Abstract
γ-Radiation is a potent inducer of apoptosis. There are multiple pathways regulating DNA damage-induced apoptosis, and we set out to identify novel mechanisms regulating γ-radiation–induced apoptosis in hematopoietic cells. In this report, we present data implicating the cyclin B1 protein as a regulator of apoptotic fate following DNA damage. Cyclin B1 is the regulatory subunit of the cdc2 serine/threonine kinase, and accumulation of cyclin B1 in late G2 phase of the cell cycle is a prerequisite for mitotic initiation in mammalian cells. We find that abundance of the cyclin B1 protein rapidly increases in several mouse and human hematopoietic cells (Ramos, DP16, HL60, thymocytes) undergoing γ-radiation–induced apoptosis. Cyclin B1 accumulation occurs in all phases of the cell cycle. Antisense inhibition of cyclin B1 accumulation decreases apoptosis, and ectopic cyclin B1 expression is sufficient to induce apoptosis. These observations are consistent with the idea that cyclin B1 is both necessary and sufficient for γ-radiation-induced apoptosis.
The cyclin B protein and its binding partner, the cdc2 serine/threonine kinase, regulate mitotic initiation in vertebrate cells.1-3 The cyclin B/cdc2 heterodimer induces mitosis by phosphorylating and activating enzymes regulating chromatin condensation, nuclear membrane breakdown, and mitosis-specific microtubule reorganization.4 Activation of cdc2 requires changes in cdc2 phosphorylation5 as well as association with cyclin B.2 There are two human and mouse B-type cyclins, B1 and B2. Cyclin B2 is not essential for mouse development, and mice homozygous for a targeted deletion of the cyclin B2 gene are viable, fertile, and develop normally.6 Conversely, homozygous deletion of cyclin B1 leads to death in utero,6consistent with the idea that cyclin B1 is likely the primary regulator of mammalian mitosis.
Regulation of intracellular cyclin B levels is one of the mechanisms controlling mitotic initiation. In human cells, an inhibition of cyclin B1 transcription by the p53 tumor suppressor prevents G2/M transition.7 In Xenopus oocytes, the amount of cyclin B protein is 20- to 30-fold higher in late G2 than in G1, and a threshold level of cyclin B must be reached before mitosis can proceed.1
Cyclin B1 and cdc2 are also apoptotic regulators. Apoptosis, or programmed cell death, occurs in response to DNA damage,8in limb development, and during hematopoiesis and lymphopoiesis.9-11 Furthermore, apoptosis regulates the cytotoxicity of the anticancer agents γ-radiation, Adriamycin, 5-fluorouracil, etoposide, and cisplatin.12,13 The apoptosis induced by granzyme B, taxol, Fas, camptothecin, Nerve Growth Factor (NGF) withdrawal, human immunodeficiency virus infection, and T-cell activation are all associated with cdc2 activation and/or accumulation of cyclin B1 protein.14-20Thus, cdc2 and cyclin B1 are likely to be involved in multiple apoptotic pathways.
In this report, we present data suggesting that cyclin B1 protein abundance regulates the apoptosis caused by γ-radiation. We have found that cyclin B1 protein levels rapidly increase during γ-radiation–induced apoptosis in several mouse and human hematopoietic cells (Ramos, DP16, HL60) as well as in primary thymocytes. Consistent with a role for cyclin B1 in apoptosis, we have found that antisense inhibition of cyclin B1 accumulation prevents γ-radiation–induced apoptosis and that ectopic cyclin B1 expression is sufficient to induce apoptosis. These results suggest that the cellular decision to enter into apoptosis is regulated, at least in part, by the abundance of the cyclin B1 protein.
Materials and methods
Cell culture
Ramos is a human Burkitt's lymphoma line and HL-60 is a human promyelocytic leukemia, both maintained in RPMI 1640 medium (Gibco BRL), 10% fetal bovine serum (FBS) (Gibco BRL), and 2% penicillin-streptomycin (Sigma). Both lines were obtained from the American Type Culture Collection (ATCC). The mouse erythroleukemia cell line DP-16 is derived from a radiation/2J mouse infected with the polycythemia strain of Friend murine virus and was maintained in Dulbecco's modified minimal essential medium supplemented with 10% FBS (Gibco BRL) and 2% penicillin-streptomycin (Sigma). Thymocytes were obtained by removing the thymus from an 8-week-old C57Bl/6 mouse and gently squeezing out the cells with forceps. Thymocytes were washed in phosphate-buffered saline (PBS) and resuspended in RPMI supplemented with 10% FBS. All cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cell viability was determined by trypan blue.
Cell-cycle analysis
Cells (106) were suspended in Tris buffer (0.10 mol/L Tris, 0.10 mol/L NaCl in deionized H20, pH 7.6), ice-cold lysis solution (0.01 mol/L glycine, 0.3 mol/L NaCl, 0.10% v/v Triton-X, pH10), RNase A (100 μL of 1 mg/mL), and ethidium bromide (50 μL of 0.1 mg/mL, 0.013 mmol/L) were incubated for 10 minutes at 4°C. Cell-cycle profiles of no less than 20 000 cells were generated on a Coulter EPICS IV Profile or an EPICS XL flow cytometer. Data were analyzed by the MCYCLE program for cell cycle distribution histograms (Phoenix Flow Systems).
Elutriation of cell-cycle fractions
At least 107 cells were suspended in 10 mL of PBS containing 5 mmol/L glucose, 10 mmol/L sodium citrate, and 5% w/v albumin and loaded into a Beckman J2-21 centrifuge equipped with a JE-6B elutriation system and rotor. Flow rate was kept constant at 17 mL/min. Rotor speed was initially set at 4000 rpm until loading was complete and then decreased by 100 rpm for each consecutive sample. Samples of 100 mL were collected, starting at 2500 rpm and continuing until the rotor speed was at 1000 rpm. DNA profiles for each sample were analyzed by flow cytometry.
Cyclin B1 antisense and transfection
Scrambled (5′AGGTTTGATGGCGACCTGTGA) and antisense (5′ CATCGGGCTTGGAGAGGGATT) oligonucleotides were prepared by McMaster University MOBIX Sequencing Center. Cells were transfected with 5 μg scrambled/antisense or mock-control vectors using 5 μL of Superfect reagent (Qiagen) for 3 hours, fresh media were added, and cells irradiated with 600 rads of γ-radiation. Delivery efficiencies were observed for each experiment by transfecting a control population with fluorescein isothiocyanate-labeled sense/antisense oligonucleotides and observing the number of transfected cells under the fluorescent microscope. Overexpression studies were conducted in a similar manner, using either 5 μg of cyclin B1/cytomegalovirus (generous gift of Dr Phil Hinds, Harvard) or control pDNA3 with 5 μL superfect reagent. Transfections were left for 5 hours, fresh media were added, and cells were irradiated 3 hours later with 200 rads of γ-radiation.
Apoptosis detection
Redistribution of phosphatidylserine to the outer plasma membrane was visualized by incubating the cells with either fluorescein isothiocyanate-conjugated human recombinant Annexin V (Immunotech) or Cy3 as per the manufacturers' directions. For the detection of DNA fragmentation, 1 × 106 cells were centrifuged at 14 000g for 10 seconds and the media aspirated. Cells were resuspended in 200 μL of 100 mmol/L Tris (pH 7.5), 10 mmol/L EDTA, 100 mmol/L NaCl, 0.5% SDS, and 10 μg/mL proteinase K and incubated at 55°C overnight. Extracts were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, visualized under UV light, and photographed using a Strategen Eagle Eye video camera.
Immunoblotting
To prepare cells for immunoblotting, cells were washed with PBS once and resuspended in 300 μL lysis buffer (1% NP40, 50 mm Tris HCl, pH 7.4, 5 mmol/L EDTA, 150 mmol/L NaCl, 2 μmol/L leupeptin, 400 μm phenylmethylsulfonyl fluoride, and 5 μg/mL aprotinin). Samples were left on ice for 20 minutes, centrifuged at 14 000g for 10 minutes at 4°C and the supernatant collected. Protein concentration was determined using the bicinchoninic acid method (Pierce, Rockford, IL), and 10 μg of total protein was electrophoresed on a 10% SDS-polyacrylamide gel and transferred to nitrocellulose paper. The blot was then incubated in 5% nonfat dried milk/TBST, 25 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% Tween20 for 1 hour at room temperature, then incubated with a primary antibody diluted 1:1000 in the same buffer for 1 hour also at room temperature. The blot was washed three times in TBST and horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse or anti-rabbit immunoglobulin G, Kirkegaard & Perry Laboratories) added (diluted 1:1000) for 1 hour. Primary antibodies included mouse monoclonal anti-human cyclin B1 and cyclin A antibodies (Oncogene Science) and mouse monoclonal anti-mouse cyclin B1 (Santa-Cruz). Where necessary, immunoblots were stripped by incubating in 100 mmol/L mercaptoethanol, 2% sodium dodecyl sulfate, 62.5 mmol/L Tris-HCl, pH 6.7 for 30 minutes at 50°C followed by two washes in TBST. For immunoprecipitation, cell lysates were mixed with 10 μL of primary antibody and incubated at 4°C overnight. The samples were then mixed with 50 μL protein A sepharose beads (Pharmacia Biotech) and rotated for at least 1 hour, centrifuged, and washed with 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP-40. Immunoprecipitated proteins were used for immunoblotting or for kinase assay as indicated.
Cdc2 kinase assay
The in vitro cdk1 kinase uses phosphorylation of Histone H1 as a measure of cdk1 activity. Immunoprecipitated cdk1 (10 μL) was incubated with 10 μL of 2mg/mL Histone H1, 10 μL of a non-cdk1 kinase inhibitor cocktail, 20 μmol/L PKC inhibitor peptide, 2 μmol/L protein kinase A inhibitor peptide, and 20 μmol/L compound R2571. Histone H1 and the inhibitors were made up in 20 mmol/L MOPS, ph7.2, 25 mmol/L glycerol phosphate, 5 mmol/L EGTA, 1 mmol/L sodium orthovanadate, and 1 mmol/L dithiothreitol. The reaction was started by adding 9 μL magnesium/adenosine triphosphate (ATP) cocktail (75 mmol/L magnesium chloride and 500 μmol/L ATP) containing 1 μL of 100 μCi [32P]ATP (3000 Ci/mmol; Amersham Life Sciences) and incubated at 30°C for 10 minutes. The reaction was stopped by pipetting 25 μL of the reaction mixture onto P81 phosphocellulose paper. The radiolabeled substrate was allowed to bind to the filter paper for 30 seconds before immersing the paper into a beaker containing 0.75% phosphoric acid. The papers were washed with up to 10 rinses of 0.75% phosphoric acid. After washing, acetone was added for 2 minutes. Bound radioactivity was quantitated by adding scintillation cocktail and counting on a Beckman scintillation counter for 2 min/vial. Equal loading was determined by Western blotting the immunoprecipitated protein and probing the membranes with [125I]-protein A.
Results
Accumulation of cyclin B1 and cdc2 activation during γ-radiation–induced apoptosis
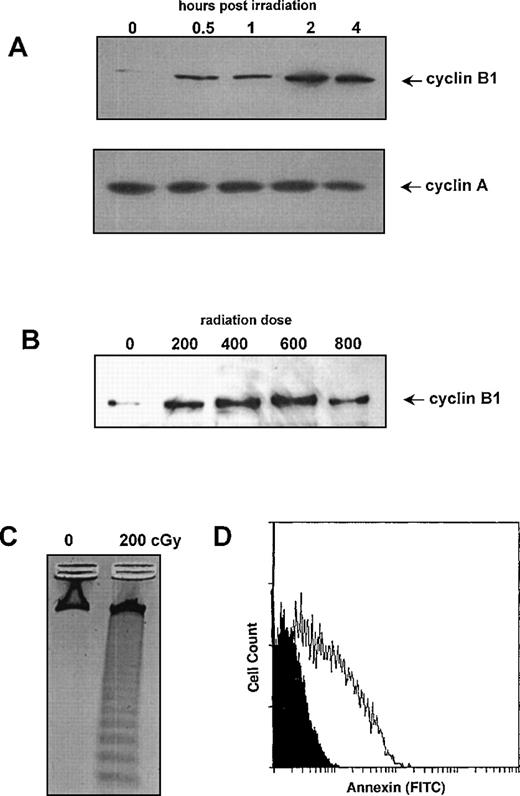
To determine whether cyclin B1 is involved in the γ-radiation response, we initially measured protein levels of cyclin B1 in human Ramos cells undergoing γ-radiation–induced apoptosis. As shown in Figure 1A, 200 cGy of γ-radiation stimulated the accumulation of cyclin B1 protein but did not alter the levels of cyclin A protein. Cyclin B1 protein is first detectable at 0.5 hour postirradiation, with levels reaching a plateau at 2 hours. The level of cyclin B1 protein is sustained for 24-48 hours (not shown). Induction of cyclin B protein occurs in a dose range of 200-800 cGy (Figure 1B). A dose of 200 cGy of radiation is sufficient to induce nucleosomal fragmentation (Figure 1C) and Annexin V staining (Figure 1D) in Ramos cells. Annexin V staining, the result of phosphatidylserine translocation from the inner to the outer plasma membrane in apoptotic cells, is commonly used as a marker for early stages of apoptosis.21
Effect of γ-radiation on cyclin B1 protein levels in Ramos cells.
(A) Western blots of protein lysates (10 μg/lane) were prepared at the indicated time points following treatment with 200 cGy of γ-radiation. Blots were probed with either cyclin A or cyclin B1 antibodies. (B) Western blot of protein lysates was prepared 4 hours following treatment with indicated doses of γ-radiation. Blots were probed with cyclin B antibodies. (C) DNA fragmentation was measured 24 hours following treatment with 200 cGy of γ-irradiation. Control cells were not irradiated but maintained under identical conditions. (D) Annexin V staining of cells 24 hours following treatment with 200 cGy of γ-radiation (white profile) relative to mock irradiated cells (black profile).
Effect of γ-radiation on cyclin B1 protein levels in Ramos cells.
(A) Western blots of protein lysates (10 μg/lane) were prepared at the indicated time points following treatment with 200 cGy of γ-radiation. Blots were probed with either cyclin A or cyclin B1 antibodies. (B) Western blot of protein lysates was prepared 4 hours following treatment with indicated doses of γ-radiation. Blots were probed with cyclin B antibodies. (C) DNA fragmentation was measured 24 hours following treatment with 200 cGy of γ-irradiation. Control cells were not irradiated but maintained under identical conditions. (D) Annexin V staining of cells 24 hours following treatment with 200 cGy of γ-radiation (white profile) relative to mock irradiated cells (black profile).
Cyclin B1 is the regulatory subunit of the cyclin dependent kinase, cdc2. To determine if γ-radiation alters cdc2 activity, we measured kinase activity in γ-irradiated Ramos cells. As shown in Figure2A, 200 cGy of radiation leads to a reproducible 2-fold increase in cdc2 kinase activity. Increased cdc2 activity is first detectable 30 minutes postirradiation and peaks after 1 hour. Activity of cdc2 falls below control levels by 12 hours postirradiation (Figure 2A). This activation of cdc2 is not due to an alteration in the levels of cdc2 protein (Figure 2B).
Effect of radiation on cdc2 activity.
Cdc2 was immunoprecipitated from human Ramos cells at various time points following treatment with 200 cGy of γ-radiation. (A, B) H1 kinase activity of cdc2 over time. (C) Western blot of cdc2 immunoprecipitate probed with [125I]-Protein A.
Effect of radiation on cdc2 activity.
Cdc2 was immunoprecipitated from human Ramos cells at various time points following treatment with 200 cGy of γ-radiation. (A, B) H1 kinase activity of cdc2 over time. (C) Western blot of cdc2 immunoprecipitate probed with [125I]-Protein A.
Cyclin B1 accumulation in cell lines and primary thymocytes
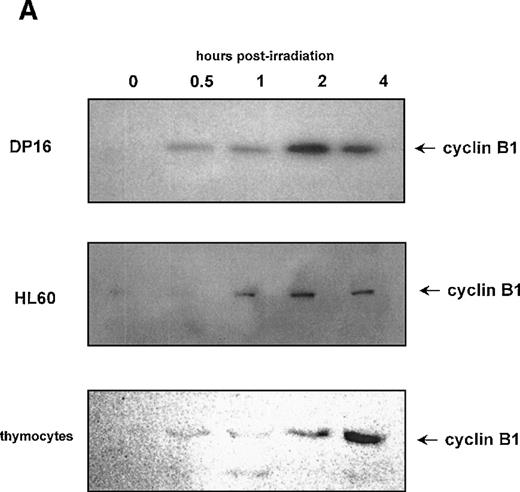
Figures 1 and 2 suggest a role for cyclin B1 and cdc2 in the radiation response. To determine whether radiation-induced accumulation of cyclin B1 is a common property of cells undergoing apoptosis, we measured cyclin B1 protein levels in the mouse DP16 erythroleukemia cell line, the human promyelocytic leukemia line HL60, and primary mouse C57 bl/6 thymocytes following γ-radiation. These cells lines were chosen as a broad spectrum of human and mouse hematopoietic cell types. As shown in Figure 3A, all of these cell lines displayed a radiation-induced accumulation of cyclin B1 protein. Although the kinetics of cyclin B1 accumulation in each cell line is somewhat different, cyclin B1 levels increase in all cells 4 hours post–γ-irradiation. These cell lines all undergo apoptosis as measured by DNA fragmentation and Annexin V staining (Figure 3B, 3C). To determine whether cyclin B1 accumulation may be a component of other thymocyte apoptosis programs, we determined whether cyclin B1 protein levels increased during dexamethasone-induced apoptosis. Dexamethasone and γ-radiation are known to induce thymocyte apoptosis through independent pathways.22 As shown in Figure 3D, cyclin B1 protein levels increase during the apoptosis (Figure 3E) caused by the steroid.
Effect of γ-irradiation on cyclin B1 accumulation in hematopoietic cell lines.
(A) Protein lysates (10 μg/lane) of DP16, HL60, and primary mouse thymocytes were prepared at various time points following γ-irradiation, and the levels of cyclin B1 protein measured by Western blot. Cells were treated with 200 cGy of radiation. (B) DNA fragmentation 24 hours following treatment with γ-radiation (+ lanes). Control cells (- lanes) were mock irradiated and maintained under identical conditions. (C) Annexin staining of DP16 and HL60 24 hours following γ-irradiation (white profiles) relative to mock irradiated cells (black profiles). (D) Stimulation of cyclin B1 protein accumulation in primary thymocytes by both 200 rad of γ-radiation and 1 μg of dexamethasone. (E) Thymocyte DNA fragmentation stimulated by radiation and dexamethasone.
Effect of γ-irradiation on cyclin B1 accumulation in hematopoietic cell lines.
(A) Protein lysates (10 μg/lane) of DP16, HL60, and primary mouse thymocytes were prepared at various time points following γ-irradiation, and the levels of cyclin B1 protein measured by Western blot. Cells were treated with 200 cGy of radiation. (B) DNA fragmentation 24 hours following treatment with γ-radiation (+ lanes). Control cells (- lanes) were mock irradiated and maintained under identical conditions. (C) Annexin staining of DP16 and HL60 24 hours following γ-irradiation (white profiles) relative to mock irradiated cells (black profiles). (D) Stimulation of cyclin B1 protein accumulation in primary thymocytes by both 200 rad of γ-radiation and 1 μg of dexamethasone. (E) Thymocyte DNA fragmentation stimulated by radiation and dexamethasone.
Cyclin B1 accumulation is not the result of a change in cell-cycle position
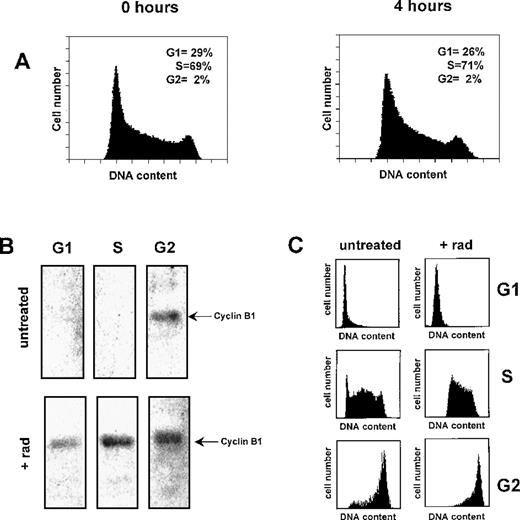
Cyclin B1 protein accumulates in late G2 phase of the cell cycle, the combined result of increased transcription and messenger RNA (mRNA) stabilization. It is possible that the observed accumulation of B1 protein in γ-irradiated Ramos cells is the result of an accumulation of cells in G2 phase. To investigate this possibility, we measured the cell-cycle position of Ramos cells during the time period for which we observe cyclin B1 accumulation. As shown in Figure4A, a γ-radiation dose (200 cGy) that induces B1 protein accumulation (Figure 1) has no observable affect on the percentage of cells in G1, S, or G2/M 4 hours postirradiation.
Cyclin B1 accumulates in a cell-cycle independent manner following γ-irradiation.
(A) Flow cytometry histograms of human Ramos cells 4 hours following treatment with 200 cGy radiation. (B) Effect of γ-radiation on cyclin B1 protein levels at different phases of the cell cycle. Ramos cells were irradiated and 4 hours later separated into G1, S, and G2 cell-cycle fractions by centrifugal elutriation. Total protein lysates of each fraction were probed with cyclin B1 antibodies (5 μg total protein per lane). Untreated cells were mock irradiated and maintained under identical culture conditions to the irradiated Ramos cells. (C) Cell cycle histograms from control and γ-irradiated cell fractions.
Cyclin B1 accumulates in a cell-cycle independent manner following γ-irradiation.
(A) Flow cytometry histograms of human Ramos cells 4 hours following treatment with 200 cGy radiation. (B) Effect of γ-radiation on cyclin B1 protein levels at different phases of the cell cycle. Ramos cells were irradiated and 4 hours later separated into G1, S, and G2 cell-cycle fractions by centrifugal elutriation. Total protein lysates of each fraction were probed with cyclin B1 antibodies (5 μg total protein per lane). Untreated cells were mock irradiated and maintained under identical culture conditions to the irradiated Ramos cells. (C) Cell cycle histograms from control and γ-irradiated cell fractions.
To further establish that the γ-radiation–induced accumulation of cyclin B1 is not the result of cell-cycle alterations, we separated γ-irradiated and control unirradiated Ramos cells into their respective G1, S, and G2 cell-cycle phases. The level of cyclin B1 protein from each phase was then measured by Western blotting. As shown in Figure 4B, cyclin B1 protein is only detectable in unirradiated Ramos cells in G2 phase. However, 4 hours after Ramos has been γ-irradiated, cyclin B1 protein is detectable in G1, S, and G2 cells. Cell-cycle profiles of each of the elutriated phases in control and irradiated Ramos cells are shown in Figure 4C. Thus, γ-irradiation causes the expression of cyclin B1 in all phases of the cell cycle.
Cyclin B1 is necessary and sufficient for radiation-induced apoptosis
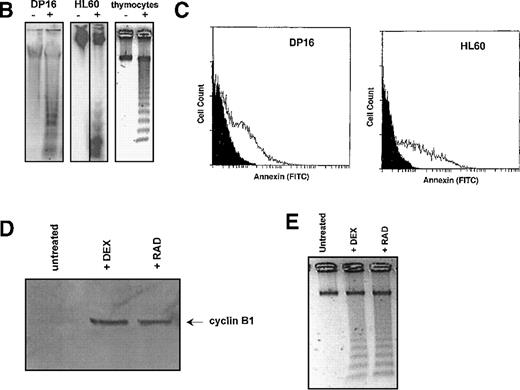
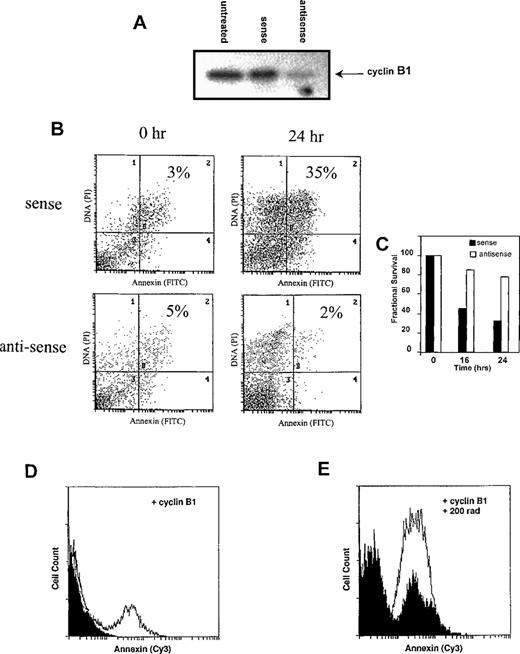
To determine whether cyclin B1 accumulation was necessary for γ-radiation–induced apoptosis, we reduced cyclin B1 protein levels in Ramos cells using antisense oligonucleotides. As shown in Figure5A, treatment of Ramos cells with antisense cyclin B1 oligonucleotides substantially reduced the amount of cyclin B1 protein following irradiation. Control oligonucleotides had no such effect. As shown in Figure 5B and 5C, the antisense-mediated reduction in cyclin B1 levels leads to a reduction in Annexin V staining apoptotic cells and a concomitant increase in Ramos cell viability, relative to sense-treated and control-untreated Ramos cells. Similar results were seen with DP16 and HL60 (not shown).
Cyclin B1 is both necessary and sufficient for γ-radiation–induced apoptosis.
(A-C) Cyclin B1-antisense oligonucleotides inhibit γ-radiation–induced apoptosis. Ramos cells were treated with 5 μmol/L sense or antisense oligonucleotides and subjected to γ-irradiation 4 hours later. Four hours following γ-irradiation, (A) cyclin B1 protein levels were measured by Western blot, indicating that antisense oligonucleotides, but not the sense control, prevent cyclin B1 protein accumulation following γ-irradiation. Untreated cells were irradiated but not exposed to sense or antisense oligonucleotides. (B) Apoptosis, as measured by Annexin staining (quadrants 2 and 4), is decreased by antisense treatment relative to the sense control. Results are representative of three experiments. (D-E) Ectopic cyclin B1 expression is sufficient to induce apoptosis. Ramos cells were transfected with 5 μg of either a control pCDNA3 plasmid or cyclin B1. (D) Transfection of cyclin B1 (white profile), relative to the pCDNA3 control (black profile), resulted in an increase of apoptosis, as measured by an increase in the number of Annexin staining cells. (E) Twenty-four hours after transfection with cyclin B1 or pCDNA3, cells were exposed to 200 rad of γ-radiation. Transfection of cyclin B1 increased the number of Annexin staining cells following irradiation (white profile) relative to the control pCDNA3 transfected cells (black profile).
Cyclin B1 is both necessary and sufficient for γ-radiation–induced apoptosis.
(A-C) Cyclin B1-antisense oligonucleotides inhibit γ-radiation–induced apoptosis. Ramos cells were treated with 5 μmol/L sense or antisense oligonucleotides and subjected to γ-irradiation 4 hours later. Four hours following γ-irradiation, (A) cyclin B1 protein levels were measured by Western blot, indicating that antisense oligonucleotides, but not the sense control, prevent cyclin B1 protein accumulation following γ-irradiation. Untreated cells were irradiated but not exposed to sense or antisense oligonucleotides. (B) Apoptosis, as measured by Annexin staining (quadrants 2 and 4), is decreased by antisense treatment relative to the sense control. Results are representative of three experiments. (D-E) Ectopic cyclin B1 expression is sufficient to induce apoptosis. Ramos cells were transfected with 5 μg of either a control pCDNA3 plasmid or cyclin B1. (D) Transfection of cyclin B1 (white profile), relative to the pCDNA3 control (black profile), resulted in an increase of apoptosis, as measured by an increase in the number of Annexin staining cells. (E) Twenty-four hours after transfection with cyclin B1 or pCDNA3, cells were exposed to 200 rad of γ-radiation. Transfection of cyclin B1 increased the number of Annexin staining cells following irradiation (white profile) relative to the control pCDNA3 transfected cells (black profile).
To determine if cyclin B1 accumulation is sufficient to induce apoptosis, cyclin B1 under the control of the cytomegalovirus promoter was transiently transfected into Ramos cells. As shown in Figure 5D, transfection of cyclin B1 increased the number of Annexin V-positive cells relative to the pCDNA3 vector control. Furthermore, ectopic cyclin B1 expression increases the number of apoptotic cells in response to γ-radiation (Figure 6E). Similar results are seen with HL60 and DP16 cells (not shown). Transfection efficiencies in these experiments were on the order of 30%-40%. Taken together, Figures 5A-E suggest that cyclin B1 is both necessary and sufficient for γ-radiation–induced apoptosis.
Discussion
Cyclin B1 and cdc2 are known to be regulators of a variety of apoptotic stimuli.14-20 In this report, we show that abundance of the cyclin B1 protein has a key role in controlling γ-radiation–induced apoptosis. Cyclin B1 protein levels increase in hematopoietic cells undergoing apoptosis, and inhibition of this increase with antisense oligonucleotides inhibits apoptosis. Moreover, ectopic expression of cyclin B1 is sufficient to induce apoptosis, consistent with the idea that cyclin B1 is both necessary and sufficient for γ-radiation–induced apoptosis.
One of the major regulators of the apoptotic response to γ-radiation is the p53 protein. p53 is a tumor suppressor, mutated in 50% of human cancers,23 which regulates cell growth and the sensitivity to γ-radiation and multiple anticancer agents.8 Loss of p53 function in Li-Fraumeni patients or in experimental mouse models leads to a loss of γ-radiation–induced apoptosis and the development of a radiation-resistant cellular phenotype.8 However, many p53-deficient cell lines retain the ability to undergo γ-radiation–induced apoptosis, suggesting that there are p53-independent pathways of apoptosis.24 The cell lines that we have used to demonstrate cycle B1 accumulation have either a p53 mutation (Ramos) or have lost p53 entirely (DP16, HL60).25-27 Furthermore, we have observed accumulation of cyclin B1 in thymocytes undergoing dexamethasone-induced apoptosis, a process known to be p53 independent.22 Thus, accumulation of cyclin B1 is likely to be a p53-independent event, and cyclin B1 accumulation is likely to be a critical component of p53-independent apoptosis.
Not all cell lines accumulate cyclin B1 in response to γ-radiation. It has previously been reported that γ-radiation exposure decreases total B1 protein and an inactivation of cdc2 in human HeLa.28-31 This decrease is one of the factors responsible for DNA damage-induced G2/M arrest in HeLa cells, and ectopic cyclin B1 expression partially rescues the G2/M arrest.32 Moreover, exposure of HeLa cells to γ-radiation leads to cdc2 inactivation; HeLa cells have a general resistance to DNA damage-induced apoptosis and die by necrosis in response to radiation.33 It is possible that the failure of HeLa and other cells to undergo apoptosis in response to radiation may be directly related to their failure to accumulate cyclin B1.
How does γ-radiation lead to cyclin B1 accumulation? Cyclin B1 protein abundance is tightly regulated and, during the normal cell cycle, it is highest in late G2 phase.1 Cyclin B1 abundance is controlled by (i) activity of the B1 promoter, (ii) stability of the B1 mRNA, and (iii) ubiquitin-mediated proteolysis of the B1 protein.29,34-36 We have found that γ-radiation–induced accumulation of cyclin B1 occurs in all phases of the cell cycle, suggesting that some or all of these processes may be affected. Cyclin B1 transcription is activated by the USF and NF-Y transcription factors34,37 and can be inhibited by p53 and MyoD. However, it remains to be established if USF and NF-Y activity is increased by γ-radiation. γ-Radiation is known to activate several signaling cascades, including activation of Abl, BTK, JNK, Lyn, Fyn, Raf-1, and Src kinases.28 38-41 Activation of any or all of these signaling molecules by γ-radiation may result in an increase in cyclin B1 transcription. Determining which cascades are important in activating cyclin B1 following γ-radiation will be important in understanding the mechanisms of γ-radiation–induced apoptosis and in understanding why some cells apoptose and others do not.
We have found that the accumulation of cyclin B1 following irradiation is associated with an activation of the cdc2 kinase. Activation of cdc2 and/or cdk2 is associated with multiple forms of apoptosis, including the cell death induced by FAS,42 TNF,43 and granzyme B.15 Furthermore, granzyme B- and Myc-induced apoptosis is associated with accumulation of cyclin A protein and mRNA.44,45 However, there is some controversy as to the absolute requirement for cdc2 and cdk2 in apoptosis. Although dominant negative forms of both cdk2 and cdc2 can inhibit apoptosis,46 indicating a requirement for these two kinases, other investigators have reported that inhibition of cdc2 activity actually increases apoptosis in some cells47 and that cdc2 and cdk2 activation are not sufficient for triggering apoptosis.48-50 Thus, it is likely that there are both cdc2-dependent and -independent forms of apoptosis.
It has yet to be established how cyclin B1 induces apoptosis. Apoptosis is a pathway of programmed cell death involving mitochondrial alteration, cysteine protease activation, and DNA cleavage.51-54 Conceivably, cyclin B1 and cdc2 could regulate apoptosis by phosphorylating and activating the molecules that regulate any or all or these processes. A further investigation into cyclin B1 will likely shed new light onto these questions.
Acknowledgments
We thank Steve Innocente, Anthony Bruce, and Drs John Hassell, Maria Rozakis-Adcock, Michael Rudnicki, and Peter Whyte for helpful discussions and critical reading of this manuscript. We also thank Susan Sweet for providing her expertise in the cell cycle analysis as well as Kathryn Adams and Sarka Lohtak for technical assistance with the flow cytometer.
Supported by a grant from the Medical Research Council of Canada (J.M.L.). L.A.P. is the recipient of a studentship from the Leukemia Research Fund of Canada.
Reprints:Jonathan M. Lee, Hamilton Regional Cancer Center, 699 Concession St, Hamilton, Ontario, Canada L8V 5C2; e-mail:jonathan.lee@hrcc.on.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Effect of radiation on cdc2 activity. / Cdc2 was immunoprecipitated from human Ramos cells at various time points following treatment with 200 cGy of γ-radiation. (A, B) H1 kinase activity of cdc2 over time. (C) Western blot of cdc2 immunoprecipitate probed with [125I]-Protein A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2645/4/m_bloo00818002w.jpeg?Expires=1769786394&Signature=dYyLvGr4C803SzN09BniH~IfbYXpiB1alVd1WV2-SvY1xRVFGy9X7nC~aKBYeMQB7w5GyZjVrhMW8gdLj3JxtQuWqcFE1z9~W451Iciq2jX8KT-w0bkmSqOyWnEQ8IXM7Gk8oYvu37vr3VLWooD5of-GpMbeZ6wg7K-dJaWqgAe4Idp-aizXpVnsv-ckwxloJSQdU5mbcpOfiLbDmsRGCO9wshV7OnxNDFMlF6EjE7vWYwe8NOXuPBqn-WyzYtsVYh4~qbiwXbW7sStGyNJIMAn9vDp-93TTMxrx9t-UX8v44K4xUZdD5lsQorZrejkP8J~zhH3eW05nCfYEPZlp~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal