CML patients examined at diagnosis (in CP), several years after diagnosis but still in CP, and in BP are shown in Figure3. As indicated in Table 1, telomere fluorescence in CD3+ cells (15.0 ± 0.4 kMESF, n = 51 from 42 patients) was significantly (P < .001) higher than in total leukocytes from the same individual patients (10.3 ± 0.4 kMESF). No significant difference in telomere length between lymphocytes and granulocytes from the blood of age-matched healthy controls was observed in this particular age range (data not shown).
Flow-FISH analysis of leukocytes and cultured T-lymphocytes from 3 representative individual patients with CML at different stages of the disease.
Total nucleated cells from peripheral blood (A and C) and bone marrow (B) as well as cultured T lymphocytes from the same specimen in each case (D-F) were analyzed with or without FITC-(C3TA2)3 PNA (respectively dark gray and light gray histograms). Total leukocytes (A-C) show shorter telomeres than the respective cultured T lymphocytes (D-F).
Flow-FISH analysis of leukocytes and cultured T-lymphocytes from 3 representative individual patients with CML at different stages of the disease.
Total nucleated cells from peripheral blood (A and C) and bone marrow (B) as well as cultured T lymphocytes from the same specimen in each case (D-F) were analyzed with or without FITC-(C3TA2)3 PNA (respectively dark gray and light gray histograms). Total leukocytes (A-C) show shorter telomeres than the respective cultured T lymphocytes (D-F).
Telomere fluorescence of CML cells from patients at different stages of disease
We next investigated whether any significant changes in telomere length of CML cells could be correlated with the clinical stage of the disease at the time the sample was obtained. Telomere fluorescence in cells obtained from patients in AP/BP was significantly lower than in cells from patients in CP (P = .02) and also significantly lower than in cells from patients in CR (P = .03). Compared to samples obtained in AP (8.6 ± 0.8 kMESF, n = 8), telomere fluorescence tended to be higher in samples obtained in BP (10.5 ± 1.1 kMESF, n = 7); however, this difference did not reach statistical significance.
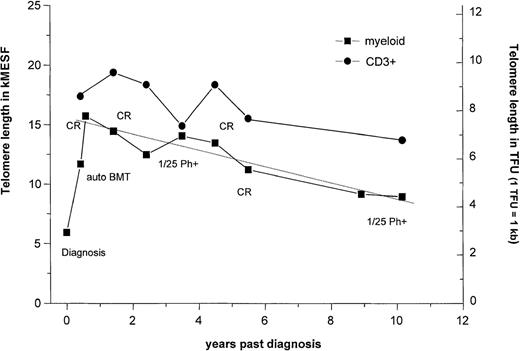
We also investigated the possibility of a correlation between telomere length in CP and remaining duration of CP (rdCP) before onset of either AP or BP disease. Because a substantial proportion of the analyses had been performed on fresh samples from recently diagnosed patients, information on the rdCP was only available for 76 samples derived from 22 patients. Of these 76, the rdCP was less than 2 years for 27 samples (from 15 patients), whereas for the other 49 samples (from 11 patients), the rdCP was more than 2 years. Some samples from the same patient fell in either group. Samples with the shorter rdCP showed significantly (P < .05) lower telomere fluorescence (10.9 ± 0.8 kMESF) compared to patients with rdCP longer then 2 years (12.6 ± 0.5 kMESF). A number of samples that had been obtained from patients before and after treatment at our center with culture-purged autologous BMT57 were also included in the present study. Some of these patients achieved long-lasting cytogenetic remissions after BMT either with or without α-interferon maintenance (M.J. Barnett, unpublished data, December 1999). Thus, the patients in the CR group represent a bias toward patients with long disease duration (average time after diagnosis, 6.7 years compared to 2.9 years for the whole data set). However, the availability of sequential samples from selected patients of this cohort for longitudinal studies allowed problems related to the variation in telomere length between individuals to be minimized. Figure 4 shows the results of a series of telomere length measurements performed on 1 example of such a patient (UPN 399 reported in Barnet et al57) who received culture-purged autologous BMT soon after diagnosis. A long-lasting cytogenetic remission (> 9 years) was achieved starting 1 month after autologous BMT. This patient developed cytogenetically detectable Ph+ cells 3 years after BMT (1/25 metaphases Ph+), but these became undetectable after treatment with low-dose interferon-α. As can be seen, reduction of Ph+ cells in this patient by the transplant procedure and their replacement by predominantly normal (Ph−) hematopoiesis was accompanied by an increase in telomere fluorescence from 11.7 kMESF (before BMT) to 15.7 kMESF. The latter value is in the normal range for blood cells of age-adjusted healthy individuals. Following the return of normal hematopoiesis in this patient, telomere fluorescence of the low-density blood cells steadily decreased at a rate of 0.72 kMESF/y (corresponding to 340 bp/y) as shown by linear regression analysis (R = 0.95, P = .0004). For comparison, telomere length in expanded T lymphocytes from this patient measured in parallel in 7 of 10 samples did not decline significantly over the time period investigated after BMT.
Telomere length dynamics in a single patient who received a transplant of culture-purged marrow.
Telomere length measurements in thawed low-density cells from bone marrow or peripheral blood (squares) and from ex vivo expanded T cells (circles) are shown. The average rate of telomere shortening after transplantation was calculated by linear regression analysis and the slopes expressed in base pairs per year. Note that the patient showed an increase in telomere length after transplantation followed by a decline thereafter and that T lymphocytes always showed longer telomeres and seemed to decline at a slightly slower rate than the PBL in the 10 years after transplantation.
Telomere length dynamics in a single patient who received a transplant of culture-purged marrow.
Telomere length measurements in thawed low-density cells from bone marrow or peripheral blood (squares) and from ex vivo expanded T cells (circles) are shown. The average rate of telomere shortening after transplantation was calculated by linear regression analysis and the slopes expressed in base pairs per year. Note that the patient showed an increase in telomere length after transplantation followed by a decline thereafter and that T lymphocytes always showed longer telomeres and seemed to decline at a slightly slower rate than the PBL in the 10 years after transplantation.
Correlation between telomere shortening and predominance of Ph+ LTC-IC
It has previously been demonstrated that Ph−LTC-IC are usually readily detectable in patients with CML. However, in a small proportion of patients, this early progenitor compartment is dominated by Ph+ cells even at the time of diagnosis.8 58 To determine whether this biologic difference might correlate with telomere length measured on more mature progeny, patients were segregated into 2 groups based on the Ph status of their LTC-IC. For the 33 samples on which cytogenetic data were available for analysis, 15 were in the Ph+ LTC-IC group and 16 were in the Ph− LTC-IC group. In this analysis, cells from patients with predominantly Ph+ LTC-IC tended to have shorter telomeres (11.2 ± 1.1 kMESF) than those with predominantly Ph− LTC-ICs (12.6 ± 1.0 kMESF). However, this difference did not reach statistical significance.
Discussion
Telomere length differences indicate enhanced stem cell turnover of Ph+ HSC in patients with CML
According to prevalent models of hematopoiesis, mature peripheral blood cells are derived from rapidly dividing progenitor cells that, in turn, are derived from HSCs. In adults, the number of cell divisions that separate the bulk of the mature blood cells from the HSC population is assumed to be roughly constant unless the system is perturbed. Thus, considering the relatively short half-life of mature cells, the average length of their telomeres should reflect that of the HSC a limited number of cell generations earlier.52 The present investigations of telomere length in leukocytes from a large series of patients with CML have shown that in CP CML cells, telomere length was approximately 1 kb shorter than in age-matched controls. Assuming that roughly 100 bp (50-200 bp) are lost per cell division in somatic cells,28,39 the reduced telomere length in CP CML cells would indicate that their leukemic stem cells would have undergone on average 10 more divisions than normal HSC during the development of the clone before diagnosis. With disease progression telomere length progressively shortens leading to a significantly increased difference of 2 kb between cells from samples acquired in AP or BP compared to age-adjusted controls. Further evidence of an increased rate of turnover of the Ph+ stem cell population is provided by the finding that the mature Ph+ cells showed significantly shorter telomeres than ex vivo expanded Ph− T lymphocytes. In a previous study, we showed that the average telomere length in (naive) T lymphocytes and granulocytes of healthy individuals of approximately the same age range (16-80 years) is similar.52
We and others have previously shown that committed Ph+progenitors are more actively cycling than their counterparts in normal individuals (reviewed in Eaves59) and more recently have extended this observation to the Ph+ LTC-IC compartment.13 However, such differences would predict a much faster rate of turnover of the leukemic stem cell compartment than indicated by the telomere length measurement described here, assuming that the average interval over which the neoplastic clone develops before diagnosis is several years.60 This apparent paradox, however, may be reconciled by our demonstration in CP CML patients of a quiescent subset of CML cells61 and a decreased probability of self-renewal by those stimulated to proliferate.9 16
An increase in telomere length after recovery from autografting was demonstrated in 1 patient with CML who was followed after having received a purged autologous BMT. This was not unexpected given the predominantly normal genotype of the hematopoietic cells regenerated and maintained for several years after transplant in this individual. However, the rate of telomere shortening during this period averaged 340 bp/y, which is 8-fold faster than the rate we have measured for normal blood cells (42 bp/y) in a cross-sectional study in healthy individuals.52 Data on allogeneic BMT recipients suggest a fixed loss of telomere repeats after regeneration has occurred in the recipient as compared to the donor, but no accelerated rate of shortening thereafter.46 47 The patient described here showed an increased rate of telomere shortening compared to healthy donors throughout the whole period examined after BMT. This observation could reflect a low number of normal stem cells contributing to hematopoiesis following autotransplantation. Because, in our study, the majority of samples in Ph− CR were derived from patients after autologous BMT, the telomere length deficit in such cells relative to age-matched healthy controls suggests that continuing accelerated telomere shortening in residual normal cells following autotransplant may be a more general phenomenon. Additional studies will be of interest to test this prediction.
Telomere length in CML cells correlates with disease evolution
A major purpose of this study was to investigate whether telomere length measurements of the neoplastic cells from patients with CML using the recently developed flow-FISH method might have prognostic importance. Three different approaches to address this question were used in this study. First, we compared the telomere length of cells taken from patients at different stages in their disease. Average telomere fluorescence was highest in patients in Ph−CR and in CP followed by significantly lower telomere fluorescence in AP/BP samples. This finding is in line with recent data showing telomere shortening in all 12 patients who were serially studied in CP and BP48 as well as in another cross-sectional study comparing telomere length in CP versus BP,49 both of which used Southern blot analysis. Secondly, we compared the telomere length in CP samples with a remaining duration of CP of less than 2 years with those in samples from patients with a remaining duration of CP of more than 2 years. A significant difference between the 2 groups was found (P < .05). Thirdly, we compared telomere length in samples from patients grouped according to the prevalence of leukemic or normal elements in their LTC-IC population. At the time of diagnosis most CML patients have predominantly normal LTC-IC.8,13 62 However, in a proportion of CP patients, Ph+ LTC-IC predominate. This analysis showed that the samples with predominantly Ph+ LTC-IC tended to have shorter telomeres than samples with Ph− LTC-IC. However, the sample size in this particular subanalysis was too small to draw definitive conclusions. In summary, these findings support the hypothesis that progressive telomere shortening is correlated with disease progression in CPCML.
It had been reported that CML patients with long telomeres also respond better to treatment with interferon-α and it was suggested that these patients might be at an “earlier” stage of the disease.50 Similarly, patients with short telomeres seemed to have higher levels of telomerase expression than patients with long telomeres,50 and high levels of telomerase expression were correlated with a high frequency of additional cytogenetic abnormalities.49 Furthermore, levels of telomerase expression seemed to be normal40 or only slightly elevated49 in CP samples. However, a significant increase in telomerase expression was found in samples that were obtained during BP. Taken together, these findings support an association between progressive telomere shortening in CP and up-regulation of telomerase activity or a relative increase in cells expressing telomerase occurring late in disease evolution of CML.
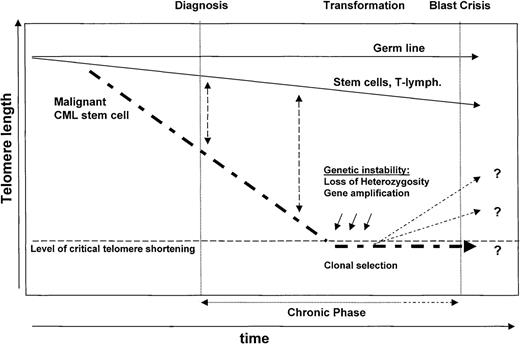
Model of telomere biology in CML
Based on the data reported so far by other groups and the results presented here, we propose the following model of telomere biology in CML (see also Figure 5). An increased rate of turnover in the leukemic HSC clone will result in a shorter telomere length of Ph+ compared to polyclonal Ph−stem cells at diagnosis. Similar to normal HSCs, the level of telomerase expression in Ph+ stem cells is apparently unable to prevent replication-dependent telomere shortening at this stage of the disease. During CP and with continued proliferation of the Ph+ stem cells, the differences in telomere length as compared to normal cells are expected to become more pronounced. Eventually, the telomeres in the cells of the neoplastic clone become critically short and this may precipitate progression of the disease to AP/BP. Genetic instability is suspected to be of major importance in the progression of many tumors,63 and the acquisition of additional cytogenetic alterations is a hallmark of AP/BP CML. Mechanisms underlying genetic instability, like gene amplification, aneuploidy, and loss of heterozygosity, may directly result from telomeric associations arising from cells with short telomeres,29,64,65 thus linking telomere shortening with genetic instability.33 One mechanism by which late CP CML cells might escape replicative senescence is through activation or up-regulation of telomerase. Because telomerase is expressed at low levels in normal hematopoietic cells,42 a separate genetic event may not be required for selection of cells expressing high levels. Telomerase-positive subclones might either re-elongate their telomeres or continue to grow without a net lengthening of their telomeres.66 The selective growth advantage of Ph+ cells with increased levels of telomerase following selection of cells mediated by telomere-mediated genetic instability would thus be expected to result in CML BP.
Model of telomere biology in CML.
Note that telomeres are shorter in Ph+ cells as compared to Ph− normal cells at diagnosis compatible with a greater number of accumulated cell divisions in the Ph+stem cell clone. During CP, and with continued proliferation of the Ph+ stem cells, the differences in telomere length between Ph+ and Ph− cells are expected to become more pronounced. Eventually, the telomeres in the cells of the neoplastic clone become critically short. The resulting chromosomal instability may facilitate progression of the disease to AP/BP. Up-regulation of telomerase at this stage could eliminate telomere-related restrictions in cellular proliferation and is expected to prevent further telomere-mediated genetic instability.
Model of telomere biology in CML.
Note that telomeres are shorter in Ph+ cells as compared to Ph− normal cells at diagnosis compatible with a greater number of accumulated cell divisions in the Ph+stem cell clone. During CP, and with continued proliferation of the Ph+ stem cells, the differences in telomere length between Ph+ and Ph− cells are expected to become more pronounced. Eventually, the telomeres in the cells of the neoplastic clone become critically short. The resulting chromosomal instability may facilitate progression of the disease to AP/BP. Up-regulation of telomerase at this stage could eliminate telomere-related restrictions in cellular proliferation and is expected to prevent further telomere-mediated genetic instability.
This model suggests that telomere length measurements in normal Ph− and malignant Ph+ cells from patients with CML should provide prognostic information relevant to the clinical management of individual patients. Further assessment of this approach awaits validation in association with a prospective clinical trial. The present studies establish the feasibility and utility of flow-FISH as a suitable methodology for this purpose because repeated quantitative measurements of normal and Ph+ cells from the same patient can be reproducibly collected.
Acknowledgments
Gloria Shaw and Elizabeth Chavez are thanked for cytogenetic analysis, Edwin Mak for help with the statistical analysis of the data, Jennifer Mak and Pamela Austin for technical assistance, Karen Lambie for help with retrieval of patient samples, Daphne Brockington for collection of patient information, and Colleen MacKinnon for editorial work on the manuscript. The authors also thank Cangene, Novartis, and StemCell Technologies for valuable gifts of reagents.
Supported by grants AI29524 and GM56162 from the National Institutes of Health and by a grant from the National Cancer Institute of Canada with funds from the Terry Fox Run. T.H.B. is funded by a grant from the Deutsche Forschungsgemeinschaft. T.L.H. holds a Senior Lecturership from the United Kingdom Leukemia Research Foundation. N.R. is a recipient of a fellowship from the Fonds National Suisse. C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
Reprints:Peter M. Lansdorp, Terry Fox Laboratory, BC Cancer Agency, 601 West 10th Ave, Vancouver, British Columbia, V5Z 1L3, Canada; e-mail: plansdor@bccancer.bc.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal