Abstract
Natural killer T (NKT) cells have recently been shown to play an important role in the rejection of malignant tumors and in the regulation of autoimmune diseases. Potent antitumor effects of the marine sponge–derived NKT cell ligand KRN7000 were observed in mice. Therefore, the elucidation of the natural ligand of NKT cells, which is currently still unknown, might have important clinical consequences for the treatment of cancer and autoimmune diseases. Analysis of cord blood mononuclear cells from healthy term infants demonstrated that in sharp contrast with the vast majority of cord blood lymphocytes, human NKT cells have already acquired a memory-activated phenotype before birth. This observation indicates that NKT cells encounter a natural ligand during fetal life and that this ligand is unlikely to be of microbial origin.
Natural killer T (NKT) cells have recently been identified as a novel lymphocyte lineage. In humans these cells are characterized by an extremely restricted T-cell receptor (TCR) repertoire consisting of a Vα24 chain preferentially paired with a Vβ11 chain.1-3 NKT cells have been implicated as playing crucial roles in various immune responses including protective immunity against malignant tumors and the regulation of autoimmune diseases.4-7 The natural ligand of NKT cells is still unknown. The NKT cell ligand KRN7000 is an α-galactosylceramide that was originally isolated from a marine sponge. The fact that ligand KRN7000 exhibits potent antitumor activity in mice8suggests that the elucidation of the natural ligand will be of critical importance for understanding the physiological role of NKT cells. The natural ligand might provide clues for designing better treatments for conditions such as autoimmune diseases and cancer. It has been demonstrated that adult human Vα24+Vβ11+ T cells (NKT cells) express enhanced levels of the memory marker CD45RO compared with mainstream αβ T cells. This indicates that NKT cells are, at some point, exposed to an antigen. A continuous antigen exposure seems, however, unlikely because these NKT cells did not appear to be constitutively activated.9
Strong expression of CD45RA and weak expression of memory marker CD45RO, illustrative of the naive status of the fetal immune system, characterizes mononuclear cells from human cord blood. Activation of naive T cells, which coexpress CD45RA and the lymph node homing receptor L-selectin (CD62L),10 results in loss of the CD45RA epitope, acquisition of the CD45RO epitope, and loss of CD62L.11 In this study we compared the expression of various activation and memory markers on human T cells, NK cells, and NKT cells from both cord and adult peripheral blood and demonstrated that human NKT cells are unique in acquiring a memory-activated phenotype before birth. It appears that NKT cells therefore encounter a natural ligand during fetal life, which might indicate that these cells already exert an important immune regulatory function before birth. Study design
Cell preparation
Cord blood of 10 healthy term infants (6 males and 4 females) and peripheral blood of 5 healthy adult volunteers (3 males and 2 females; mean age, 32 years) were obtained after informed consent. Mononuclear cells were prepared by density gradient centrifugation (Lymphoprep; Nycomed Pharma AS, Oslo, Norway).
Flow cytometry
Flow cytometric expression of cell-surface markers was assessed (FACStar plus; Becton Dickinson, San Jose, CA). T cells were characterized by expression of CD3, NK cells by expression of CD16, and NKT cells by coexpression of the T-cell receptor Vα24 and Vβ11 chain. The following reagents were used: phycoerythrin-labeled (PE-labeled) CD3 and CD16 and fluorescein isothiocyanate–labeled (FITC-labeled) CD69 (Becton Dickinson); PE-labeled CD25, CD45RA, and CD45RO (PharMingen, San Diego, CA); PE- and FITC-labeled Vα24 and PE- and biotin-labeled Vβ11 (Immunotech, Marseilles, France); PE-Cyanin 5.1 (Cy5)-labeled CD62L (gift from Beckman Coulter, Mijdrecht, The Netherlands); and R-PE-Cy5–labeled streptavidin (DAKO, Glostrup, Denmark).
Statistical analysis
Statistical analysis was performed using the paired Studentt test; P < .05 was considered significant.
Results and discussion
The naive status of the fetal immune system is illustrated by the fact that the vast majority of human cord blood mononuclear cells strongly expresses CD45RA and weakly expresses memory marker CD45RO. During activation of naive T cells, which coexpress CD45RA and CD62L,10 the CD45RA/RO conversion is accompanied by an initial up-regulation of CD62L expression, which is thereafter heterogeneously down-regulated, leading to CD45RO+CD62L+ and CD45RO+CD62L− memory-effector cells.12 Surprisingly, when analyzing human cord blood mononuclear cells from healthy term infants, we found that in sharp contrast with both T cells and NK cells, NKT cells strongly expressed CD45RO and weakly expressed CD45RA (Table1). Accordingly, CD62L expression was significantly reduced on cord blood NKT cells when compared with T cells. Expression of CD62L on NK cells was moderate, in line with a previous report that showed limited CD62L expression on NK cells.13 These data indicate that NKT cells are unique in acquiring a memory phenotype during fetal life and that they have been activated, at some moment in fetal life, by encountering a natural ligand.
NKT cells and a memory-activated phenotype
| . | Cord Blood . | Adult Blood . | ||||
|---|---|---|---|---|---|---|
| T Cells . | NK Cells . | NKT Cells . | T Cells . | NK Cells . | NKT Cells . | |
| CD45RAbright | 64.4 ± 10.1 | 98.0 ± 2.7 | 9.4 ± 7.5* | 50.3 ± 5.4 | 97.1 ± 2.1 | 3.4 ± 1.3* |
| CD45RAdim | 33.2 ± 9.0 | 1.9 ± 2.8 | 42.9 ± 2.1 | 27.4 ± 6.2 | 2.7 ± 2.0 | 79.7 ± 8.2* |
| CD45RO | 25.4 ± 5.3 | 2.7 ± 2.7 | 93.7 ± 5.6* | 53.4 ± 8.4 | 3.7 ± 1.9 | 99.2 ± 0.7† |
| CD62L | 79.7 ± 18.7 | 22.6 ± 10.6 | 41.3 ± 16.3‡ | 67.9 ± 9.6 | 28.0 ± 10.5 | 15.4 ± 11.2‡ |
| CD69 | 0.2 ± 0.1 | 1.8 ± 2.6 | 0.5 ± 0.8 | 0.5 ± 0.3 | 1.5 ± 1.6 | 8.5 ± 8.9 |
| CD25 | 7.4 ± 2.8 | 1.3 ± 1.5 | 60.8 ± 23.8* | 17.0 ± 6.1 | 2.7 ± 1.7 | 55.7 ± 25.21-153 |
| . | Cord Blood . | Adult Blood . | ||||
|---|---|---|---|---|---|---|
| T Cells . | NK Cells . | NKT Cells . | T Cells . | NK Cells . | NKT Cells . | |
| CD45RAbright | 64.4 ± 10.1 | 98.0 ± 2.7 | 9.4 ± 7.5* | 50.3 ± 5.4 | 97.1 ± 2.1 | 3.4 ± 1.3* |
| CD45RAdim | 33.2 ± 9.0 | 1.9 ± 2.8 | 42.9 ± 2.1 | 27.4 ± 6.2 | 2.7 ± 2.0 | 79.7 ± 8.2* |
| CD45RO | 25.4 ± 5.3 | 2.7 ± 2.7 | 93.7 ± 5.6* | 53.4 ± 8.4 | 3.7 ± 1.9 | 99.2 ± 0.7† |
| CD62L | 79.7 ± 18.7 | 22.6 ± 10.6 | 41.3 ± 16.3‡ | 67.9 ± 9.6 | 28.0 ± 10.5 | 15.4 ± 11.2‡ |
| CD69 | 0.2 ± 0.1 | 1.8 ± 2.6 | 0.5 ± 0.8 | 0.5 ± 0.3 | 1.5 ± 1.6 | 8.5 ± 8.9 |
| CD25 | 7.4 ± 2.8 | 1.3 ± 1.5 | 60.8 ± 23.8* | 17.0 ± 6.1 | 2.7 ± 1.7 | 55.7 ± 25.21-153 |
Expression of cell-surface markers on T, NK, and NKT cells in cord and adult peripheral blood. Data represent mean percentage ± SD of positive cells within each cell population. NKT cells represented 0.08 ± 0.05% and 0.08 ± 0.04% of T cells in cord and adult peripheral blood, respectively.
Indicates significant compared to T cells (P < .0001) and NK cells (P < .0001).
Indicates significant compared to T cells (P = .0002) and NK cells (P < .0001).
Indicates significant compared to T cells (P < .0001).
Indicates significant compared to T cells (P = .0376) and NK cells (P = .0095).
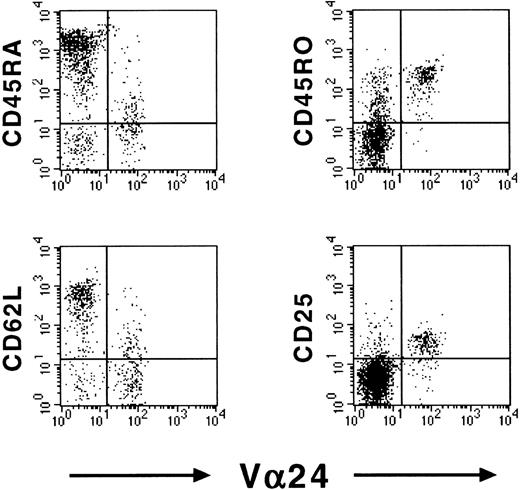
To determine whether NKT cells showed signs of activation in cord blood, we assessed the expression of CD25 and CD69 on cord blood NKT cells. Although we observed negligible expression of the early activation marker CD69, a high proportion of cord blood NKT cells expressed the activation marker CD25, which indicates previous antigen contact. As the TCR repertoire of a cell population is skewed upon such contacts, it is not surprising that NKT cells already exhibit a restricted TCR repertoire in cord blood.14Representative dot-plots of CD45RA, CD45RO, CD62L, and CD25 expression in cord blood Vβ11+ T cells are shown in Figure1.
Memory-activated phenotype of cord blood NKT cells.
Expression of memory markers CD45RA, CD45RO, CD62L, and CD25 on cord blood Vβ11+ T cells. Expression was assessed using flow cytometry. Representative dot-plots of 1 donor are shown.
Memory-activated phenotype of cord blood NKT cells.
Expression of memory markers CD45RA, CD45RO, CD62L, and CD25 on cord blood Vβ11+ T cells. Expression was assessed using flow cytometry. Representative dot-plots of 1 donor are shown.
The memory phenotype of NKT cells, as determined by the expression of CD45RA, CD45RO, and CD62L, was similar on NKT cells in adult peripheral blood. However, the expression of CD62L appeared to have been further down-regulated on adult NKT cells when compared with cord blood NKT cells. Of note, NKT cells in adult peripheral blood were also found to express distinct but highly variable levels of CD25 expression (Table1). These findings indicate that the as-yet unknown natural ligand(s) can continue to stimulate NKT cells in healthy adult individuals.
CD1 molecules present lipid, glycolipid, or hydrophobic peptide antigens and have been implicated in playing an important role in the presentation of microbial products.15 The NKT cell ligand KRN7000 was shown to be presented to NKT cells by the evolutionary highly conserved CD1d molecule.16-18 Therefore cells expressing CD1d (eg, cells from the hematopoietic lineage, hepatocytes,19 and trophoblast cells20) are candidates for the observed fetal activation of NKT cells. Possible natural ligands would be α-anomeric monoglycolipids or phytosphingosine with glycolipid structures, which have been detected in certain bacteria and in some conditions of mammalian tissues such as cancer cells and fetal cells (summarized in Kawano et al16).
Our data strongly suggest that NKT cells encounter a natural ligand during fetal life. The germ-free environment of a fetus makes it very unlikely that this ligand is of microbial origin. The reason for the activation of NKT cells in utero is at this time still speculative, but it might illustrate an important regulatory function. An essential role for NKT cells in the development of immune tolerance was recently demonstrated in the anterior chamber–associated immune deviation model. When an antigen was placed in the anterior chamber of the eye, NKT cells were found to be critical for the induction of systemic tolerance through the induction of regulatory T cells.21 Because defects in NKT cell development and/or function have been reported to be involved in the development of several autoimmune diseases, it seems possible that the activation of NKT cells is a more general mechanism through which regulatory T cells are generated.
We provide evidence that NKT cells acquire a memory phenotype before birth. This observation, combined with the proposed regulatory roles of this cell population, might indicate that NKT cells already play an important role in the regulation of immune responses and in the maintenance of self-tolerance in utero. Elucidation of the natural ligand(s) of NKT cells will be crucial for a better understanding of the putative physiologic function(s) of NKT cells and might have important clinical consequences in tumor immunotherapy and in the treatment of autoimmune diseases.
Partially supported by a SPINOLA grant from the Netherlands Organization for Scientific Research (NWO), The Netherlands.
Reprints:R.J. Scheper, Department of Pathology, University Hospital Vrije Universiteit, De Boelelaan 1117, 1081 HV, Amsterdam, The Netherlands; e-mail: rj.scheper@azvu.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal