Abstract
Fc-receptors, such as FcR and FcγRII, play an important role in leukocyte activation, and rapid modulation of ligand binding (“activation”) is critical for receptor regulation. We have previously demonstrated that ligand binding to Fc-receptors on human eosinophils is dependent on cytokine stimulation. Utilization of pharmacological inhibitors provided evidence that the phenomenon of interleukin (IL)-5 induced immunoglobulin A (IgA) binding to human eosinophils requires activation of phosphatidylinositol 3-kinase (PI3K). However, eosinophils are refractory to manipulation by molecular techniques such as DNA transfection or viral infection. Here we utilize an IL-3 dependent pre-B cell line to investigate the molecular mechanism of cytokine-mediated ligand binding to FcR. In this system, IgA binding is dependent on IL-3, similarly to the requirement for IL-5 of eosinophils. We show that IL-3-mediated activation of FcR (CD89) requires the activation of PI3K, independent of p21ras activation. Co-expression of dominant negative (▵p85) and active (p110_K227E) forms of PI3K demonstrate that the affinity switch regulating FcR activation requires PI3K. Moreover, overexpression of PI3K is both necessary and sufficient for activation of FcR. Furthermore, we show that IL-3/IL-5/GM-CSF induced inside-out signaling pathways activating FcR require the involvement of protein kinase C downstream of PI3K. Finally, we show that these inside-out signaling pathways responsible for Fc-receptor modulation require CD89, independent of its association with the FcRγ chain.
Transmembrane receptors specific for the Fc-region of immunoglobulins, Fc-receptors (FcRs), play an important role in leukocyte activation by the recognition and binding of opsonized targets during inflammatory processes.1 Interaction of FcRs on effector cells with immunoglobulins present on opsonized particles triggers a variety of processes, including phagocytosis, superoxide generation, antibody-dependent cytotoxicity, and release of inflammatory mediators and cytokines.2 FcR function has also been demonstrated to be involved in a variety of immune disorders.3 4 For example, engagement of functional FcRs on phagocytes triggers the destruction of autologous erythrocytes or platelets in the presence of auto-antibodies directed against these cells.
FcRs exist for all five classes of human immunoglobulins. The best-studied FcRs are the leukocyte receptors for immunoglobulin G (IgG) (FcγR) and IgE (FcεR), due to early isolation of their genes and the availability of anti-FcR antibodies.2,5,6Relatively little is known about the receptors for IgA (FcαR/CD89), despite the fact that IgA is the most abundant human immunoglobulin isotype.5 IgA appears to play a critical role in protecting the host against environmental pathogens and antigens encountered at mucosal surfaces. Failure to clear IgA complexes has been proposed to lead to their deposition in the kidney where they are associated with inflammation and chronic tissue damage.6 Although FcαR has been described to be expressed on many cell types, including monocytes/macrophages, neutrophils, and eosinophils,7-9 at present little is known concerning the activation of FcαR and its functioning.
We have previously demonstrated that activation of the FcRs for IgA (FcαR) and IgG (FcγRII) on primary human eosinophils is regulated by Th2-derived cytokines, such as interleukin (IL)-4 and IL-5.10,11 Cytokine stimulation leads to an increase in ligand binding without changing the levels of receptor expression, suggesting that stimulation with cytokines regulates either the affinity or avidity of FcRs.10-12 Utilization of specific pharmacological inhibitors led to the suggestion that selective regulation of either FcαR or FcγRII on eosinophils is dependent on cytokine-induced activation of distinct signal transduction pathways.13 Because human eosinophils are refractory to manipulation by molecular techniques such as DNA transfection and viral infection, utilization of a model system was required to further analyze the signal transduction pathways involved in FcαR activation. Therefore, we have studied the regulation of the human FcR for IgA (FcαR/CD89) by cytokines in a murine pre-B (Ba/F3) model system. In addition to utilizing specific pharmacological inhibitors, receptor mutants and dominant negative and active mutants of critical signaling components could be analyzed in this model system. By using Ba/F3_FcαR cells, we studied the involvement of various signaling pathways in FcαR activation as suggested for eosinophils. Here we show that activation of phosphatidylinositol 3-kinase (PI3K) is critical for IL-5/IL-3/GM-CSF induced FcαR activation. Furthermore, inhibition of downstream targets of PI3K suggests that PI3K exerts its role in FcαR modulation most likely through activation of protein kinase C (PKC).
Materials and methods
Reagents and antibodies
Purified human serum IgA (>20 mg/mL) was obtained from Cappel (Malvern, PA). It contained no detectable trace of IgG, IgM, or non-immunoglobulin serum proteins. Recombinant mouse IL-3 was produced in COS cells.14 For the detection of FcαR (CD89) with a FACS vantage flow cytometer, we used a specific PE-conjugated monoclonal antibody A59 (A59-PE, Pharmingen). Pharmacological inhibitors wortmannin, LY294002, PD98059, and SB203580 were purchased from BioMol (Plymouth Meeting, PA). LXSN-vectors containing Ras mutants (RasV12, RasN17, RasV12S35, and RasV12C40) and PI3K constructs (p110-K227E and Δp85) were a kind gift of Dr J. Downward (ICRF, London, UK).
FcR constructs
FcαRwt was cloned into a pMT2 vector, containing a VSV-epitope tag. Human FcαR (in pSG513)15 was used as a template for polymerase chain reaction (PCR), using the following primers: FcαRwt (Fwt: GCTGTCAGCACGATGGAC and Rwt: TTCACCTCCAGGTGTTTA). A FcαR (R209D) mutant was constructed via a two-step PCR mutagenesis: primers sets Fwt/RR > D (GATCAAGTTCTGCGTCGT) and FR > D (CGCAGAACTTGATCGATATGGCCGTGGCAG)/Rwt were used for the first step. Subsequently, the products of this PCR reaction were annealed at 38°C, and a second PCR was performed with primers to Fwt/Rwt to obtain a full-length FcαRR209D.
Generation of stable transfectants
Ba/F3 cells were cultured at a cell density of 105-106 cells/mL in RPMI 1640 supplemented with 8% Hyclone serum (Gibco) and recombinant mouse IL-3. For the generation of polyclonal transfectants, pMT2_VSV containing FcαRwt or FcαRR209D were electroporated into Ba/F3 cells (0.28 V; capacitance 960 μFD) together with pSG5-CMV-Hygro containing the hygromycin resistance gene. Cells were cultured in the presence of IL-3 and selected in 500 μg/mL hygromycin (Boehringer Mannheim, Germany). After 2 weeks of selection, cells were tested for FcαR expression, and positive cells were sorted with a FACS vantage flow cytometer (Becton & Dickinson immunocytometry systems, Mountain View, CA). Briefly, FcαR transfected Ba/F3 cells were incubated with the PE-conjugated monoclonal antibody A59 (A59-PE) for 30 minutes at 4°C. Fluorescence of the cells was quantified with the flow cytometer, and A59-positive cells were sorted and cultured. Polyclonal cell lines were generated, expressing either FcαRwt or the substitution mutant R209D. Ba/F3_FcαRwt cells, expressing FcαRwt_VSV, were subsequently used for transfection of pLXSN (neo) containing mutants of H-Ras (RasV12, RasV12C40, RasN17)16or PI3K (p110_K227E, Δp85α)17 18 or for transfection of myc-PTEN (Dijkers et al, submitted). Cells were cultured with mouse IL-3 and 500 μg/mL G418 (Boehringer Mannheim, Germany) to select for resistance. Stable cell lines were grown continually on mIL3, G418, and Hygromycin. Expression of FcαR was checked regularly with the flow cytometer.
IgA-binding assays
IgA-binding assays were performed either with cytokine-starved Ba/F3 cells or with purified human eosinophils. For IL-3 starvation, Ba/F3 cells were washed twice with phosphate-buffered saline and left in medium (RPMI 1640 with 0.5% serum) without IL-3 for 4 hours. Prior to performing a binding assay, Ba/F3 cells or purified eosinophils were washed with Ca++-free incubation buffer containing 0.5 mmol/L ethylene glycol bis (β-amino ethylether) N, N, N′, N′-tetra acetic acid (EGTA) and brought to a concentration of 8 × 106 cells/mL. A cell suspension of 50 μL (0.4 × 106 cells) was incubated at 37°C, with or without cytokines. Ba/F3 cells were pre-incubated with IL-3 (1:1000; 15 minutes). Human eosinophils were stimulated with IL-5 with a final concentration of 10−9 mol/L. After stimulation of the cells, dynabeads coated with serum IgA (10 mg/mL) as described previously10 were added in a ratio of 3.5 beads/cell. After briefly mixing, the cells and beads were pelleted for 15 seconds at 100 rpm and incubated for 30 minutes at 37°C. After incubation, cells were resuspended vigorously, and IgA binding was evaluated under a microscope. All cells that had bound two beads or more were defined as rosettes. One hundred cells were scored, and the number of beads that were bound to the cells was counted. The amount of beads bound to a total of 100 cells (bound and unbound to beads) was designated as the rosette index.
Inhibition of IgA binding with pharmacological inhibitors or peptides
For inhibition studies, cytokine-starved cells were pre-incubated with specific inhibitors prior to incubation with IL-3. Cells were incubated with PI3K inhibitors, wortmannin, or LY294002 for 15 minutes at final concentrations of 20 nmol/L and 1 μmol/L, respectively. The p38 inhibitor SB203580 was incubated for 15 minutes at a concentration of 1 μmol/L, while incubation with the MEK inhibitor PD98059 was for 30 minutes at a concentration of 50 μmol/L. Rapamycin, the p70S6K inhibitor, was incubated for 10 minutes at a concentration of 20 ng/mL. PKC inhibitors GF109203X and Ro31-8220 were used at a concentration of 1 μmol/L for 10 minutes.
STAT5, PKB, ERK2, and p38 MAPK phosphorylation
Ba/F3 cells were washed twice with phosphate-buffered saline and left in IL-3-depleted medium (RPMI 1640 with 0.5% serum) for 4 hours. To investigate the effect of IL-3 stimulation on activation of STAT, MAPK, and PI3K pathways, cells were stimulated at 37°C, for a time course as indicated (0-30′). For detection of phosphorylation of STAT5, ERK, p38 MAPK, or PKB, Ba/F3 cells (0.2 × 106 per condition) were washed twice in ice-cold phosphate-buffered saline after stimulation and lyzed in lysis buffer (1% Triton-X100, 50 mmol/L Tris-Cl, pH 8.0, 100 mmol/L NaCl) with phosphatase inhibitors. Subsequently, 5x Laemmli sample buffer was added, and the lysates were boiled for 5 minutes. Total cell lysates were analyzed on 15% SDS-polyacrylamide gels. Proteins were transferred to Immobilon-P and incubated with blocking buffer (Tris Buffered Saline/Tween 20 supplemented with 1 mmol/L EDTA and 0.6% bovine serum albumin) with either polyclonal phospho-STAT5 (Tyr694), phospho-p38 MAPK (Thr180/182), phospho-ERK1/2, or phospho-PKB (Ser473) antisera (NEB). Detection was with ECL (Amersham, UK).
Results
Murine Ba/F3 cells as a model for FcR regulation
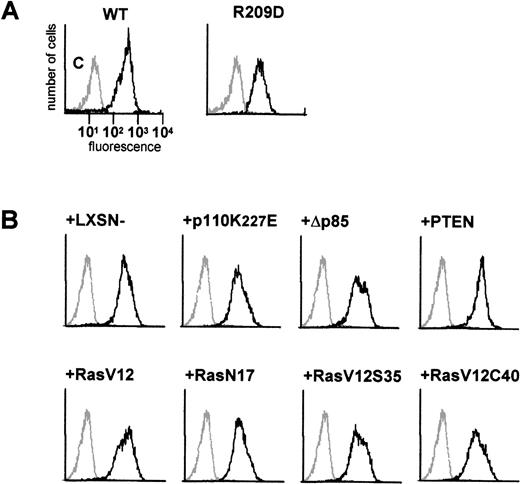
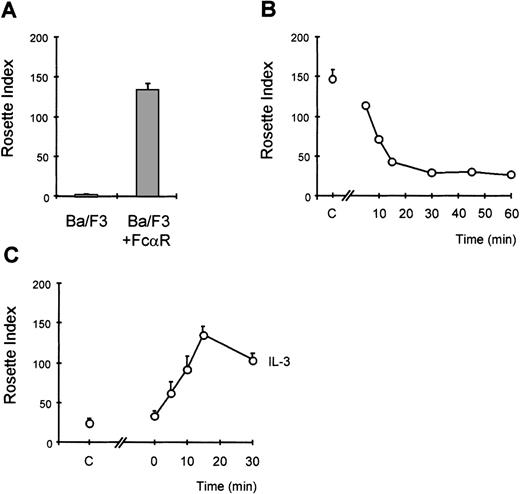
We have previously demonstrated that the IgA receptor on human eosinophils can be regulated by cytokines, such as IL-5 and GM-CSF, to become optimally functional.10,13,19 To understand the mechanism by which cytokines can modulate FcαR (CD89) function, we have utilized a model system to study FcR activation. The murine pre-B cell line, Ba/F3, lacks CD89 expression, and these cells require IL-3 to survive, similarly to the requirement for IL-5/IL-3/GM-CSF for human eosinophil survival. Cells transfected with FcαR were stained with CD89 antibody (A59-PE) and sorted with a FACS flow cytometer to obtain polyclonal cell lines expressing high levels of FcαR (CD89) (Figure 1A). To confirm the functionality of the receptor expressed by Ba/F3 cells, we performed IgA-binding assays as previously described.10,13,19 Although untransfected cells did not bind IgA-coated particles, Ba/F3_FcαR did bind IgA beads (Figure2A). To determine whether IL-3 was necessary for this IgA binding, comparable to IL-5 induced IgA binding to human eosinophils, Ba/F3_FcαR cells were cytokine-deprived, and we investigated the effect of IL-3 stimulation. As shown in Figure 2, removal of IL-3 led to a dramatic decrease in rosette formation within 15 minutes (Figure 2B), whereas binding of IgA-coated beads is rapidly restored after addition of IL-3 to the cytokine-starved cells (Figure2C). These findings suggest that the “default” binding state of the receptor is high but suppressed in unstimulated cells. Cytokine stimulation may release or overrule this suppression, switching the receptor to a ligand-binding state. As shown in Figure 2B, this cytokine-dependent increase of FcαR functionality was maximal within 15 minutes of IL-3 stimulation. This time course is identical to IL-5-induced IgA binding to human eosinophils,10 thus Ba/F3_FcαR cells serve as a model to study the molecular mechanisms of cytokine-mediated regulation of the human FcαR.
FACS analysis of FcR expression in transfected Ba/F3 cells.
In (A), expression of FcαR and a FcαR mutant, FcαR_R209D, was analyzed by flow cytometry. In (B), the effect of co-expression of Ras, phosphatidylinositol 3-kinase, PTEN, and gag constructs on FcαR_wt expression was analyzed. Polyclonal stable cell lines were tested for expression of FcαR with PE-labeled A59, a monoclonal antibody against FcαR (CD89). Levels of FcαR expression are indicated as relative fluorescence, and nontransfected Ba/F3 cells were used as a control in each panel (gray line).
FACS analysis of FcR expression in transfected Ba/F3 cells.
In (A), expression of FcαR and a FcαR mutant, FcαR_R209D, was analyzed by flow cytometry. In (B), the effect of co-expression of Ras, phosphatidylinositol 3-kinase, PTEN, and gag constructs on FcαR_wt expression was analyzed. Polyclonal stable cell lines were tested for expression of FcαR with PE-labeled A59, a monoclonal antibody against FcαR (CD89). Levels of FcαR expression are indicated as relative fluorescence, and nontransfected Ba/F3 cells were used as a control in each panel (gray line).
Cytokine-induced activation of FcR in Ba/F3 cells.
Immunoglobulin A (IgA)-binding assays were performed with Ba/F3_FcαR cells. In (A), the binding of IgA beads to Ba/F3_FcαRwt cells was compared with that of untransfected cells (Ba/F3). To study the effect of interleukin 3 (IL-3) on IgA binding, cells were washed and resuspended in IL-3-free medium with 0.5% fetal calf serum. In (B), time points indicate the period of IL-3 withdrawal prior to performing the assay (n = 2). In (C), the effect of IL-3 addition is shown, and the time points indicate for how long cells were stimulated with IL-3 prior to being incubated with IgA beads (n = 3). Subsequent to cytokine stimulation, IgA binding was performed. In all panels, results are expressed as rosette index (number of beads/100 cells) and as means ± SE.
Cytokine-induced activation of FcR in Ba/F3 cells.
Immunoglobulin A (IgA)-binding assays were performed with Ba/F3_FcαR cells. In (A), the binding of IgA beads to Ba/F3_FcαRwt cells was compared with that of untransfected cells (Ba/F3). To study the effect of interleukin 3 (IL-3) on IgA binding, cells were washed and resuspended in IL-3-free medium with 0.5% fetal calf serum. In (B), time points indicate the period of IL-3 withdrawal prior to performing the assay (n = 2). In (C), the effect of IL-3 addition is shown, and the time points indicate for how long cells were stimulated with IL-3 prior to being incubated with IgA beads (n = 3). Subsequent to cytokine stimulation, IgA binding was performed. In all panels, results are expressed as rosette index (number of beads/100 cells) and as means ± SE.
IL-3 induced IgA binding requires activation of PI3K but not MAPKs
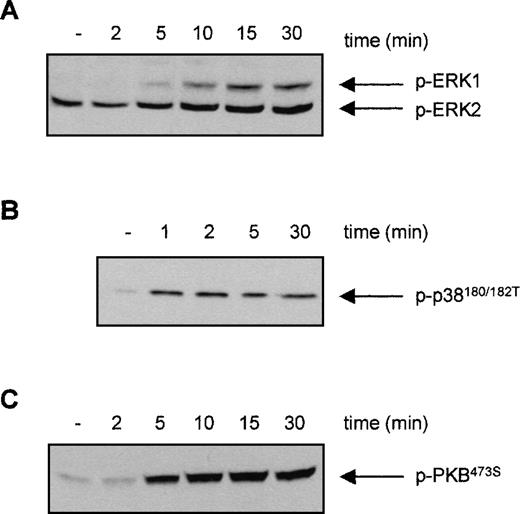
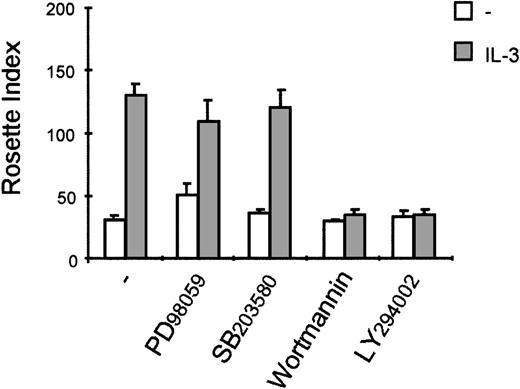
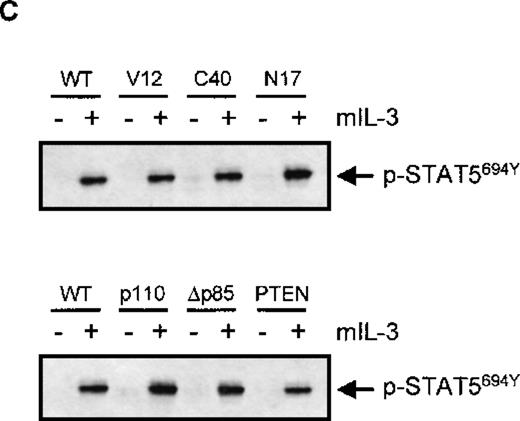
For eosinophils, we have shown that activation of distinct signal transduction pathways was required for specific cytokine-induced activation of different FcRs. IL-5 stimulation results in a fast activation of several signal transduction pathways in human eosinophils, including the phosphorylation and activation of ERK2 and p38 MAPK as well as PI3K13 20 To investigate whether the PI3K, p38 MAPK, or ERK1/2 could be involved in the IL-3-induced activation of FcαR on Ba/F3 cells, we first studied the ability of IL-3 to activate these pathways. Cytokine-starved Ba/F3 cells were stimulated with IL-3, and phosphorylation of ERK1/2 and p38 MAPK was detected. As shown in Figure 3, phosphorylation of ERK2 was already detected in unstimulated cells, which could be slightly further increased by IL-3 stimulation. Furthermore, IL-3 stimulation of cytokine-starved Ba/F3 cells resulted in a rapid phosphorylation of ERK1 (Figure 3A) and p38 MAPK (Figure3B). Detection of phosphorylated protein kinase B (PKB), a downstream effector of PI3K, was used as a measurement for PI3K activation. As shown in Figure 3C, IL-3 stimulation also resulted in rapid phosphorylation of PKB, suggesting that PI3K was activated on cytokine stimulation. To investigate the relevance of the activation of these signaling pathways in IL-3 mediated FcαR functioning in Ba/F3 cells, we studied the effect of specific pharmacological inhibitors on receptor-ligand interactions. IL-3-induced binding of IgA beads to Ba/F3_FcαR cells was not blocked by inhibitors of MAPKs (Figure4). Ba/F3_FcαR cells incubated with either the MEK inhibitor PD98059 (50 μmol/L), or with the p38 MAPK inhibitor SB203580 (1 μmol/L), showed normal IgA binding after IL-3 stimulation (Figure 4). In contrast, the effect of IL-3-induced IgA binding was completely abrogated by incubation with the PI3K inhibitors, LY294002 and wortmannin. These findings suggest that, although IL-3 is able to activate multiple pathways in Ba/F3 cells, activation of PI3K, but not ERK or p38 MAP kinases, is necessary for IL-3-induced activation of FcαR.
Effect of interleukin 3 (IL-3) stimulation on the phosphorylation of ERK1/2, p38 MAPK, and protein kinase B (PKB) in Ba/F3 cells.
Cytokine-starved Ba/F3 cells were stimulated with IL-3 for the indicated time. After stimulation, cells (0.2 × 106per sample) were washed with ice-cold phosphate-buffered saline, lyzed in lysis buffer, and heated for 5 minutes after addition of 5x sample buffer. Phosphorylation of ERK1/2 (A), p38 MAPK (B), and PKB (C) was detected, using polyclonal anti-phospho-ERK1/2, anti-phospho-p38, or anti-phospho-PKB antiserum for Western blotting.
Effect of interleukin 3 (IL-3) stimulation on the phosphorylation of ERK1/2, p38 MAPK, and protein kinase B (PKB) in Ba/F3 cells.
Cytokine-starved Ba/F3 cells were stimulated with IL-3 for the indicated time. After stimulation, cells (0.2 × 106per sample) were washed with ice-cold phosphate-buffered saline, lyzed in lysis buffer, and heated for 5 minutes after addition of 5x sample buffer. Phosphorylation of ERK1/2 (A), p38 MAPK (B), and PKB (C) was detected, using polyclonal anti-phospho-ERK1/2, anti-phospho-p38, or anti-phospho-PKB antiserum for Western blotting.
Interleukin 3 (IL-3)-induced immunoglobulin A (IgA) binding is inhibited by the phosphatidylinositol 3-kinase (PI3K) inhibitors, wortmannin and LY294002.
Cytokine-starved Ba/F3_FcαR cells were pretreated for 15 minutes at 37°C with buffer, 50 μmol/L MEK inhibitor PD98059, 1 μmol/L p38 MAPK inhibitor SB203580, 20 nmol/L wortmannin, or 1 μmol/L LY294002, and subsequently stimulated at 37°C with buffer (white bars) or IL-3 (1:1000; gray bars) for 15 minutes. Binding of IgA beads to these cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
Interleukin 3 (IL-3)-induced immunoglobulin A (IgA) binding is inhibited by the phosphatidylinositol 3-kinase (PI3K) inhibitors, wortmannin and LY294002.
Cytokine-starved Ba/F3_FcαR cells were pretreated for 15 minutes at 37°C with buffer, 50 μmol/L MEK inhibitor PD98059, 1 μmol/L p38 MAPK inhibitor SB203580, 20 nmol/L wortmannin, or 1 μmol/L LY294002, and subsequently stimulated at 37°C with buffer (white bars) or IL-3 (1:1000; gray bars) for 15 minutes. Binding of IgA beads to these cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
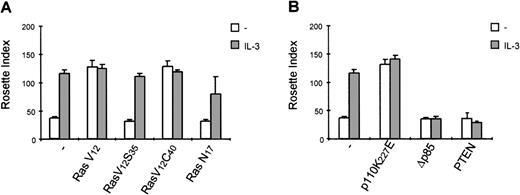
Because p21ras has been demonstrated to activate multiple downstream signaling events,21 we evaluated the role of p21ras signaling in the activation of FcαR. We stably overexpressed dominant negative and constitutively activated p21ras constructs (RasN17 and RasV12, respectively) and studied the effect of their expression on FcαR functioning. As shown in Figure 5A, expression of RasV12 greatly enhanced IgA binding to levels comparable with cytokine stimulation, and this binding could not be further enhanced by cytokine treatment. However, overexpression of dominant negative Ras (RasN17) only partly reduced IL-3-mediated IgA binding. Because overexpression of p21ras is known to activate a plethora of intracellular signaling pathways, we also used two Ras effector mutants that have specific mutations within the amino-terminal effector domain (amino acids 32-40 in Ha-Ras), eliminating binding to specific effectors without disturbing binding to others.21 In an activated RasV12 context, the mutants Ha-RasV12S35 and Ha-RasV12C40 retain only the ability to interact with either Raf122,23or p110-PI3K,22,24 respectively. Stable cell lines were generated, overexpressing either RasV12S35 or RasV12C40 (Figure 1B). As shown in Figure 5A, the IL-3-dependent IgA binding was unaffected in Ba/F3_FcαR (RasV12S35) cells, suggesting no role for Raf/MEK/ERK. This finding is in line with the lack of PD98059 inhibition of IL-3-induced FcαR activation (Figure 4). In contrast, overexpression of RasV12C40, which specifically activates PI3K signaling, conferred cytokine-independent IgA binding. These data suggest that enhanced IgA binding seen by overexpression of active Ras is due to activation of PI3K and not via activation of the Raf/MEK/ERK signaling. To determine if PI3K activation is not only necessary but also sufficient for the cytokine-mediated regulation of FcαR activation, we overexpressed p110K227E, a catalytic subunit mutant, that acts as a constitutively active form of PI3K.18 As shown in Figure 5B, overexpression of this active PI3K construct led to an IL-3-independent activation of FcαR. Moreover, inhibition of PI3K signaling by either co-expression of dominant negative Δp85 adapter subunit17 or of the recently described PI-lipid phosphatase PTEN25 resulted in inhibition of IL-3-mediated IgA binding (Figure 5B).
Activation of phosphatidylinositol 3-kinase (PI3K) is critical for interleukin 3 (IL-3)-mediated immunoglobulin A (IgA) binding.
IgA-binding studies were performed with Ba/F3_FcαR cells, co-expressing p21ras mutants (RasV12, V12S35, V12C40, or N17) (A), PI3K mutants (p110K227E or ▵p85) or active PTEN (B). Cells were cytokine-starved for 4 hours and treated with buffer (white bars) or IL-3 (1:1000; gray bars) for 15 minutes. Binding of IgA beads to the cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 4). (C) Cytokine-starved Ba/F3 stable cell lines were stimulated with or without IL-3 for 15 minutes. After stimulation, cells (0.2 × 106 per sample) were washed with ice-cold phosphate-buffered saline, lyzed in lysis buffer, and heated for 5 minutes after addition of 5x sample buffer. Phosphorylation STAT5 was detected, using polyclonal anti-phospho-STAT5 (Tyr694) antiserum for Western blotting.
Activation of phosphatidylinositol 3-kinase (PI3K) is critical for interleukin 3 (IL-3)-mediated immunoglobulin A (IgA) binding.
IgA-binding studies were performed with Ba/F3_FcαR cells, co-expressing p21ras mutants (RasV12, V12S35, V12C40, or N17) (A), PI3K mutants (p110K227E or ▵p85) or active PTEN (B). Cells were cytokine-starved for 4 hours and treated with buffer (white bars) or IL-3 (1:1000; gray bars) for 15 minutes. Binding of IgA beads to the cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 4). (C) Cytokine-starved Ba/F3 stable cell lines were stimulated with or without IL-3 for 15 minutes. After stimulation, cells (0.2 × 106 per sample) were washed with ice-cold phosphate-buffered saline, lyzed in lysis buffer, and heated for 5 minutes after addition of 5x sample buffer. Phosphorylation STAT5 was detected, using polyclonal anti-phospho-STAT5 (Tyr694) antiserum for Western blotting.
To be sure that the effects observed by co-transfection of active and dominant negative signaling molecules was not simply due to an aspecific block in IL-3 signaling in general, we analyzed the phosphorylation of STAT5 by IL-3 in the various cell lines. As is clearly demonstrated in Figure 5C, there was no effect on IL-3-induced STAT5 tyrosine phosphorylation in the various cell lines utilized, arguing against any aspecific modulation of IL-3 signal transduction in these Ba/F3 lines.
Downstream targets of PI3K involved in IL-3-induced FcR activation
We further investigated which pathway(s) downstream of PI3K could be involved in the IL-3-induced FcαR activation. Known targets of PI3K are p70 S6 Kinase (p70S6K),26 PKB,27 and PKC isoforms.28 29 We excluded PKB as a major effector of PI3K-mediated FcαR activation, since overexpression of active or dominant negative PKB-mutants did not influence IgA binding (data not shown). A role for p70S6K in FcαR stimulation is also unlikely, since inhibition of p70S6K activation by rapamycin did not affect IgA binding to Ba/F3_FcαR (Figure6). Interestingly, however, treatment with either GF109203X or RO31-8220 resulted in a decreased IgA binding to IL-3-stimulated cells (Figure 6A). Furthermore, these PKC inhibitors also blocked the binding of IgA beads to Ba/F3_FcαR (p110_K227E) cells (Figure 6B), suggesting that activation of PKC downstream of PI3K is necessary for FcαR activation.
Inhibition of protein kinase C (PKC) results in abolished immunoglobulin A (IgA) binding on interleukin 3 (IL-3) stimulation.
IL-3-starved Ba/F3_FcαR (A) or Ba/F3_FcαR (p110K227E) cells (B) were pretreated for 10 minutes with buffer (white), p70S6K inhibitor rapamycin (20 ng/mL; gray), or PKC inhibitors GF109203X (1μmol/L; black) or Ro-31-8220 (1μmol/L; arched) and subsequently incubated with or without IL-3 for 15 minutes. Binding of IgA beads to these cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
Inhibition of protein kinase C (PKC) results in abolished immunoglobulin A (IgA) binding on interleukin 3 (IL-3) stimulation.
IL-3-starved Ba/F3_FcαR (A) or Ba/F3_FcαR (p110K227E) cells (B) were pretreated for 10 minutes with buffer (white), p70S6K inhibitor rapamycin (20 ng/mL; gray), or PKC inhibitors GF109203X (1μmol/L; black) or Ro-31-8220 (1μmol/L; arched) and subsequently incubated with or without IL-3 for 15 minutes. Binding of IgA beads to these cells was measured, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
IL-3-mediated regulation of FcR does not involve the FcRγ-chain
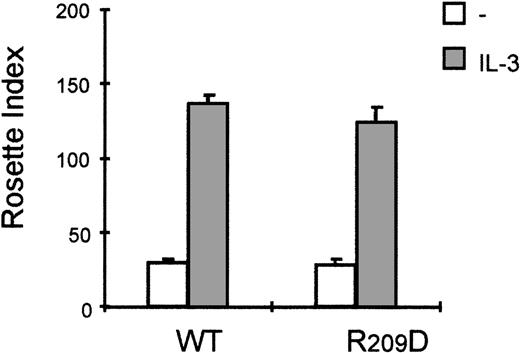
It is clear that the activation of PI3K and PKC is critical for FcαR activation by cytokines. FcαR has been shown to be associated with a FcRγ-chain homodimer,30,31 and it is suggested that formation of FcαR/γ chain complex is necessary for FcαR functioning.32 Although the short intracellular domain of FcαR does not contain any known signaling motifs, the FcRγ chain contains specific immunoreceptor tyrosine-based activation motifs that might be essential for FcαR-mediated signal transduction. The association between FcαR and FcRγ occurs via a positively charged arginine in the predicted transmembrane domain of FcαR.30To rule out the possibility that the cytokine-mediated activation of FcαR occurs via the FcRγ chain, which is present on Ba/F3 cells (not shown), we constructed a cell line expressing a FcαR_R > D mutant. In this mutant, the positively charged arginine (Arg209) in the transmembrane region of FcαR was substituted by a negatively charged aspartic acid, resulting in a FcαR mutant that cannot associate with the FcRγ-chain homodimer.30 As shown in Figure7, stimulation with IL-3 leads to comparable levels of IgA binding to both cells expressing the wild type FcαR or Ba/F3_FcαR-R > D cells. Therefore, association of FcαR with the FcRγ chain is not necessary for cytokine-induced modulation of the receptor, suggesting that the PI3K-mediated mechanism by which IL-3/IL-5/GM-CSF regulate FcαR functioning specifically requires the Fcα chain.
Interleukin 3 (IL-3)-induced FcαR activation is independent of association with the Fc-receptor (FcR) γ chain.
The effect of cytokine stimulation on immunoglobulin A (IgA) binding was investigated in Ba/F3 cells expressing the FcαR_R209D mutant, which is unable to interact with the FcRγ chain. Cells were cytokine-starved for 4 hours and treated for 15 minutes with IL-3 (gray bars) or left untreated (white bars), and subsequently IgA-binding assays were performed. Binding of IgA beads was scored under a microscope, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
Interleukin 3 (IL-3)-induced FcαR activation is independent of association with the Fc-receptor (FcR) γ chain.
The effect of cytokine stimulation on immunoglobulin A (IgA) binding was investigated in Ba/F3 cells expressing the FcαR_R209D mutant, which is unable to interact with the FcRγ chain. Cells were cytokine-starved for 4 hours and treated for 15 minutes with IL-3 (gray bars) or left untreated (white bars), and subsequently IgA-binding assays were performed. Binding of IgA beads was scored under a microscope, and results are expressed as rosette index (number of beads/100 cells) and as means ± SE (n = 3).
Discussion
FcRs are present on both lymphoid- and myeloid-derived hematopoietic cells and provide a crucial link between the humoral and cellular branches of the immune system. Activation of FcRs on inflammatory effector cells results in the triggering of immune responses. It is known that immunoglobulins are potent triggers of granulocyte activation, and binding of immunoglobulin to FcRs results in the activation of cellular responses such as degranulation, respiratory burst, and antibody-dependent cytotoxicity.12,33 Inappropriate activation of human granulocytes can lead to local tissue damage of the respiratory epithelium and airway hyperresponsiveness as observed during allergic inflammatory reactions. Because uncontrolled activation of effector cells can be deleterious, regulation of cellular activation is crucial for correct functioning of the immune system. Cytokines, such as interleukins, are important mediators of cellular activation, and it has been described for human eosinophils that cytokines are involved in the regulation of many effector functions (reviewed in34).
For the FcRs for IgA (FcαR) and IgG (FcγRII), we have previously shown that binding of immunoglobulin-coated targets to FcRs on eosinophils is dependent on cytokine stimulation of the cells.10,13,19 Although the FcRs on eosinophils do not bind monomeric ligand, the functional status of both FcαR and FcγRII for complexed ligand is altered by Th2-derived cytokines such as IL-5. Because this cytokine-mediated modulation is very rapid,10this switch is not likely to be due to de novo receptor synthesis. Moreover, analysis by flow cytometer revealed that levels of receptor expression on the membrane are not altered by cytokine stimulation11 (unpublished results).
In this study, we have utilized Ba/F3 cells as a model to study the molecular mechanism of cytokine-induced ligand binding to FcαR (CD89). In contrast with other cell lines commonly used for FcR studies, such as the murine pre-B IIA1.6 cells, Ba/F3_FcαR cells interact with IgA-coated targets in a cytokine-dependent fashion, providing an excellent system to study FcR regulation. In addition to the utilization of pharmacological inhibitors, overexpression of dominant negative or constitutively active signaling molecules has made it possible to analyze the involvement of specific signaling pathways in IL-3-mediated inside-out signaling regulating FcαR. We demonstrate that overexpression of active Ras (V12) can enhance FcαR dramatically. In contrast, ectopic expression of dominant negative RasN17 can only partially inhibit IL-3-induced IgA binding (Figure 5A), suggesting that activation of p21ras is sufficient but not necessary to activate FcαR. This finding is in line with the observation that incubation with the MEK inhibitor PD98059 did not influence IL-3-stimulated IgA binding. Therefore, activation of the Ras/Raf/ERK pathway is not required to mediate IL-3-induced FcαR regulation. Indeed, utilization of specific activated p21ras effector mutants (RasV12S35 via Raf1 and RasV12C40 via PI3K) revealed that p21ras-mediated FcαR activation occurs only when p21ras can activate PI3K (Figure 4A). The p110 catalytic subunit of PI3K has been described as a direct target of p21ras,21 whereby the level of PI3K activity obtained by direct p21ras stimulation is dependent on the p110-isoform.35 Interaction of PI3K with p21ras probably targets p110 to the membrane, allowing access to phospholipid substrates. However, PI3K activation is not wholly dependent on p21ras, since recruitment to the membrane can also occur via translocation of the regulatory p85 subunit to phosphotyrosine residues of protein tyrosine kinase receptors36 or via Gβγ subunits to G-protein coupled receptors. Because p21ras is not the only intermediate utilized to activate PI3K, it explains the observation that RasN17 only partially inhibited IL-3-induced IgA binding.
In addition to pharmacologically inhibiting PI3K activation and overexpression of constitutively active and dominant negative forms of PI3K, we also analyzed the effect of ectopically expressing the recently identified phosphatidylinositol lipid phosphatase PTEN.25 This phosphatase has been shown to dephosphorylate the 3-phospholipid products of PI3K, thus counteracting the lipid kinase, but not the protein kinase activity of PI3K and preventing activation of downstream targets. Indeed, blocking the PI3K pathway by inhibitors or by overexpression of either the dominant negative Δp85 or PTEN completely abolished the effect of IL-3 on IgA binding. These data indeed demonstrate PI3K is necessary for IL-3-stimulated FcαR function. Moreover, activation of PI3K is sufficient for FcαR activation, since expression of a constitutively active p110_K227E results in a functional FcαR. Because PTEN counteracts the lipid, but not the protein kinase activity, it suggests that the production of 3-phospholipids is involved in the FcαR activation.
On activation of PI3K at the membrane, downstream targets can be recruited to the membrane and activated by phosphorylation. PI3,4,5P3-dependent kinases are direct targets of PI3K-generated products and are responsible for the phosphorylation of recruited PI3K effectors, including PKB27 and p70 S6 Kinase (p70S6K).26 Also activation of PKC isoforms have recently been described to be activated by PI3,4,5P3-dependent kinases,28,29including Ca++-independent PKCs (PKCδ/ε/η) and the atypical PKCζ.37 PI3K-mediated FcαR regulation appears to require activation of a PKC isoform and not PKB or p70S6 kinase, since IgA binding was not influenced by overexpression of active or negative PKB mutants (not shown) nor by incubation with rapamycin (Figure 6). However, PKC inhibitors RO31-8220 and GF109203X did inhibit the cytokine-induced IgA binding (Figure 6). Moreover, these PKC inhibitors also inhibit the IgA binding to Ba/F3_FcαR (p110_K227E) cells (Figure 6, right panel), suggesting that activation of PKC downstream of PI3K indeed is required for IL-3-induced FcαR activation.
Mechanisms of FcαR activation by cytokines may include regulation of FcαR via direct activation on the receptor, including processes such as phosphorylation, conformational changes, or association with additional proteins involved in signal transduction. An alternative explanation would suggest indirect activation of FcαR, when activation of PI3K-PKC results in the phosphorylation or cytoskeletal reorganization, leading to clustering or activation of FcRs. Also, regulation of FcαR activation might occur via the associated γ-chain homodimer. Because the FcRγ chain could be detected on Ba/F3 cells (unpublished observations), it needed to be investigated whether cytokine-mediated FcαR activation relied on the association with this subunit. Substitution of the transmembrane residue 209R to an aspartic acid prevents FcαR to associate with the γ chain.30 As shown in Figure 7, cytokine-dependent IgA binding to a cell line expressing FcαR_R209D was similar to binding to FcαR. These data suggested that an interaction with the γ chain is not critical for cytokine-induced ligand binding. Therefore, it is likely that the induction of IgA binding via IL-3-induced inside-out signaling is mediated via the FcαR, while FcRγ is likely to be critical for ligand-induced outside-in signaling of FcαR. This is the first publication demonstrating a critical role for the Fcα chain (CD89) in the regulation of ligand binding.
Cytokine-induced inside-out signaling switches FcαR to an active state and subsequent ligand binding will lead to FcRγ chain mediated outside-in signaling, resulting in cell activation. In this way, leukocytes can respond very rapidly and efficiently on their environment, a process that requires tight regulation. A greater understanding of cytokine-mediated modulation of FcR functioning on leukocytes will generate insight into the regulation of leukocyte activation and the pathogenesis of inflammation, possibly providing novel therapeutic options.
Acknowledgments
The authors would like to thank Pascale Dijkers for generating the PTEN constructs and critically reading the manuscript, Jan van der Linden and Deon Kanters for assistance with the FACS and cell sorting, and Julian Downward for the Ras and PI3K constructs.
Supported by a research grant of the Netherlands Asthma Foundation (AF 94.44).
Reprints:L. Koenderman, Dept. of Pulmonary Diseases, F02.333, University Medical Centre, Heidelberglaan 100 3584 CX Utrecht, The Netherlands; e-mail: L.Koenderman@hli.azu.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal