We compared 48-hour urinary iron excretion after a twice-daily subcutaneous bolus injection of deferoxamine and after 12 hours of subcutaneous continuous infusion of the drug in 27 patients with iron overload (mean age, 55.7 years). In most patients, the iron overload was due to multiple transfusions administered during chemotherapy or as part of supportive care for a hematologic or oncologic disorder. One patient had sickle cell anemia and 1 had hereditary hemochromatosis and spherocytosis. Similar urinary iron excretion was observed with the 2 methods of administration; mean ± SD values were 6935.3 ± 3832.3 μg/48 hours with subcutaneous bolus injection and 6630.4 ± 3606.9 μg/48 hours with subcutaneous continuous infusion (P = .3). Twenty-six patients (96.3%) chose to continue therapy with bolus injection. The long-term efficacy of bolus injection was evaluated by measuring the serum ferritin concentration at regular intervals for a follow-up time of 20.1 ± 4.5 months. Ferritin concentration decreased to below 1000 μg/L in 73% of the patients and to below 500 μg/L in 42% and became normal in 26%. Best results were obtained in patients who were no longer receiving blood transfusions when chelation therapy was initiated. Three of 26 patients (11.5%) had mild, transient side effects after bolus injection. Larger prospective, randomized studies must be conducted before deferoxamine bolus injection can be routinely recommended for patients with iron overload.
Iron overload is a severe problem for patients who are receiving regular blood transfusions or who are homozygous for the hereditary hemochromatosis gene. Typical patients are those affected by thalassemia major in whom a variety of iron-related complications develop during their lifetime and whose main cause of death is iron-induced cardiac disease.1 In addition, with the introduction of new chemotherapy regimens and improvements in supportive care over the past few years, there has been an increase in survival of adult patients with premalignant or malignant conditions who require or previously required regular blood transfusions. These patients constitute a new population of patients with iron overload who need chelation.2-6 Iron chelation is also needed by patients with hereditary hemochromatosis coexistent with other congenital or acquired disorders that prevent the use of phlebotomy.7
Deferoxamine mesylate (DFO) remains the only first-line iron-chelating agent. Unfortunately, because it has a short half-life and is poorly absorbed by the gastrointestinal tract, DFO must be administered parenterally,8-14 usually by daily subcutaneous continuous infusion administered over 8 to 12 hours with use of a battery-operated portable pump. However, studies have shown that subcutaneous bolus injection of DFO is effective in the short term and is well tolerated both in pediatric patients with thalassemia15,16and in adults with hematologic or oncologic disorders.17 In this study, we compared urinary iron excretion after subcutaneous bolus injection of DFO and after subcutaneous continuous infusion of the agent in 27 adult patients with hereditary or acquired hemochromatosis. We also evaluated the long-term efficacy of subcutaneous bolus administration by measuring the serum ferritin concentration, an indirect but widely used method of assessing iron stores, in all patients during approximately 2 years of follow-up.
Patients and methods
Patients
From January 1996 to March 1999, 27 consecutively seen adult patients with iron overload were enrolled in a prospective study. Their mean (± SD) age was 55.7 ± 17.0 years (range, 18-77 years). The ratio of men to women was 1.1 (14 men and 13 women). Twenty-six patients were or had been dependent on transfusions. The patients' characteristics are shown in Tables 1 and2. Patient 20 had hereditary hemochromatosis (homozygous for the C282Y mutation in the HFEgene) and hereditary spherocytosis that prevented him from undergoing phlebotomy because of the presence of anemia.
Response to deferoxamine (DFO) therapy of patients who continued to receive transfusions during treatment
| Patient No. . | Diagnosis . | Age, y/Sex . | Initial Ferritin Level (μg/L)* . | TIL before Chelation (mg/kg)† . | TIL during Chelation (mg/kg)† . | UIE after DFO Bolus (μg/48 h) . | UIE after DFO Infusion (μg/48 h) . | Follow-up Time (mo) . | Last Ferritin Value (μg/L)‡ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IMF | 61/F | 1705 | 113.1 | 228.1 | 8450 | 6870 | 21 | 570 |
| 3 | MDS, RAEB | 77/M | 2230 | 174.0 | 145.0 | 17 640 | 14 510 | 25 | 1070 |
| 4 | IMF | 61/M | 2100 | 261.0 | 116.0 | 7880 | 11 530 | 21 | 1110 |
| 5 | MDS, RAEB-t | 77/M | 2710 | 152.3 | 186.4 | 4243 | 2556 | 24 | 1320 |
| 6 | CML-CP | 48/F | 1670 | 195.8 | 226.2 | 13 000 | 11 390 | 26 | 930 |
| 7 | NHL-LG | 77/F | 1130 | 95.2 | 181.6 | 4144 | 3737 | 25 | 820 |
| 8 | MDS, RA | 51/F | 685 | 89.8 | 214.9 | 7703 | 6790 | 28 | 615 |
| 9 | MDS, RA | 76/F | 1186 | 93.2 | 118.7 | 10 990 | 8262 | 22 | 620 |
| 10 | IMF | 66/M | 660 | 87.0 | 216.0 | 4360 | 3400 | 20 | 530 |
| 12 | SCA | 24/M | 835 | 96.0 | 121.5 | 5610 | 7360 | 19 | 520 |
| 14 | MDS, RA | 56/F | 2714 | 268.9 | 126.5 | 3800 | 3420 | 16 | 1450 |
| 15 | MDS, RAEB-t | 67/F | 1080 | 87.0 | 101.5 | 7600 | 3610 | 14 | 670 |
| 17 | MDS, RAS | 63/F | 2153 | 232.0 | 130.5 | 11 050 | 13 480 | 19 | 1320 |
| 19 | MDS, RA | 72/F | 2280 | 164.3 | 116.0 | 9920 | 8780 | 12 | 1470 |
| 22 | MDS, RAEB | 73/M | 1390 | 149.1 | 105.6 | 3170 | 3230 | 19 | 730 |
| 1635.2 ± 701.9 | 150.6 ± 64.4 | 155.6 ± 47.6 | 7970.7 ± 4046.4 | 7261.7 ± 3999.9 | 20.7 ± 4.5 | 916.3 ± 347.2 |
| Patient No. . | Diagnosis . | Age, y/Sex . | Initial Ferritin Level (μg/L)* . | TIL before Chelation (mg/kg)† . | TIL during Chelation (mg/kg)† . | UIE after DFO Bolus (μg/48 h) . | UIE after DFO Infusion (μg/48 h) . | Follow-up Time (mo) . | Last Ferritin Value (μg/L)‡ . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IMF | 61/F | 1705 | 113.1 | 228.1 | 8450 | 6870 | 21 | 570 |
| 3 | MDS, RAEB | 77/M | 2230 | 174.0 | 145.0 | 17 640 | 14 510 | 25 | 1070 |
| 4 | IMF | 61/M | 2100 | 261.0 | 116.0 | 7880 | 11 530 | 21 | 1110 |
| 5 | MDS, RAEB-t | 77/M | 2710 | 152.3 | 186.4 | 4243 | 2556 | 24 | 1320 |
| 6 | CML-CP | 48/F | 1670 | 195.8 | 226.2 | 13 000 | 11 390 | 26 | 930 |
| 7 | NHL-LG | 77/F | 1130 | 95.2 | 181.6 | 4144 | 3737 | 25 | 820 |
| 8 | MDS, RA | 51/F | 685 | 89.8 | 214.9 | 7703 | 6790 | 28 | 615 |
| 9 | MDS, RA | 76/F | 1186 | 93.2 | 118.7 | 10 990 | 8262 | 22 | 620 |
| 10 | IMF | 66/M | 660 | 87.0 | 216.0 | 4360 | 3400 | 20 | 530 |
| 12 | SCA | 24/M | 835 | 96.0 | 121.5 | 5610 | 7360 | 19 | 520 |
| 14 | MDS, RA | 56/F | 2714 | 268.9 | 126.5 | 3800 | 3420 | 16 | 1450 |
| 15 | MDS, RAEB-t | 67/F | 1080 | 87.0 | 101.5 | 7600 | 3610 | 14 | 670 |
| 17 | MDS, RAS | 63/F | 2153 | 232.0 | 130.5 | 11 050 | 13 480 | 19 | 1320 |
| 19 | MDS, RA | 72/F | 2280 | 164.3 | 116.0 | 9920 | 8780 | 12 | 1470 |
| 22 | MDS, RAEB | 73/M | 1390 | 149.1 | 105.6 | 3170 | 3230 | 19 | 730 |
| 1635.2 ± 701.9 | 150.6 ± 64.4 | 155.6 ± 47.6 | 7970.7 ± 4046.4 | 7261.7 ± 3999.9 | 20.7 ± 4.5 | 916.3 ± 347.2 |
TIL indicates total iron load; UIE, urinary iron excretion; IMF, idiopathic myelofibrosis; MDS, myelodysplastic syndrome; RAEB, refractory anemia with excess of blast cells; RAEB-t, refractory anemia with excess of blast cells in transformation to acute myeloid leukemia; CML-CP, chronic myeloid leukemia-chronic phase; NHL-LG, non-Hodgkin lymphoma-low grade; RA, refractory anemia; SCA, sickle cell anemia; and RAS, refractory anemia with ring sideroblasts. Plus-minus values are mean ± SD.
Twelve patients (1-10, 12, 15) were already undergoing DFO iron chelation administered by subcutaneous infusion using a portable pump.
TIL before chelation therapy is expressed as the total amount of iron transfused per kilogram of body weight; TIL during chelation therapy is expressed as the total amount of iron transfused during the follow-up time (months) per kilogram of body weight.
Normal range of serum ferritin concentration, 15-250 μg/L.
Response to deferoxamine (DFO) therapy of patients who did not receive blood transfusions during treatment
| Patient No. . | Diagnosis . | Age, y/Sex . | Initial Ferritin Level (μg/L) . | TIL before Chelation (mg/kg)* . | UIE after DFO Bolus (μg/48 h) . | UIE after DFO Infusion (μg/48 h) . | Follow-up Time (mo) . | Last Ferritin Value (μg/L)† . |
|---|---|---|---|---|---|---|---|---|
| 11 | RCA | 69/F | 2600 | 267.7 | 13 885 | 11 940 | 18 | 1210 |
| 13 | MDS, RA | 55/M | 1022 | 74.0 | 7836 | 8842 | 18 | 63 |
| 16 | AML, CR | 61/F | 2214 | 135.9 | 2325 | 2625 | 24 | 452 |
| 18 | ALL, CR | 57/M | 1557 | 137.8 | 3600 | 4200 | 14 | 490 |
| 20 | S/H | 45/M | 2360 | — | 6200 | 5780 | 28 | 740 |
| 21 | AML, CR | 36/M | 1625 | 112.8 | 5470 | 6660 | 21 | 250 |
| 23 | AML, CR | 35/M | 1460 | 123.2 | 4580 | 4970 | 18 | 440 |
| 24 | ALL, CR | 18/M | 980 | 94.2 | 2240 | 2470 | 12 | 230 |
| 25 | ALL, CR | 22/M | 1170 | 101.5 | 4600 | 4760 | 20 | 210 |
| 26 | ALL, CR | 47/M | 1320 | 118.1 | 3800 | 3460 | 22 | 240 |
| 27 | MM | 57/F | 1570 | 108.7 | 4430 | 4170 | 16 | 690 |
| 1625.3 ± 543.3 | 127.4 ± 52.9 | 5360.5 ± 3256.6 | 5443.4 ± 2827.1 | 18.3 ± 3.9 | 455.9 ± 325.6 |
| Patient No. . | Diagnosis . | Age, y/Sex . | Initial Ferritin Level (μg/L) . | TIL before Chelation (mg/kg)* . | UIE after DFO Bolus (μg/48 h) . | UIE after DFO Infusion (μg/48 h) . | Follow-up Time (mo) . | Last Ferritin Value (μg/L)† . |
|---|---|---|---|---|---|---|---|---|
| 11 | RCA | 69/F | 2600 | 267.7 | 13 885 | 11 940 | 18 | 1210 |
| 13 | MDS, RA | 55/M | 1022 | 74.0 | 7836 | 8842 | 18 | 63 |
| 16 | AML, CR | 61/F | 2214 | 135.9 | 2325 | 2625 | 24 | 452 |
| 18 | ALL, CR | 57/M | 1557 | 137.8 | 3600 | 4200 | 14 | 490 |
| 20 | S/H | 45/M | 2360 | — | 6200 | 5780 | 28 | 740 |
| 21 | AML, CR | 36/M | 1625 | 112.8 | 5470 | 6660 | 21 | 250 |
| 23 | AML, CR | 35/M | 1460 | 123.2 | 4580 | 4970 | 18 | 440 |
| 24 | ALL, CR | 18/M | 980 | 94.2 | 2240 | 2470 | 12 | 230 |
| 25 | ALL, CR | 22/M | 1170 | 101.5 | 4600 | 4760 | 20 | 210 |
| 26 | ALL, CR | 47/M | 1320 | 118.1 | 3800 | 3460 | 22 | 240 |
| 27 | MM | 57/F | 1570 | 108.7 | 4430 | 4170 | 16 | 690 |
| 1625.3 ± 543.3 | 127.4 ± 52.9 | 5360.5 ± 3256.6 | 5443.4 ± 2827.1 | 18.3 ± 3.9 | 455.9 ± 325.6 |
TIL indicates total iron load; UIE, urinary iron excretion; RCA, red cell aplasia; MDS, myelodysplastic syndrome; RA, refractory anemia; AML, acute myeloid leukemia; CR, complete remission; ALL, acute lymphoblastic leukemia; S/H, spherocytosis and hemochromatosis; and MM, multiple myeloma. Plus-minus values are mean ± SD.
TIL before chelation therapy is expressed as the total amount of iron transfused per kilogram of body weight; TIL during chelation therapy was not measured in these patients because they did not receive transfusions during DFO therapy.
Normal range of serum ferritin concentration, 15-250 μg/L.
DFO test administration
Informed consent was obtained from all patients before enrollment in the study. Side effects occurring during the test were recorded. DFO was administered subcutaneously in the abdominal wall in all patients. Patients were randomly selected to start with either subcutaneous continuous infusion or bolus injection. The 2 tests were carried out 2 weeks apart, 48 hours before transfusion (if transfusion had to be given). Twelve of the 27 patients (44.4%) were already undergoing iron chelation by means of subcutaneous infusion of DFO from a portable pump (Table 1). In these patients, chelation therapy was discontinued 48 hours before the test began. With continuous infusion, DFO was administered for 12 hours subcutaneously with use of a battery-powered syringe pump. The test dose of DFO was 2000 mg diluted in 20 mL of distilled water. With bolus injection, the same daily dose of DFO was administered in 2 doses given 12 hours apart so that each dose consisted of 1000 mg of DFO diluted in 10 mL of distilled water. The injection was performed with a 25-gauge scalp needle at the rate of about 1 mL/minute or greater, as tolerated. Particular care was taken to avoid direct injection into a vessel.
Urine collection
A 48-hour urine collection was begun at the time of the initial infusion or injection. The 48-hour DFO-induced urinary iron excretion after subcutaneous infusion was compared with the 48-hour DFO-induced urinary iron excretion after subcutaneous bolus injection. Urinary iron excretion was measured by atomic absorption spectrophotometry (Perkin-Elmer Corp, Norwalk, CT).
Evaluation of long-term efficacy of DFO injection
After the test of both methods of DFO administration, each patient decided which method to use to start or to continue chelation therapy. Only the patients who chose bolus injection were included in the study protocol. They received 30 mg/kg of body weight per day of DFO administered by subcutaneous bolus injection in 2 separate doses given 12 hours apart on 5 days a week. Every 2 months, serum ferritin concentration, liver-enzyme levels (serum alanine aminotransferase and aspartate aminotransferase), and the main inflammation indices (erythrocyte sedimentation rate, C-reactive protein, and fibrinogen) were measured. Because inflammation and cytolysis may increase serum ferritin levels, we included in the study only the ferritin values obtained when the values for the inflammation indices were within the normal ranges. Ferritin was measured by immunoassay using direct chemiluminometry (Chiron Diagnostics Corp, East Walpole, MA). No vitamin C supplementation was given. Side effects were recorded. The study included no measure of compliance with subcutaneous injections. All statistical analyses were done with paired t tests.
Results
The mean 48-hour DFO-induced urinary iron excretion was 6935.3 ± 3832.3 μg/48 hours (range, 2240-17 640 μg/48 hours) after the 2 daily subcutaneous bolus injections of DFO and 6630.4 ± 3606.9 μg/48 hours (range, 2470-14 510 μg/48 hours) after the subcutaneous infusion. Although the average urinary excretion of iron was higher after bolus injection than after infusion, there was no significant difference between the 2 methods of administration (P = .3). The order in which subcutaneous infusion and bolus injection was given did not influence the urinary iron excretion.
Twenty-six of the 27 patients tested (96.3%) chose to continue DFO therapy with bolus injection. Twelve patients (patients 1-10, 12, and 15; Table 1) were already undergoing DFO therapy with continuous subcutaneous infusion (for a mean of 12.8 ± 5.5 months [range 3-24 months]); however, because of poor compliance, 6 of them had been treated intermittently. Eleven of the 12 patients (91.6%) elected to continue chelation with bolus injection. Patient 2, a 54-year-old woman with myelodysplastic syndrome and a initial 48-hour urinary iron excretion after subcutaneous bolus injection of 8728 μg and a 48-hour urinary iron excretion after subcutaneous pump infusion of 10 218 μg, chose to continue chelation therapy with subcutaneous continuous infusion and was therefore excluded from the analysis of the effect of DFO injection on serum ferritin level. The mean follow-up time among the 26 patients treated with subcutaneous bolus injection of DFO was 20.1 ± 4.5 months (range, 12-28 months). The average serum ferritin concentration in the 26 patients at the beginning of the study was 1631.5 ± 627.7 μg/L (range, 660-2714 μg/L). At the end of the follow-up period, it was 721.5 ± 404.7 μg/L (range, 63 to 1470 μg/L).
Transfusion-dependent patients
Fifteen of the 26 patients (57.7%) had transfusions regularly (Table 1). Their average total iron load (TIL) before chelation therapy was 150.6 ± 64.4 mg/kg (range, 87.0-268.9 mg/kg), and their average TIL during chelating therapy was 155.6 ± 47.6 mg/kg (range, 101.5-228.1 mg/kg). The TIL per year in these patients was 92.9 ± 21.6 mg/kg (range, 64.8-130.3 mg/kg). The mean serum ferritin level at the start of the study was 1635.2 ± 701.9 μg/L (range, 660-2714 μg/L). By the end of the follow-up period (mean follow-up time, 20.7 ± 4.5 months), the mean serum ferritin level had fallen to 916.3 ± 347.2 μg/L (range 520-1470 μg/L). The ferritin concentration did not return to normal in any of these patients.
Patients not receiving transfusions during chelation
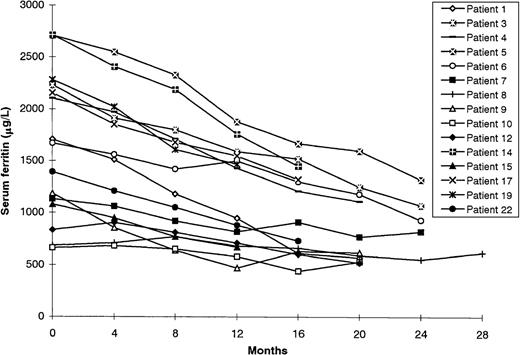
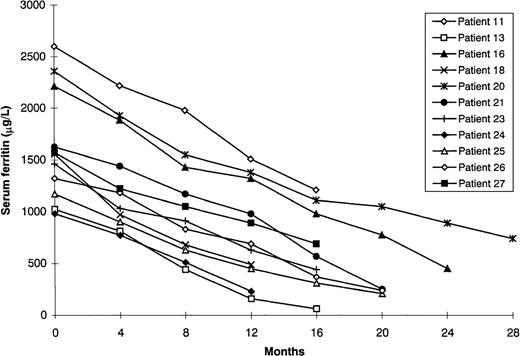
The remaining 11 patients did not receive additional blood transfusions during DFO treatment using bolus injection (Table 2). All but 1 had hematologic or oncologic disease and had undergone transfusion as part of supportive care or during chemotherapy (TIL before chelation, 127.4 ± 52.9 mg/kg; range, 74.0-267.7 mg/kg). In the 1 patient who had hemochromatosis and hereditary spherocytosis, all attempts at phlebotomy were prevented by anemia. Mean initial serum ferritin levels in the 11 patients were 1625.3 ± 543.3 μg/L (range, 980-2600 μg/L). By the end of the follow-up period (mean 18.3 ± 3.9 months), they had decreased to 455.9 ± 325.6 μg/L (range, 63 to 1210 μg/L). In 5 patients (45.4%), ferritin levels became normal (normal range, 15-250 μg/L). The difference in the decrease in mean serum ferritin concentration in the transfusion-dependent patients and that in the patients not receiving transfusions during chelation was significant (P = .001; Figures 1 and2).
Response to bolus deferoxamine therapy of patients who continued to receive blood transfusions during treatment.
Response to bolus deferoxamine therapy of patients who continued to receive blood transfusions during treatment.
Response to bolus deferoxamine therapy of patients who did not receive blood transfusions during treatment.
Response to bolus deferoxamine therapy of patients who did not receive blood transfusions during treatment.
Six measurements of serum ferritin were excluded from the analysis because of increased inflammatory variables. Mild, painless swelling that appeared during injection and disappeared within 10 to 15 minutes was reported by all patients. Three patients (11.5%) reported other side effects. One patient reported redness and pain at the injection site during the bolus test. These symptoms disappeared rapidly after drug administration and did not reappear with later injections. Another patient described soreness and pain during injection after 3 months of treatment. The symptoms disappeared when the infusion rate was reduced to 0.5 mL/minute. The third patient reported, 6 months after beginning the treatment regimen, nausea that developed immediately after DFO administration. This disappeared when the dose was reduced from 30 mg/kg per day to 20 mg/kg per day.
Discussion
Since its introduction in 1976, subcutaneous continuous infusion of DFO using a portable pump has proved to be the most effective and safest method of preventing or treating iron overload.11,12Its widespread use has greatly improved survival in patients with thalassemia undergoing long-term transfusion therapy.9,10DFO infusion therapy, however, is very demanding and requires patients' compliance for 8 to 12 hours daily. As a result, a sizable number of patients are not treated properly and have complications due to iron overload. To improve compliance, an alternative approach using a twice-daily subcutaneous bolus injection of DFO has been developed.15-19 Urinary iron excretion after subcutaneous DFO bolus injection was shown to be similar to that after continuous infusion.16 17 Our results confirm that the short-term efficacy of both methods is similar (6935.3 ± 3832.3 μg/48 hours with subcutaneous bolus injection compared with 6630.4 ± 3606.9 μg/48 hours with subcutaneous continuous infusion; P = .3).
Because only urinary iron excretion was measured in our comparative study, the results provide no information about the effect of bolus injection on biliary iron excretion, a major route of iron clearance with prolonged parenteral infusion. Therefore, there remains the possibility that bolus administration may be less effective than conventional therapy.
We also studied the long-term efficacy of subcutaneous bolus injection of DFO by periodically measuring the serum ferritin concentration in 26 patients during a mean follow-up time of 20.1 ± 4.5 months. The bolus injection rapidly reduced iron loading in patients not dependent on transfusions: in 45.4% of them, ferritin concentration decreased to normal levels after a mean follow-up time of 18.6 months. Only 3 of the 26 patients (11.5%) reported injection-associated side effects; these were mild and did not require discontinuation of treatment. The bolus-injection method of chelation was acceptable to patients. In fact, 26 of the 27 patients (96.3%) who took part in the initial trial, including 11 of 12 patients who were already undergoing subcutaneous continuous infusion, elected to continue treatment by subcutaneous bolus injection. No patient receiving treatment with bolus injection requested a switch to continuous infusion. The long-term response that we observed is encouraging.
It is important to point out that our population of patients, all adults affected by hematologic or oncologic diseases, differs greatly from patients with thalassemia major. The patients in our study generally had lower transfusion requirements or did not continue to receive transfusions while taking DFO. Even those who continued to undergo transfusions received only about half of the amount of iron (92.9 ± 21.6 mg/kg per year) generally received by patients with thalassemia major (∼180 mg/kg per year).20 Furthermore, the dose of DFO used (30 mg/kg per day), is lower than that prescribed (50-60 mg/kg per day) for many patients with thalassemia major. Therefore, an assessment of the tolerability, effectiveness, and toxicity of the bolus-injection method of DFO administration in patients with higher transfusion requirements who need larger doses of DFO is essential.
Assessment of serum ferritin concentration is not an accurate method of measuring iron stores, although serial measurements decrease the likelihood of error, as does the exclusion of ferritin values obtained when indicators of inflammation or liver-enzyme levels are increased. The 2 methods of administration of DFO must be compared with respect to free-iron levels, which are considered to be responsible for the majority of oxidative damage induced by iron. Preliminary results indicated that the decrease in nontransferrin-bound iron is as satisfactory, if not better, when DFO is administered as a bolus injection as when it is given as an infusion.21 Although our results must be considered preliminary, they support the need for a prospective, randomized controlled trial in a larger and more homogeneous population of patients in which hepatic, cardiac, and endocrine functions are systematically assessed.
Acknowledgment
We thank Ms Anne Holdstock-Immovilli for her assistance in reviewing this manuscript.
Supported in part by 40% and 60% grants to C.B.P. from MURST, Italy.
Reprints:Massimo Franchini, Servizio di Immunoematologia e Trasfusione, Ospedale Policlinico, Via Delle Menegone, 10-37134 Verona, Italy; e-mail:giorgio.gandini@mail.azosp.vr.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal