Abstract
Electron microscopy was used to study the internalization and delivery of ligands for complement receptor type 2 (CR2, CD21) to endocytic compartments of B-lymphoblastoid Raji cells. Opsonized antigen was mimicked with purified C3dg conjugated to colloidal gold. C3dg-gold bound specifically to the cell surface in a time-dependent manner, and preincubation of the cells with a monoclonal antibody blocking the CR2 ligand-binding site completely inhibited any C3dg-gold binding. Notably, the binding of C3d-gold was confined to cell surface protrusions, eg, microvilli. C3dg-gold was apparently internalized through coated pits located at the bases of microvilli and could be traced to different compartments of the endocytic pathway. The morphologic characteristics and intracellular distribution of these multivesicular or multilaminar structures were compatible with those of compartments known to harbor major histocompatibility complex (MHC) class II molecules. Immunolabeling showed that the internalized C3dg-gold colocalized with MHC class II in these structures. These data provide the first ultrastructural evidence that complement-coated antigens are endocytosed by antigen-nonspecific B cells by CR2 and are delivered to the compartments in which peptide loading for antigen presentation occurs. They support the notion that CR2 may play a role in antigen presentation by B cells regardless of B-cell receptor specificity.
Typically, the uptake of antigens into antigen-presenting cells (APC) is a first step that eventually leads to a specific immune response. Among APC, B cells are relatively inefficient in fluid-phase pinocytosis compared with, for example, immature dendritic cells (DC). B cells can compensate for this by the presence of antigen-specific cell surface receptors. In addition, all APC express “semispecific” receptors1 to take up molecules that have been tagged as foreign by effector mechanisms of the innate immune system. One important mechanism is activation of the complement system. The critical step for interaction with immune cells is the activation of C3 and the deposition of C3b on the antigen. The receptors for C3 fragments, CR1 to CR4, are members of 2 distinct large protein families. CR1 (CD35) and CR2 (CD21) belong to C3b/C4b-binding proteins controlling complement activation, whereas CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are heterodimeric β2-integrins.2CR3, CR4, or both are expressed on macrophages and DCs, and promote the uptake of iC3b-coated antigen. CR1 expressed on blood cells, and notably on erythrocytes, mediates the removal of C3-coated immune complexes from the blood circulation by binding to C3b. Additionally, CR1 is a potent cofactor for C3b inactivation, which is reflected in its use as a recombinant soluble molecule to control C activation.3
CR2 has the most restricted expression pattern and is found in higher amounts only on mature B cells and follicular dendritic cells and only in low density on T cells, thymocytes, or epithelial cells in certain areas of the body.4,5 The extracellular part of CR2 consists of 15 to 16 short consensus repeats (SCR), strongly conserved structural units found with C3b/C4b regulating proteins.6The first 2 most membrane-distal SCRs are involved in binding of the C3-fragment C3dg.7 On B cells, CR2 forms a noncovalent receptor complex together with CD19 and CD81.8 The CR2/CD19/CD81 complex dramatically lowers the threshold for B-cell receptor (BCR)-triggered B-cell activation in the case of low-abundant but complement-coated antigen.9
In addition to this well-established role of CR2 for the proliferation of B cells that have encountered their specific antigen, CR2 has been discussed to promote antigen presentation by antigen nonspecific B cells. Endocytosis and presentation of C3b-coupled tetanus toxoid (TT) by B cells enhances the proliferation of TT-specific T cells compared to TT alone.10 The effect is diminished by antibodies against CR2 and, to a lesser extent, against CR1, which are the only complement receptors on B cells.10,11 Compared with TT alone, TT attached to C3 fragments is cleaved later in the endocytic pathway, resulting in more effective presentation of TT peptides.12 C3-fragment–coupled TT cannot be cleaved by endosomal cathepsin D, but it can be cleaved by lysosomal cathepsin B, which suggests an additional direct effect of C3-fragments on the antigen-processing machinery.12 This is corroborated by the observation that even CR2-negative HLA-DR–transfected fibroblasts are more effective in presenting C3b-TT than TT alone.13 Using CR1−/CR2+ Raji B-lymphoblastoid cells, Boackle et al14 have shown that the presentation of influenza virus peptides by Raji to T cells stimulates T-cell proliferation when the virus is coated with C3-fragments after classical pathway activation by incubation in high-titer immune serum. As shown also for keyhole limpet hemocyanin (KLH), primary antigens may become coated with complement in this way by natural antibodies of low affinity. KLH incubated with sera containing natural antibodies against KLH forms immune complexes (IC), including iC3b and C3dg, that are efficiently taken up by CR2 and CR1 and are presented by both KLH-specific and non–KLH-specific B cells. Remarkably, proliferation of KLH-specific T cells is in a comparable range either after the incubation of KLH-IC without C3-fragments with KLH-specific B cells or after the incubation of KLH-IC-C3 with non–KLH-specific B cells.15 Taken together, this points to an important role of CR2 for nonspecific B cells in antigen presentation in addition to the presentation by specific B cells shown earlier.16 17
The characterization of the intracellular compartments in which the loading of peptides onto MHC class II molecules occurs is still a subject of intense investigation and debate.1,18 Kleijmeer et al18 investigated whether B cells do use specialized endocytic compartments to this end. On the basis of electron microscopic studies, they suggested that peptide loading occurs within several types of endocytic compartments characterized by morphologic and biochemical parameters; however, these compartments are not unique but are present in cells other than professional antigen-presenting cells as well. On the contrary, others1 have proposed that additional criteria may be essential to characterize unequivocally the class II loading compartment.
Here we show the delivery of CR2 ligands to endocytic compartments of B-lymphoblastoid Raji cells by means of electron microscopy using colloidal gold conjugated to CR2-ligands as a model complement-coated antigen. The gold conjugates bound specifically to cell surface protrusions, became internalized, and could be traced throughout the endocytic pathway where they colocalized with MHC class II antigens.
This study for the first time provides ultrastructural evidence that CR2-mediated endocytosis without involvement of the BCR is able to forward antigens to class II loading compartments.
Materials and methods
Cell lines, complement components, and antibodies
The Raji B-lymphoblastoid cell line (EBV+, sIgM−, CD19+, CR2+, CR1−) was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI 1640 (BioWhittaker, Verviers, Belgium) with 10% fetal calf serum (Kibbutz Beth Haemek, Israel).
C3dg was prepared from outdated human plasma as described.19 CR2-specific monoclonal antibodies (mAbs) used were HB5, IgG2a,20 provided by ATCC; FE8, IgG121; 1F8, IgG1, obtained from DAKO (Glostrup, Denmark)22; IIB9, IgG1, binds to SCR 3, SCR 4, or both (W.M.P., unpublished data). FE8 has been recently described to block the binding of C3dg-coated ligands to CR2 more efficiently than the previously available mAb OKB7.21 Other mAbs used were VD3, IgG2a anticomplement factor H23 and 7F7, IgG2a directed against intercellular adhesion molecule 1 (ICAM-1).24 HLA-DR CR3/43, IgG1, antihuman-HLA-DP, DQ, DR (DAKO) mAbs were isolated by protein G–affinity chromatography (Pharmacia, Uppsala, Sweden) from ascitic fluid or supplied by the manufacturer as IgG purified from hybridoma supernatant. Rabbit antihuman C3d was from DAKO.
Preparation and coupling of gold particles
Colloidal gold sols were prepared and labeled with proteins according to De Mey.25 Gold of 8- to 10-nm diameter was generated by the modified citrate method, and 60-nm gold particles were made by the standard citrate method. The protein-gold probes were prepared at the isoelectric points of the respective proteins (C3dg, pH 5.5; bovine serum albumin [BSA], pH 9.0; mAbs, pH 9.0) using the minimal protecting amounts of proteins necessary to prevent flocculation. After secondary stabilization with BSA at pH 9.0, the probes were pelleted, washed with Tris-buffered saline (50 mmol/L Tris, 145 mmol/L NaCl, pH 7.4) containing 0.1% BSA and fractionated by centrifugation on a continuous glycerol (10% to 30%) gradient. Fractions were collected, and the quality was screened by electron microscopy; in particular, probes were immobilized onto formvar-carbon coated electron microscopy grids (1 minute at room temperature [RT]), air dried, and viewed by transmission electron microscopy. Monodisperse gold probes without particle clustering were achieved and stored in Tris-buffered saline with 1% BSA at 4°C. The C3dg concentration in the probes was determined by a standard ELISA to be 6.25 μg/mL. A gold-conjugated lectin (wheat germ agglutinin (WGA), 15 nm; EY Laboratories, San Mateo, CA) was used in parallel for binding experiments.
Binding and endocytosis of gold conjugates by Raji B cells
2 × 106 Raji cells were incubated in 1 mL RPMI + 10% FCS with the gold conjugates (final concentration of gold-coupled C3dg corresponding to 100 ng/mL) in a 1.7-mL polypropylene microcentrifuge tube at 37°. The incubation was carried out for 7, 15, 30, 60, or 120 minutes in a humidified CO2 incubator. Tubes were gently tapped every 5 minutes for the mixing of cells and gold conjugates. The specificity of binding and uptake was assessed in parallel in experiments with gold-conjugated BSA or with the addition of mAb FE8 (1 μg/mL). To check whether active redistribution of CR2 after gold-conjugate binding (ie, capping) affected the distribution of the gold conjugates, we performed parallel experiments at 4°C or after the addition of 0.1% azide. Furthermore, 0.1% acetylated BSA (BSA-C; Aurion, Wageningen, The Netherlands) was added to compete with nonspecific binding of gold conjugates to positively charged sites.
Transmission electron microscopy
For morphologic analysis 2 complementary fixation protocols were used—ambient-temperature chemical fixation and ultrarapid cryofixation to rule out possible fixation artifacts and to optimize the preservation of dynamic structures. Chemical fixation was done with 2.5% glutaraldehyde (in 0.1 mol/L sodium cacodylate-buffer, pH 7.4, 120 minutes, RT) followed by 1% OsO4 (in double-distilled water, 60 minutes, 4°C). Cryofixation was carried out by slam freezing, followed by freeze substitution with anhydrous acetone containing 2% OsO4 (8 hours at −90°C). All samples were embedded in Epon epoxy resin. Eighty nanometer-thin sections were optionally poststained with aqueous uranyl acetate and lead citrate (30 and 3 minutes at RT, respectively). Binding of gold particles to membrane structures was quantified as particles per μm boundary length.26 Intersection counting was carried out from micrographs; the distance between the test lines was 1 μm.
For immunolabeling of thawed cryosections according to Tokuyasu,27 the cells were incubated with C3dg-gold (120 minutes, 37°C) and fixed with a mixture of 4% paraformaldehyde and 0.1% glutaraldehyde (in 0.1 mol/L HEPES, pH 7.5, 120 minutes, RT). After storage overnight in 2% buffered paraformaldehyde (at 4°C), the cells were embedded in 10% gelatin, infiltrated overnight with 20% polyvinylpyrrolidone plus 1.8 mol/L sucrose (in PBS, 4°C), and frozen in liquid nitrogen. Eighty nanometer-thin sections cut at −100°C were labeled with anticlass II mAb or rabbit anti-C3d (diluted 1:50 and 1:100, respectively, in PBS containing 3% BSA, 60 minutes, RT). Bound antibodies were detected with goat antimouse IgG or goat antirabbit IgG, coupled to 5 nm gold (British BioCell, Cardiff, UK). Eventually, the sections were embedded in methyl cellulose containing uranyl acetate.
The sections were examined with an EM 10 (Zeiss, Oberkochen, Germany) or a JEM 1200EX (JEOL, Tokyo, Japan) electron microscope at 80 kV. In general, there were no major differences as to the distribution of gold conjugates and endosome morphology between the fixation protocols used; however, the yield of structurally well-preserved cells after ambient-temperature chemical fixation varied substantially.
Results
To follow the endocytic uptake of complement-coated ligands into CR2-expressing B cells at the ultrastructural level, gold particles were prepared and coated with C3dg, the physiological ligand of CR2. From the conditions used for stabilization of the gold colloid, each particle of 10-nm diameter was calculated to carry 6 to 8 C3dg monomers and thus should be able to mimic opsonized soluble antigen. B-lymphoblastoid Raji cells that do not express sIgM were used because they allowed the study of CR2-mediated uptake without involvement of the BCR. Plasma membrane staining with C3dg-gold of 10-nm diameter (referred to below as C3dg-gold10) was already observed after 7 minutes of incubation at 37°C and increased during the first 60 minutes (Table 1). The C3dg concentration was equivalent to 100 ng/mL. Binding was CR2 specific because it was also observed with various anti-CR2 mAbs coupled to colloidal gold, but not with BSA-gold in the same particle density. C3dg-gold10 staining was completely abrogated by preincubation of Raji cells with mAb FE8, which blocks the C3dg binding site on CR2, whereas nonblocking mAb HB5 had no influence.
Binding of C3dg-gold(10) vs. anti–ICAM-1-gold(10) to the plasma membrane of Raji cells
| . | Gold Particles Bound/ μm Boundary Length* . | SEM . |
|---|---|---|
| C3dg-gold(10), 7 min, 37°C | 3.47 | ± 0.62 (n = 6) |
| Anti–ICAM-1-gold(10), 7 min, 37°C | 11.03 | ± 1.36 (n = 9) |
| C3dg-gold(10), 60 min, 37°C | 13.53 | ± 4.45 (n = 5) |
| . | Gold Particles Bound/ μm Boundary Length* . | SEM . |
|---|---|---|
| C3dg-gold(10), 7 min, 37°C | 3.47 | ± 0.62 (n = 6) |
| Anti–ICAM-1-gold(10), 7 min, 37°C | 11.03 | ± 1.36 (n = 9) |
| C3dg-gold(10), 60 min, 37°C | 13.53 | ± 4.45 (n = 5) |
Quantification was performed on micrographs from 150-nm–thin sections as described in “Materials and methods.”
n, number of cells counted.
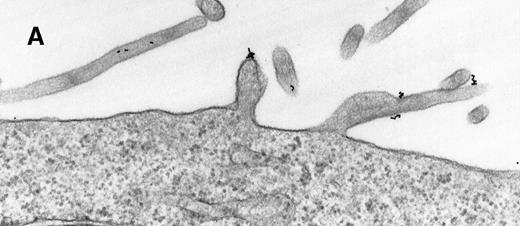
As a constant feature, staining C3dg-gold conjugates was confined to surface protrusions such as microvilli (Figures1A and 1B). These patterns did not change when the cells were incubated with C3dg-gold conjugates at 4°C or when sodium azide or excess BSA-C was added to the culture medium. Only on prolonged incubation (120 minutes) at 37°C were aggregations of gold particles detected, possibly equivalent to flocculation of gold resulting from proteolytic cleavage of C3dg from C3dg-gold.10
Raji cell surface protrusions stained with colloidal gold conjugated to C3dg.
(A) C3dg-gold10 incubated for 7 minutes at 37°C; scale bar, 0.2 μm. (B) C3dg-gold60 incubated for 60 minutes at 37°C; scale bar, 0.6 μm.
Raji cell surface protrusions stained with colloidal gold conjugated to C3dg.
(A) C3dg-gold10 incubated for 7 minutes at 37°C; scale bar, 0.2 μm. (B) C3dg-gold60 incubated for 60 minutes at 37°C; scale bar, 0.6 μm.
Various anti-CR2 mAbs coupled to colloidal gold showed similar plasma membrane binding patterns. A comparable distribution, but with a 3 times higher staining intensity, was obtained with anti-ICAM-1-gold10 (Table 1). WGA-gold15binding was uniform across the cell surface and thus contrasted with the C3dg-gold pattern seen. A gold-coupled control mAb (VD3) did not bind at all.
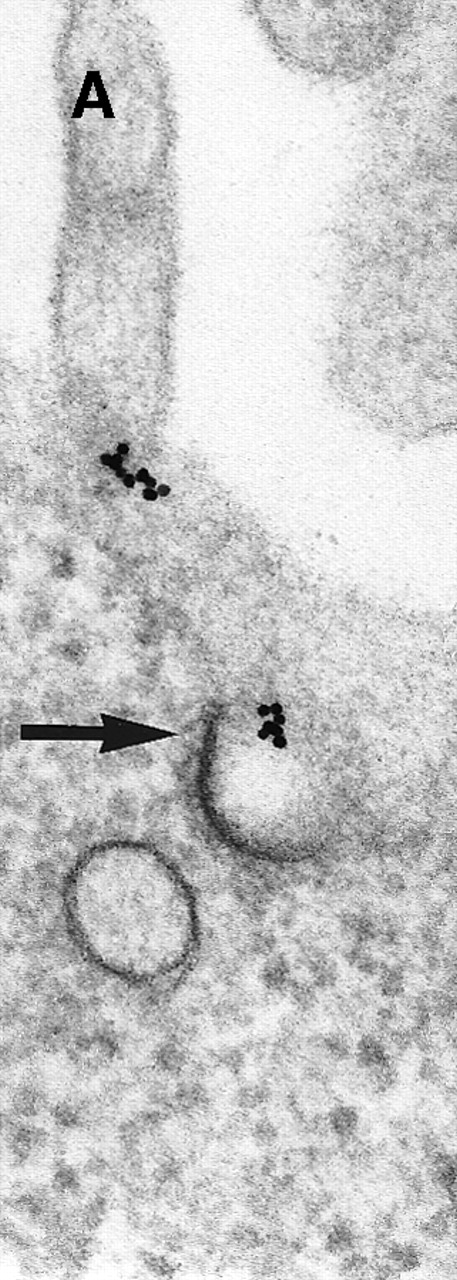
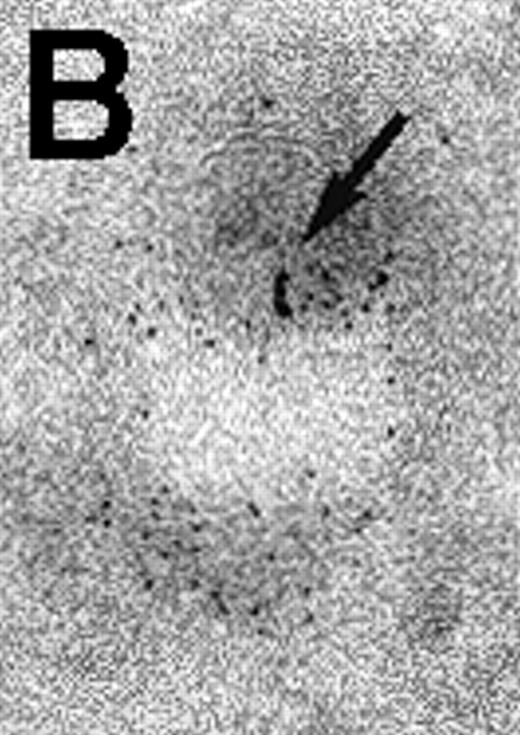
Next, the early steps of internalization of C3dg-gold conjugates by Raji cells were investigated. C3dg-gold10 was already detected after 7 minutes in close association with coated pits (Figure2A) preferentially located at the bases of microvilli. At the same time, C3dg-gold10 was also found in the cell periphery, ie, within uncoated tubules and vacuoles measuring up to 120 nm in diameter (Figures 2B and 2C). Together, this suggests that ligands for CR2 may be taken up by coated pits. However, it must be mentioned that even cryofixation failed in demonstrating C3dg-gold10 inside short-lived, small transport vesicles, the first structures to receive internalized material.
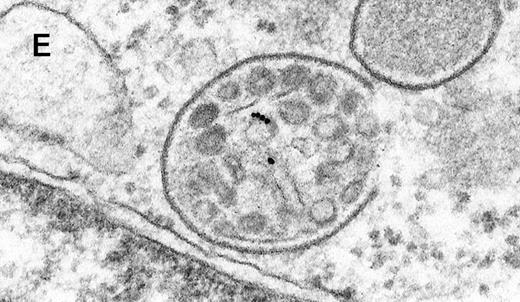
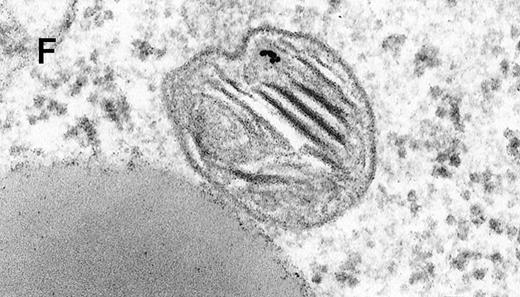
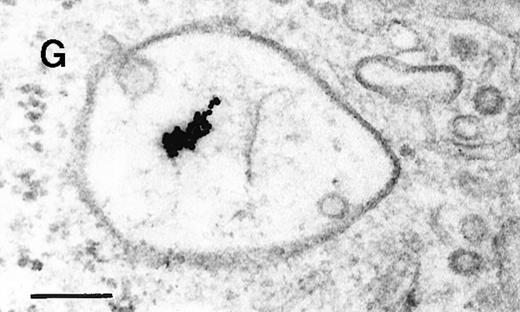
Binding of C3dg-gold10 to the plasma membrane and tracing to endocytic compartments of Raji cells.
(A) C3dg-gold10 in close vicinity to a coated pit marked by an arrow (incubation for 7 minutes at 37°C); scale bar, 0.1 μm. (B, C) Within peripherally located intracellular vacuoles (7 minutes at 37°C); (B) Cryofixation; scale bar, 0.15 μm. (D) Irregularly shaped multivesicular body in the cell periphery (30 minutes at 37°C); scale bar, 0.15 μm. (E) Multivesicular body in the vicinity of the nucleus (120 minutes at 37°C); scale bar, 0.15 μm. (F) Multilaminar body (120 minutes at 37°C); scale bar, 0.15 μm. (G) Multivesicular/multilaminar body harboring aggregations of gold particles indicating flocculation caused by proteolysis of gold-coupled C3dg (note that such aggregations do not stain with anti-C3d; data not shown; incubation for 120 minutes at 37°C); scale bar, 0.15 μm.
Binding of C3dg-gold10 to the plasma membrane and tracing to endocytic compartments of Raji cells.
(A) C3dg-gold10 in close vicinity to a coated pit marked by an arrow (incubation for 7 minutes at 37°C); scale bar, 0.1 μm. (B, C) Within peripherally located intracellular vacuoles (7 minutes at 37°C); (B) Cryofixation; scale bar, 0.15 μm. (D) Irregularly shaped multivesicular body in the cell periphery (30 minutes at 37°C); scale bar, 0.15 μm. (E) Multivesicular body in the vicinity of the nucleus (120 minutes at 37°C); scale bar, 0.15 μm. (F) Multilaminar body (120 minutes at 37°C); scale bar, 0.15 μm. (G) Multivesicular/multilaminar body harboring aggregations of gold particles indicating flocculation caused by proteolysis of gold-coupled C3dg (note that such aggregations do not stain with anti-C3d; data not shown; incubation for 120 minutes at 37°C); scale bar, 0.15 μm.
After incubation longer than 15 minutes, gold particles were detected within larger endocytic compartments of diverse morphology. These included irregularly shaped, peripherally located vacuoles and multivesicular bodies generally considered early endosomes (Figure 2D) peripheral to juxtanuclear spherical multivesicular, multilaminar bodies representing late endosomes, lysosomes, or both (Figures 2E, 2F and 2G), and, rarely, vesiculotubular structures in the vicinity of the Golgi apparatus.
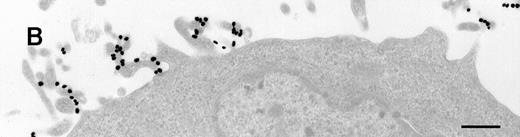
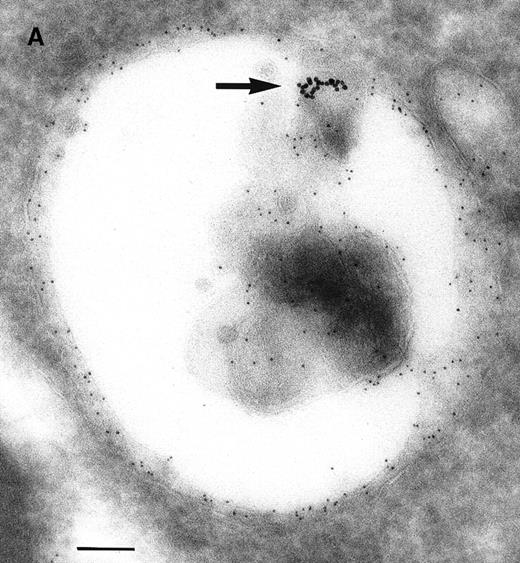
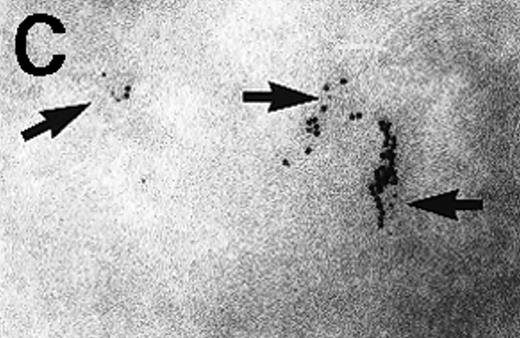
Immunoelectron microscopy revealed that the gold particles in endosomal and lysosomal structures clearly colocalized with MHC class II antigens; in particular, more than 70% of the gold particles internalized after 120 minutes were found in compartments showing strong specific labeling with anti-HLA-DR (Figures3A and 3B). Moreover, in approximately 30% of endosomes or lysosomes containing internalized gold, these gold particles could still be immunolabeled with anti-C3d (Figure 3C). Cell surface-bound gold particles stained readily with anti-C3d (Figure 3D). The specificity of the immunolabeling experiments was assessed by observing virtually no background labeling and by omitting primary antibodies or replacing them with irrelevant antibodies (data not shown).
Immunolabeling for MHC class II or C3d on thawed Tokuyasu cryosections from Raji cells having been exposed to C3dg-gold10 for 120 minutes at 37°C.
(A, B) Class II molecules are labeled with mAb HLA-DR/CR43 (anti–β-chain) visualized by antimouse immunoglobulin coupled to 5-nm gold particles. Colocalization with internalized C3dg-gold10 (arrows) occurs in juxtanuclear endosomes; scale bar, 0.1 μm. (C) A certain amount of endocytosed C3dg-gold10 stains with rabbit antihuman C3d (visualized by antirabbit immunoglobulin coupled to 5-nm gold particles; arrowheads); scale bar, 0.2 μm. (D) C3dg-gold10 bound to cell surface protrusions readily stains with rabbit antihuman C3d (visualized as in Figure 3C); scale bar, 0.2 μm.
Immunolabeling for MHC class II or C3d on thawed Tokuyasu cryosections from Raji cells having been exposed to C3dg-gold10 for 120 minutes at 37°C.
(A, B) Class II molecules are labeled with mAb HLA-DR/CR43 (anti–β-chain) visualized by antimouse immunoglobulin coupled to 5-nm gold particles. Colocalization with internalized C3dg-gold10 (arrows) occurs in juxtanuclear endosomes; scale bar, 0.1 μm. (C) A certain amount of endocytosed C3dg-gold10 stains with rabbit antihuman C3d (visualized by antirabbit immunoglobulin coupled to 5-nm gold particles; arrowheads); scale bar, 0.2 μm. (D) C3dg-gold10 bound to cell surface protrusions readily stains with rabbit antihuman C3d (visualized as in Figure 3C); scale bar, 0.2 μm.
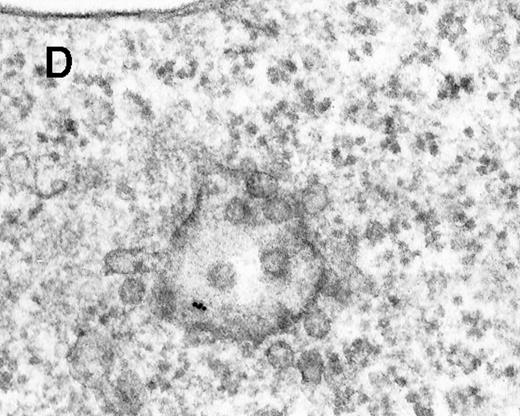
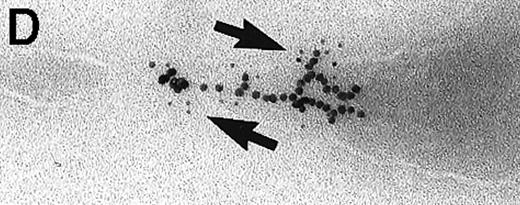
Large aggregations of gold particles (eg, Figure 2G) found in some of the late endosomes, lysosomes, and, to a minor extent, Golgi-associated structures were never attached to membranes and could not be stained with anti-C3d, which characterizes these structures as flocculated colloidal gold. Occasionally, after prolonged incubation (60 to 120 minutes), gold particles were detected within complex spherical compartments with different kinds of internal membranes and located just below the plasma membrane (Figure 4). These structures were only observed after cryofixation, indicating their transient or delicate nature.
Endocytosed C3dg-gold10 within complex multivesicular/multilaminar structures
in close vicinity to the plasma membrane (60 minutes at 37°C; cryofixation); scale bar, 0.15 μm.
Endocytosed C3dg-gold10 within complex multivesicular/multilaminar structures
in close vicinity to the plasma membrane (60 minutes at 37°C; cryofixation); scale bar, 0.15 μm.
Finally, we studied the internalization patterns of anti-CR2 mAbs with different epitope specificities coated to colloidal gold. Gold-conjugates (10 nm) prepared for each mAb (FE8, HB5, IIB9, and 1F8) yielded internalization patterns similar to C3dg-gold.101F8-gold10 showed less overall staining of endosomes, possibly because its epitope on CR2 is the most membrane proximal and perhaps less accessible or because of its relatively lower affinity. No differences were seen between C3dg-binding site-specific mAb FE8 and nonblocking mAbs HB5 and IIB9. In contrast to CR2-mAbs, colloidal gold coated with control mAb VD3 or with mAb 7F7 (anti–ICAM-1) was not endocytosed, though the latter showed intense plasma membrane staining.
Taken together, these data demonstrate that C3dg-coated soluble substrates are internalized in a BCR-negative B-cell line by receptor-mediated, ie, CR2-mediated, endocytosis.
Discussion
B cells are known to be explicitly efficient in taking up their specific antigen and processing it for MHC class II–restricted presentation of breakdown peptides to helper T cells.16,17Thus, B cells that need T cell help in addition to signals triggered by antigens through the BCR to develop into antibody-secreting cells stimulate specific helper T cells and obtain the required cytokine stimulus in return. Activation of complement can support the proliferation of specific B cells in response to antigen through interaction of antigen-bound C3dg with the CR2/CD19/CD81 receptor complex on the cell surface.5,9 B cells can thus respond to much lower concentrations of specific antigen. Additionally, signals from the BCR that influence endocytic uptake of antigen and its delivery to intracellular compartments rich in MHC class II28 may also be modulated and have an impact on antigen presentation.
A function for CR2 in antigen processing has been addressed by other investigators. Thornton et al15 have shown that B cells not specific for KLH, when incubated with C3dg-coated complexes of KLH and natural antibody, were able to stimulate KLH-specific T cells as efficiently as KLH-specific B cells incubated with KLH immune complexes devoid of C3dg. Boackle et al14 have demonstrated that influenza virus trapped in immune complexes incubated with Raji cells is more efficient in generating a specific T-cell response when the complexes contain C3-fragments. In both studies, the effect of complement was primarily mediated by CR2, whereas CR1 (on normal B cells) or Fc receptors were of minor importance. Most recently, Kozono et al29 have demonstrated that signaling by murine CR1/CR2 is sufficient to induce significant up-regulation of B7 molecules. Thus, if opsonized antigen is endocytosed by CR2 and presented by MHC class II, all requirements for the activation of TH cells will be met.
In the current study we investigated the ultrastructural basis for endocytosis of C3dg-carrying soluble antigens by B cells irrespective of BCR specificity. These data emphasize that C3dg coating of potential antigens alone is sufficient for their internalization to B cells by CR2. Interestingly, we observed binding of our model opsonized antigen C3dg-gold10 to CR2 only to microvilli or similar cell surface protrusions. Perhaps as a consequence of this preferred binding site, internalization of C3dg-gold10 occurred preferentially at the bases of microvilli, presumably through coated pits. The exact uptake mechanism (clathrin-dependent or not) is unclear, though it has been suggested that neutrophil CR1 becomes endocytosed through clathrin-coated pits30 and that CR1 and CR2 do cooperate on B-cell membranes.31 Localization of C3dg-gold in coated pits and in endocytic structures, with features characteristic of early endosomes, were already observed after short incubation periods. It is unlikely that unspecific uptake of C3dg-gold10 significantly contributed to this result because BSA-gold10 used at the same or at a 5-fold higher concentration, respectively, did not bind to the plasma membrane and was not detected intracellularly even after prolonged incubation. The low concentration of 100 ng gold-coupled protein per milliliter was used to reflect more closely the conditions in which CR2-mediated uptake would be of benefit (ie, with low levels of C3-coated antigen). Furthermore, concentrations of C3dg-gold up to 500 ng/mL used in preliminary tests caused nonspecific binding to cell debris but did not increase the amount of endocytosed gold-conjugates. These facts may also explain why, in contrast to other experimental systems, gold conjugates could not be demonstrated inside short-lived plasma membrane-derived vesicles. Anti-ICAM-1-gold10 yielded intense plasma membrane staining but was not taken up, a fact that has also been reported with other cell types.32 Interestingly, all the gold probes containing mAbs against CR2 were internalized irrespective of epitope specificities. Thus, aggregation of CR2 molecules alone or, more probably, of CR2/CD19/CD81 complexes is apparently sufficient to induce endocytosis of antigen attached to CR2. In contrast to this observation, others have reported that only OKB7, an mAb against the C3dg-binding site of CR2, was able to enhance c-fos transcription in resting B cells when cross-linked with the BCR, whereas mAb HB5 was not.33 Endocytosis through CR2 is apparently not restricted by this epitope specificity.
Antigen presentation requires the proteolytic cleavage of antigen into peptides and loading of these onto MHC class II molecules. It is still unresolved in which intracellular compartment(s) this process takes place in the different MHC class II–expressing cell types and how peptide-loaded class II reaches the plasma membrane.1,34-36For B cells, Kleijmeer et al18 proposed that class II loading involves endocytic structures of different morphology that are also found in other cell types, ie, multivesicular and multilaminar endosomes representing late endosomes or lysosomes. Our data, based on the morphology of endosomes and immunolabeling for class II and C3dg, are compatible with this report because C3dg-gold10 was shown to reach these compartments. The gold-containing multivesicular/laminar structures found after 60 to 120 minutes next to the plasma membrane might correspond to MIICs before exocytosis37; however, more work is needed to address this issue in detail.
C3b attached to tetanus toxoid (TT) leads not only to enhanced uptake through CR2 by non–TT-specific B cells but also to retarded proteolysis of TT by cathepsin B in early endosomes.12Jacquier–Sarlin et al12 reported that this feature contributed substantially to more sustained T-cell activation. In the current study, we observed flocculation or aggregation of gold particles in late endosomes or lysosomes that may be interpreted as proteolytic degradation of gold-coupled C3dg itself. C3dg-gold10 on the plasma membrane and in endosomes was frequently found in pairs or in small groups of gold particles. We think it is unlikely that this reflects an artifact: first, plasma membrane-bound gold particles stained readily with anti-C3d; second, neither incubation at 4°C nor the use of cryofixation instead of chemical fixation changed this feature. It may rather result from physiological clustering of CR2. Binding of C3dg to CR2 depends on its oligomerization or polymerization to increase the avidity because the Ka for monomeric C3dg is only 27.5 μmol/L.38Thus, cross-linking of CR2 molecules is a constant feature in the interaction with complement-coated ligands. This may well explain our observation of C3dg-gold10 in small clusters. The fact that incubation at 4°C, even in the presence of sodium azide, did not significantly alter this pattern may be explained by preclustering of CR2 complexes. The observation that C3dg-gold10 staining is confined to microvilli may support that view because CR2 in this location would be more accessible for C3-fragment carrying particles.
What may be a physiological function of CR2-mediated endocytosis in B cells shown here with regard to antigen specificity? Clearly, antigen-specific B cells can be assumed to profit most by receiving enhancing proliferation stimuli. The by far higher number of nonspecific B cells, however, could serve as presenters of “nonspecific” antigen without their being activated in a nonspecific way. Results obtained in vitro support this assumption.39 The large number of antigen-presenting B cells could be expected to increase dramatically the probability for TH cell activation, which in turn raises the chances for interaction of TH and B cells seeing the same antigen. Considering the much higher capacity of DCs, for instance, to process unselectively or “semiselectively” internalized antigen, the potential of “semispecific” antigen uptake by B cells has to be quantitated and may be different for soluble and large particulate antigens. Although B cells can ingest and process particles in the size of bacteria,40 macrophages and DC are clearly more important in this respect. Gustavsson et al41 reported that the administration of an mAb blocking the C3dg-site in murine CR1/CR2, together with sheep red blood cells (SRBC) as a particulate antigen, blocks generation of an SRBC-specific B-cell response but does not inhibit the priming of SRBC-specific TH cells in these mice. This may indicate a redundant role for antigen presentation by nonspecific B cells, at least for particulate antigens.41 Clearly, more direct experimental work has to be performed before conclusive answers can be given.
Acknowledgments
We thank Karin Gutleben, Beate Michel, Arja Strandell, Mervi Lindman, Rudolf Haring, Karin Schluifer, Martin Wurm, and Helmut Grubhofer for excellent technical assistance.
Supported by Austrian Science Fund FWF Grant F202 (W.M.P.).
M.W.H. and M.G.S. contributed equally to this work.
Reprints:Wolfgang M. Prodinger, Institut für Hygiene und Sozialmedizin, University of Innsbruck, Fritz-Pregl-Strasse 3, A-6020 Innsbruck, Austria; e-mail: wolfgang.prodinger@uibk.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal