To test the hypothesis that factor VIII expressed in the epidermis can correct hemophilia A, we generated transgenic mice in a factor VIII–deficient background that express human factor VIII under control of the involucrin promoter. Mice from 5 transgenic lines had both phenotypic correction and plasma factor VIII activity. In addition to the skin, however, some factor VIII expression was detected in other tissues that have stratified squamous epithelia. To determine whether an exclusively cutaneous source of factor VIII could correct factor VIII deficiency, we grafted skin explants from transgenic mice onto mice that are double knockouts for the factor VIII and RAG-1 genes. Two graft recipients had plasma factor VIII activity of 4% to 20% of normal and improved whole blood clotting compared with factor VIII–deficient mice. Thus, expression of factor VIII from the epidermis can correct hemophilia A mice, thereby supporting the feasibility of cutaneous gene therapy for systemic disease.
Hemophilia A is an X-linked bleeding disorder that affects 1 to 2 individuals in 10,000 male births and is caused by defects in the factor VIII gene.1 Hemophilia A is an excellent candidate for gene therapy because (1) treatment is feasible through replacement of a normal copy of the factor VIII gene; (2) factor VIII is secreted and, therefore, its expression in any of a variety of tissues could correct the deficiency; (3) factor VIII levels of 2% to 5% of normal may produce significant clinical improvement; and (4) gene therapy offers the potential for more sustained and less expensive treatment than the current standard therapy of intravenous factor VIII infusions.
For many reasons, the epidermis is an attractive target tissue for gene therapy for selected systemic diseases.2,3 First, its accessibility could facilitate gene delivery through either ex vivo or in vivo approaches. Second, the promoters of a number of genes may be used to target transgene expression to the epidermis. The epidermis is a stratified squamous epithelium consisting of basal, proliferating keratinocytes, which give rise to suprabasal, differentiating keratinocytes. Promoters such as those derived from the keratin 144 and involucrin5 genes not only direct tissue-specific expression but also restrict expression to one or the other compartment. Third, keratinocytes function as synthetic and secretory cells, and gene products produced in the epidermis can enter the systemic circulation. However, the vasculature of the skin resides in the connective tissue matrix of the dermis, which underlies the epidermis. Thus, any transgene product synthesized in the epidermis must permeate the dermis to enter the blood stream.
Several approaches to epidermal gene delivery have been described. In vivo strategies include direct injection of DNA6 or viral particles7 into the skin, ballistic particle bombardment using the “gene gun,”8 and topical application of either liposome-encased DNA9 or viral particles.10 To date, these methods have been inefficient and have led to transient expression only. However, regular administration of a therapeutic gene to the epidermis through noninvasive techniques may be desirable and allows for titration of gene delivery to meet a therapeutic need. In contrast, ex vivo gene delivery through grafting of retrovirus-transduced keratinocytes has led to highly efficient gene transfer. Moreover, recent studies have achieved persistent transgene expression in grafts on immunodeficient mice. Deng et al11 observed long-term marker gene expression using a retroviral vector designed to circumvent time-dependent transgene inactivation. Kolodka et al12demonstrated transduction of epidermal stem cells, which gave rise to persistent transgene expression.
Factor VIII is expressed predominantly in the liver as a large (265 kd) precursor protein that undergoes extensive posttranslational modification, is cleaved into 2 chains, and requires von Willebrand factor (vWF) for stability. Despite the size and complexity of factor VIII, we proposed that factor VIII expression in the epidermis could correct the coagulation defect in hemophilia A. Here we demonstrate the feasibility of this approach for factor VIII gene therapy in hemophilia A mice.
Materials and methods
Generation of pinvVIIILA transgene construct
The B domain–deleted VIIILA complementary DNA (cDNA) was liberated from the pMT2-LA plasmid13 by XhoI-SalI digestion. The ends were filled in by treatment with Klenow fragment DNA polymerase, and NotI linkers were attached by blunt-end ligation. The VIIILA insert was then subcloned into the NotI cloning site of the pH3700-pL2 plasmid,5 kindly provided by Lorne Taichman. The pinvVIIILA transgene (vIIILAcDNA driven by an involucrin promoter) was liberated from vector backbone sequences by SalI digestion prior to microinjection to generate transgenic mice.
Factor VIII transgenic and knockout mouse lines
Transgenic mice were generated using standard methods for direct microinjection of pinvVIIILA transgene DNA into male pronuclei of B6SJLF1/J mouse zygotes prior to implantation into pseudopregnant surrogate female mice. Factor VIII knockout mice generated through targeted disruption of either exon 16 or exon 17 of the factor VIII gene have less than 1% of normal factor VIII activity and have been described previously in detail.14
To generate mice that carry the pinvVIIILA transgene in a factor VIII null background, female mice bred to homozygosity for the exon 16 factor VIII knockout allele were mated with male transgenic mice from each line. All F1 male mice inherit the factor VIII knockout allele from the mother, and half of these would be predicted to inherit the transgene from the father. Subsequent matings of these transgenic male mice with factor VIII knockout female mice propagated each pinvVIIILA line in the factor VIII knockout background. Genotype determination was performed by polymerase chain reaction (PCR) analysis of tail-derived genomic DNA samples using primer pairs specific for the pinvVIIILA transgene (see “Reverse transcriptase–polymerase chain reaction analysis”) and the factor VIII exon 16 knockout allele.14
RAG-1/factor VIII double knockout mice
Male RAG-1 (recombinase activating gene-1) knockout mice15 (Jackson Laboratories, Bar Harbor, ME) were mated with female mice homozygous for the exon 17 factor VIII knockout allele to generate F1 heterozygotes. Matings of F1 mice yielded double knockout mice at a frequency approximating 1 in 8 offspring. Male and female double knockout mice were mated to each other to stably propagate this line. Genotype determination was performed by PCR analysis of tail-derived genomic DNA samples using primer pairs specific for the RAG-1 and factor VIII wild-type and knockout alleles (RAG-1 primers: wild-type allele upstream, 5′-CAGTCTCCAGTAGTTCCAGAG-3′; wild-type allele downstream, 5′-TCTGGCCAGGAAGTGACTCTT-3′); knockout allele upstream, wild-type primer; knockout allele downstream, neomycin resistance marker primer, 5′-CGCC- TTCTTGACGAGTTCTTC-3′). PCR parameters included 10′ at 94°C for denaturation, followed by 35 cycles of 60″ at 94°C, 60″ at 59°C, 1′30″ at 72°C, ending with 10′ at 72°C.
Immunofluorescence microscopy
Full-thickness neonatal skin samples were embedded in OCT and frozen in dry ice/2-methylbutane. Sections were cut to 6 μ and fixed in 1:1 methanol:acetone prior to antibody treatment. Tissue sections for factor VIII staining were treated with 1% bovine serum albumin and 1% goat serum in phosphate-buffered saline (PBS) as blocking reagents. Sections were incubated with a 1:80 dilution of primary ESH 2 monoclonal antihuman factor VIII antibody (American Diagnostica Inc, Greenwich, CT) at 4°C overnight. After rinsing in PBS, sections were treated with a 1:400 dilution of secondary Texas red-conjugated, goat antimouse immunoglobulin G antibody (Molecular Probes, Eugene, OR) at 25°C for 1 hour. Blocking reagents of 1% bovine serum albumin and 10% horse serum in PBS were used for vWF staining. Tissue sections were incubated with a 1:100 dilution of primary rabbit antihuman vWF antibody (Dako Corp, Carpinteria, CA) for 30′ at 25°C followed by a 1:200 dilution of secondary Cy3-conjugated, goat antirabbit immunoglobulin G antibody (Jackson ImmunoResearch Laboratories Inc, West Grove, PA) at 25°C for 1 hour.
Reverse transcriptase–polymerase chain reaction analysis
Total RNA was isolated from tissue samples using the Trizol method (Life Technologies Inc, Gaithersburg, MD). Deoxyribonuclease treatment and reverse transcriptase (RT) reactions for first-strand cDNA synthesis using random oligonucleotide primers were performed according to protocols provided by the supplier (Life Technologies). An upstream primer from 5′ untranslated sequences within the involucrin promoter region (5′-AAAGCCTCTGCCTCAGCCTTA-3′) and a downstream primer from human factor VIII coding sequence (5′-GAAGCAGGTGGAGAGCTC- TAT-3′) were used for PCR amplification with parameters of 10′ at 94°C for denaturation followed by 40 cycles of 30″ at 94°C, 30″ at 59°C, 60″ at 72°C, ending with 10′ at 72°C. Two intervening introns are spliced out of the pinvVIIILA transcript, thus making the RT-PCR amplification product message-specific.
Phenotype correction analysis
Wound clot formation.
Mice were anesthetised with methoxyflurane in a bell jar. A small wound was induced by snipping approximately 1 cm of distal tail tissue, and the mouse was observed for clot formation and survival.
Whole blood clotting.
Whole blood samples of roughly 100 μL were obtained from methoxyflurane-anesthetised mice by tail bleeding into Eppendorf tubes. Tubes were tapped gently approximately every 2 to 3 minutes to determine when clot formation occurred.
Plasma factor VIII activity analysis
Blood samples were collected from methoxyflurane-anesthetised mice by tail bleeding into Eppendorf tubes containing 0.1 mol/L sodium citrate, which was adjusted to 10% of the blood volume obtained. Samples were centrifuged at 2000g for 10 minutes at 25°C. The plasma fraction was removed, transferred to a fresh tube, immediately frozen on dry ice, and stored at −80°C. Samples of plasma were thawed quickly at 37°C immediately prior to use. Plasma factor VIII activity levels were measured using the COAMATIC assay16 (Chromogenix, Mölndal, Sweden) using the specifications described by the manufacturer. Control plasma samples from normal mice were pooled at the time of collection. Samples of normal plasma diluted in factor VIII knockout mouse plasma were used to generate a standard curve for factor VIII activity.
Factor VIII–specific activity determination
Factor VIII activity in transgenic mouse plasma or pooled human plasma (George King Bio-Medical Inc, Overland Park, KS) was measured using the COAMATIC assay and divided by the level of plasma factor VIII measured by an enzyme-linked immuosorbent assay (ELISA) specific for human factor VIII as previously described.17 ESH 2 monoclonal antihuman factor VIII antibody (American Diagnostica) and N771110M monoclonal antihuman factor VIII antibody (Bi∅︀design International, Saco, ME) were used to coat ELISA plates for antigen capture. CL20 035A sheep antihuman factor VIII polyclonal antibody (Cedarlane Laboratories, Hornby, ON) was used for factor VIII antigen detection followed by horseradish peroxidase–conjugated donkey antisheep immunoglobulin G (Rockland Inc, Gilbertsville, PA). O-phenylenediamine (Sigma, St Louis, MO) was used as substrate for the peroxidase reaction, which was quantified spectrophotometrically at 490 nm in an ELISA reader.
Skin grafting
Back skin of recipient factor VIII/RAG-1 double knockout mice was prepared for skin grafting by shaving and treating with a depilatory agent (Neet, Premier Inc, Greenwich, CT). Recipient mice received a single preoperative tail vein injection of 2.5 units of human factor VIII (Monoclate-P, Armour Pharmaceutical Co, Kankakee, IL), a gift from Katherine High (Children's Hospital of Philadelphia). Under sterile conditions, graft beds were prepared on the backs of methoxyflurane-anesthetised recipient mice by scissor dissection of the skin to the level of the panniculus carnosus without disturbing the underlying vascular plexus. Full-thickness shaved and depilatory-treated skin explants were harvested from transgenic donor mice. Fatty tissue was scraped off with a scalpel blade, leaving only intact epidermis and dermis. Subsequently, explants were placed directly on the prepared graft beds and secured with 6.0 nylon suture. Polysporin ointment (Warner-Lambert Consumer Healthcare, Morris Plains, NJ) was applied to the engrafted areas. A layered dressing consisting of an adhesive Tegaderm (3M Health Care, St Paul, MN) covering, zinc oxide–impregnated gauze, and Coban (3M Health Care) elastic gauze was placed circumferentially around each mouse and secured with surgical staples. Dressings were removed after 4 weeks. Due to variable take and contraction, established grafts represented less than 50% of their original size at transplantation. Nine mice received transgenic skin grafts; 7 recipients either did not maintain their grafts, bled chronically, or did not live long enough for us to determine whether their grafts were viable and supplied factor VIII to the circulation.
Results
Generation of factor VIII transgenic mice
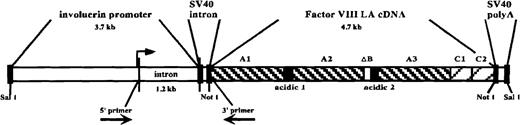
We made a transgene construct, pinvVIIILA (Figure1), which contains the human factor VIIILA cDNA that lacks most of the B domain. In this construct, the human involucrin promoter directs factor VIII expression to the suprabasal epidermis. The B domain–deleted factor VIII precursor protein is reduced to about 165 kd and generates a truncated NH2-terminal heavy chain and a normal COOH-terminal light chain. The B domain normally is processed out of the wild-type factor VIII precursor by proteolytic cleavage, and B domain–deleted factor VIII maintains procoagulant activity.13
pinvVIIILA transgene construct.
From 5′ to 3′: human involucrin promoter region (including 1.2-kilobase intron), SV40 intron sequences, human factor VIIILA (B domain–deleted) cDNA, and SV40 polyadenylation signal. The domain composition of factor VIIILA, including the retained remnant of the central B domain (ΔB), is depicted. The transgene is liberated from the vector backbone by SalI digestion prior to zygote injections. The 5′ and 3′ primers used for RT-PCR analysis flank the intron sequences as shown. The upstream primer is based in 5′ untranslated sequences derived from the involucrin promoter region, while the downstream primer is derived from factor VIII coding region. Therefore, the 210–base pair RT-PCR amplification product from this primer pair is specific for fully spliced, transgene-derived message. The transcription initiation site is designated by the arrow upstream of the first intron.
pinvVIIILA transgene construct.
From 5′ to 3′: human involucrin promoter region (including 1.2-kilobase intron), SV40 intron sequences, human factor VIIILA (B domain–deleted) cDNA, and SV40 polyadenylation signal. The domain composition of factor VIIILA, including the retained remnant of the central B domain (ΔB), is depicted. The transgene is liberated from the vector backbone by SalI digestion prior to zygote injections. The 5′ and 3′ primers used for RT-PCR analysis flank the intron sequences as shown. The upstream primer is based in 5′ untranslated sequences derived from the involucrin promoter region, while the downstream primer is derived from factor VIII coding region. Therefore, the 210–base pair RT-PCR amplification product from this primer pair is specific for fully spliced, transgene-derived message. The transcription initiation site is designated by the arrow upstream of the first intron.
Thirteen transgenic founder mice were generated, 10 of which expressed pinvVIIILA by RT-PCR analysis of total RNA (data not shown). These mice were mated with factor VIII knockout mice14 to generate mice that express the transgene but do not produce factor VIII at sites from which it is normally expressed. Lines were propagated from 5 of the founder mice that stably transmitted the factor VIII transgene. Transgenic mice from these lines appeared phenotypically identical to nontransgenic littermates.
Analysis of factor VIII transgene expression
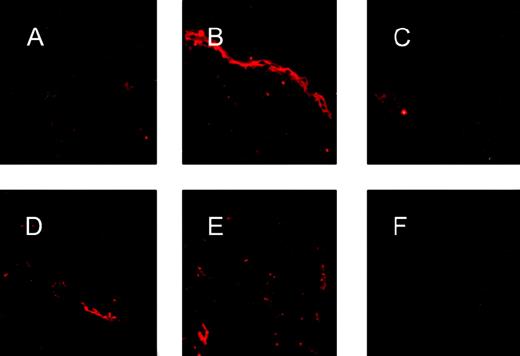
To confirm that the pinvVIIILA transgene was expressed in a compartment-specific manner in the epidermis, transgenic skin was subjected to immunofluorescence microscopy using a monoclonal antibody directed against human factor VIII (Figure2, A-C). Transgenic skin showed an intense suprabasal band not found in skin from nontransgenic littermates and transgenic skin treated only with secondary antibody. In contrast, immunofluorescence patterns of vWF expression were identical in transgenic and nontransgenic skin (Figure 2, D-F).
FVIII antigen expression in transgenic epidermis.
Immunostaining for factor VIII expression using an antihuman factor VIII antibody (A-C) showed an intense band of staining confined to the suprabasal epidermis in transgenic skin (B), whereas nontransgenic skin (A) and transgenic skin treated only with secondary antibodies (C) show no epidermal staining for factor VIII. Immunostaining for vWF using an antihuman vWF antibody that cross-reacts with murine vWF revealed focal signals confined to the dermal vascular endothelium in both transgenic (D) and nontransgenic (E) skin. Skin from a vWF knockout mouse (F) was used as a negative control and showed no positive signal.
FVIII antigen expression in transgenic epidermis.
Immunostaining for factor VIII expression using an antihuman factor VIII antibody (A-C) showed an intense band of staining confined to the suprabasal epidermis in transgenic skin (B), whereas nontransgenic skin (A) and transgenic skin treated only with secondary antibodies (C) show no epidermal staining for factor VIII. Immunostaining for vWF using an antihuman vWF antibody that cross-reacts with murine vWF revealed focal signals confined to the dermal vascular endothelium in both transgenic (D) and nontransgenic (E) skin. Skin from a vWF knockout mouse (F) was used as a negative control and showed no positive signal.
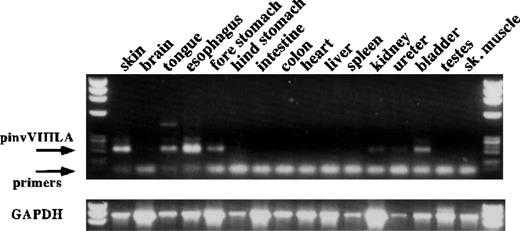
To confirm tissue-specific expression of the factor VIII transgene, we performed RT-PCR on total RNA derived from multiple tissues of transgenic mice (Figure 3). As expected, transgene expression was seen in the skin. However, expression was also observed in the upper gastrointestinal tract and the genitourinary tract, which have stratified squamous epithelia. This result is consistent with observations in other transgenic mice generated using the involucrin promoter.5 Therefore, expression in other stratified squamous epithelia may contribute in part to factor VIII replacement in pinvVIIILA mice.
RT-PCR analysis of pinvVIIILA expression in transgenic mice.
RT-PCR analysis of several tissues from a pinvVIIILA mouse is shown. The primer pair depicted in Figure 1 gives a fully spliced, message-specific product of 210 base pairs. There is evidence of transgene expression in skin and other tissues that have stratified squamous epithelia. Tissues that have no stratified squamous epithelia show no transgene expression. Control RT-PCR reactions for RNA integrity used primers specific for mouse glyceraldehyde phosphate dehydrogenase (GAPDH) message (bottom panel).
RT-PCR analysis of pinvVIIILA expression in transgenic mice.
RT-PCR analysis of several tissues from a pinvVIIILA mouse is shown. The primer pair depicted in Figure 1 gives a fully spliced, message-specific product of 210 base pairs. There is evidence of transgene expression in skin and other tissues that have stratified squamous epithelia. Tissues that have no stratified squamous epithelia show no transgene expression. Control RT-PCR reactions for RNA integrity used primers specific for mouse glyceraldehyde phosphate dehydrogenase (GAPDH) message (bottom panel).
Correction of factor VIII deficiency in pinvVIIILA mice
Mice expressing the factor VIII transgene but not endogenous factor VIII were tested for phenotypic correction and circulating factor VIII activity. To assess phenotypic correction of the coagulation defect, we determined ability to clot and survive after inducing a minor wound by tail snipping. Seventeen mice from 5 independent pinvVIIILA lines were tested, and 7 of 7 transgenic mice, but none of 10 nontransgenic littermates, were able to clot and survive.
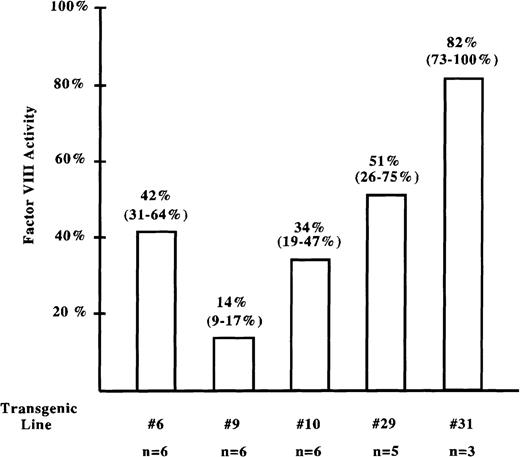
We then measured factor VIII activity in the circulation of these mice using an in vitro photometric assay specific for factor VIII activity.16 Plasma samples from transgenic mice of each pinvVIIILA line had average factor VIII activity of 14% (line no. 9) to 82% (line no. 31) of normal (Figure 4). Therefore, mice from 5 independent pinvVIIILA transgenic lines demonstrated both phenotypic correction and plasma factor VIII activity, suggesting that factor VIII expressed predominantly in the suprabasal epidermis is functional and can access the systemic circulation.
Factor VIII activity analysis in transgenic mice.
Factor VIII activity in samples of transgenic mouse plasma was measured using the COAMATIC assay. A standard curve for factor VIII activity was generated from pooled plasma of normal mice. Knockout mouse plasma was used to generate a baseline point (less than 1% of normal activity) for the standard curve. The average factor VIII activity for each line compared with normal mice is displayed, and the range of activity observed is shown in parentheses. The number of mice assayed from each line is listed in the bottom row.
Factor VIII activity analysis in transgenic mice.
Factor VIII activity in samples of transgenic mouse plasma was measured using the COAMATIC assay. A standard curve for factor VIII activity was generated from pooled plasma of normal mice. Knockout mouse plasma was used to generate a baseline point (less than 1% of normal activity) for the standard curve. The average factor VIII activity for each line compared with normal mice is displayed, and the range of activity observed is shown in parentheses. The number of mice assayed from each line is listed in the bottom row.
Determination of levels and specific activities of transgenic mouse-derived factor VIII relative to human-derived factor VIII
We determined the plasma level and specific activity of keratinocyte-derived human factor VIII for mice of 3 pinvVIIILA transgenic lines relative to factor VIII from normal humans. Factor VIII activity and ELISA assays were performed on the same plasma samples. Levels of factor VIII antigen approaching those in human plasma were observed in transgenic mouse plasma (Table1). Transgenic lines no. 6, 10, and 31 showed specific activities of 61%, 42%, and 100% of human plasma-derived factor VIII, respectively (Table 1).
Plasma levels and specific activities of transgenic mouse-derived factor VIII relative to normal human factor VIII
| Transgenic line no. | 6 | 10 | 31 |
| Plasma factor VIII antigen level (ng/mL) | 233 | 128 | 255 |
| Plasma factor VIII antigen level relative to human factor VIII | 0.83 | 0.46 | 0.91 |
| Specific activity relative to human factor VIII | 0.61 | 0.42 | 1.00 |
| n = 3 | n = 3 | n = 4 |
| Transgenic line no. | 6 | 10 | 31 |
| Plasma factor VIII antigen level (ng/mL) | 233 | 128 | 255 |
| Plasma factor VIII antigen level relative to human factor VIII | 0.83 | 0.46 | 0.91 |
| Specific activity relative to human factor VIII | 0.61 | 0.42 | 1.00 |
| n = 3 | n = 3 | n = 4 |
Plasma levels of factor VIII antigen were assayed by ELISA specific for human factor VIII. The level of factor VIII in pooled human plasma samples approximated 280 ng/mL. Specific activity was calculated by dividing factor VIII activity (measured using the COAMATIC assay) by the plasma level of factor VIII. Relative specific activity was determined by the ratio of specific activity for transgenic factor VIII to specific activity for normal human factor VIII. Results represent averages for each line based on the number (n) of mice assayed.
Correction of factor VIII–deficient mice through transplantation of transgenic skin
Because factor VIII expression in pinvVIIILA mice occurs over the entire surface of the skin and in stratified squamous epithelia at other sites, we determined whether systemic factor VIII activity could be obtained exclusively from a focal cutaneous source. Factor VIII–expressing skin explants derived from mice of transgenic line no. 29 were transplanted to factor VIII–deficient mice. To increase factor VIII expression, we bred the transgene locus to presumptive homozygosity and transplanted skin from F1 offspring showing high plasma factor VIII activity (60%-95% of normal). To generate graft recipients, we bred the factor VIII knockout mice onto the immunodeficient RAG-1 knockout background.15 Mice deficient for the RAG-1 gene product do not generate functional B or T lymphocytes because they lack recombinase activity that enables VDJ rearrangement at both immunoglobulin and T-cell receptor loci. Therefore, mice from the resulting double knockout line should not reject skin grafts based on immunologic incompatibilities and can be used to assay for factor VIII delivery to the circulation. To minimize bleeding, graft recipients received a preoperative intravenous injection of human factor VIII, which was not detectable 1 week after grafting.
Two informative graft recipients maintained approximately 4 to 6 cm2 of transplanted skin, representing about 10% to 15% of total body surface area (Figure 5). Expression of the factor VIII transgene in samples of transplanted skin was confirmed by RT-PCR analysis (data not shown). One mouse had circulating factor VIII activity of 5% of normal 2 weeks after skin grafting and maintained activity of 9% to 20% between weeks 4 and 6. However, activity decreased to about 3% at week 8. The other mouse first showed factor VIII activity significantly above background at week 6. It had activity of 5% at this time point and 4% at week 8 (Table 2). To corroborate these results, we assessed whole blood clotting as a measure of phenotypic correction 6 to 8 weeks after grafting. Whole blood samples from both mice clotted within about 15 to 25 minutes, whereas whole blood from hemophilic mice failed to clot within 60 minutes and whole blood from normal mice clotted within 8 to 15 minutes (Table 3). Together, these results demonstrate that functional factor VIII delivered to the circulation from a skin transplant can correct factor VIII deficiency in a mouse model of hemophilia A.
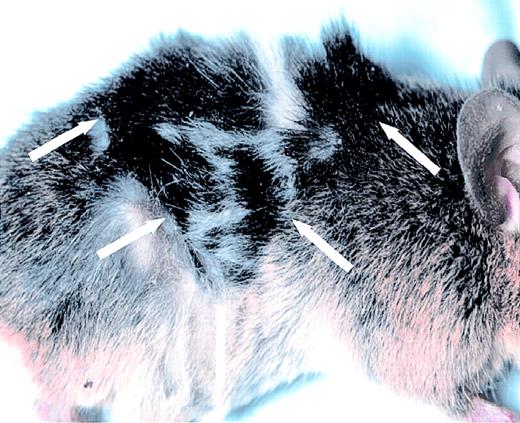
Factor VIII–expressing skin graft.
A graft of black transgenic donor skin is shown on a factor VIII/RAG-1 double knockout recipient mouse. The edges of the graft are demarcated by the arrows. A similar graft is present on the contralateral flank of this mouse. See also Table 2 and Table 3.
Plasma factor VIII activity in skin graft recipient mice
| . | Week . | 2 . | 4 . | 6 . | 8 . |
|---|---|---|---|---|---|
| Mouse | |||||
| A | 5% | 12% | 20% | 3%* | |
| B | < 1% | < 1% | 5% | 4% | |
| Control | < 1% | ————————————————————→ | |||
| . | Week . | 2 . | 4 . | 6 . | 8 . |
|---|---|---|---|---|---|
| Mouse | |||||
| A | 5% | 12% | 20% | 3%* | |
| B | < 1% | < 1% | 5% | 4% | |
| Control | < 1% | ————————————————————→ | |||
Values represent levels of plasma factor VIII activity compared with normal mice as determined using the COAMATIC assay.
Mouse A was killed 8 weeks after grafting when it appeared ill after bleeding from the rectal area. Similarly, mouse B was killed 12 weeks after grafting when it appeared ill. Diminished factor VIII activity was observed then, although no clear cause of illness was evident. Blood was not obtained at the 10-week point from this mouse.
Whole blood clotting time in skin graft recipient mice
| . | Clotting Time . |
|---|---|
| Graft recipients | 15-25′ |
| Factor VIII (−) controls | > 60′ |
| Normal mice | 8-15′ |
| . | Clotting Time . |
|---|---|
| Graft recipients | 15-25′ |
| Factor VIII (−) controls | > 60′ |
| Normal mice | 8-15′ |
Improved clotting times were observed for whole blood from both engrafted mice compared with control factor VIII–deficient mice; however, clotting times did not correct to normal levels.
Discussion
Several approaches to gene therapy for the hemophilias using different potential target tissues and vectors for transgene delivery have been examined. Some strategies have yielded promising results in preclinical studies. For example, in vivo delivery of a factor VIII–expressing adenovirus targeted to liver yielded therapeutic levels of factor VIII sustained for months in factor VIII–deficient mice18,19 and transient therapeutic levels in hemophilia A dogs.20 Furthermore, sustained factor IX expression was observed using adeno-associated virus to deliver a factor IX gene to muscle in hemophilia B dogs21 and to liver in both factor IX–deficient mice and dogs.22Nonetheless, many issues regarding efficacy, duration, potential immune responses, readministration, and long-term safety remain to be resolved in patients. Thus, no definitive approach to gene therapy for the hemophilias has yet emerged, warranting further research into alternative strategies.
Here we have demonstrated that factor VIII expressed mainly in the suprabasal epidermis can supply functional factor VIII to the systemic circulation, and we then showed that transplants of factor VIII–expressing skin can correct the phenotype of hemophilic mice. These findings support the feasibility of epidermal factor VIII gene therapy for the treatment of hemophilia A.
Although previous studies have demonstrated delivery of transgene-derived proteins from the epidermis to the systemic circulation, the present study is significant on several levels. Gene products including growth hormone (22 kd), apolipoprotein E (34 kd), alpha1-antitrypsin (56 kd), factor IX (57 kd), and various cytokines have been shown to access the systemic circulation from the epidermis in models for ex vivo and in vivo gene delivery as well as in transgenic mice.7,23-29 However, these proteins are relatively small and are not modified as extensively as factor VIII, which is large and complex. Factor VIII is heavily processed through sulfation of tyrosine residues, addition of O-linked oligosaccharides to serines and threonines, and N-glycosylation of asparagine residues.30-32 Moreover, factor VIII is cleaved into 2 chains, both of which must access the vasculature to form a stable circulating complex with vWF. Our results demonstrate that keratinocytes are capable of synthesizing and secreting factor VIII and that keratinocyte-derived factor VIII is functional and enters the circulation despite the separation of the epidermis from the dermal vasculature.
Furthermore, vWF expression in transgenic skin, as in nontransgenic skin, is limited to the dermal vascular endothelium. Therefore, prior to its entry into the circulation, factor VIII synthesized in the epidermis must exist in a form devoid of vWF stabilization. Despite this, sufficient circulating factor VIII to achieve phenotype correction can be derived from the epidermis.
For these studies, we used the B domain–deleted VIIILA variant of the human factor VIII cDNA. This cDNA produces a shortened factor VIII precursor protein that gives rise to a truncated heavy chain of about 90 kd (reduced from about 200 kd) and a normal light chain of about 80 kd. Despite removal of the B domain, both chains generated from the VIIILA cDNA are larger than other transgene products that have been shown to access the circulation from the epidermis. Furthermore, deletion of the B domain dramatically simplifies posttranslational modification of factor VIII by removing many of its potential N-glycosylation sites.13 Whether full-length factor VIII is produced and secreted in a similar fashion to B domain–deleted factor VIII has not yet been determined.
Circulating levels of factor VIII antigen approaching those in human plasma were observed in transgenic mice. However, the proportion of factor VIII synthesized in the epidermis that gains entry into the circulation is unknown. Furthermore, the specific activities for keratinocyte-derived human factor VIII in the 3 transgenic lines tested ranged from 42% to 100% of factor VIII in samples of human plasma. The variation in specific activity we observed is comparable to normal human subjects33 34 and may also reflect subtle strain differences given that the transgenic lines are not inbred.
We observed factor VIII activity and phenotype correction in 2 mice that received transgenic skin grafts. Although a limited number of informative graft recipients were obtained, these results nonetheless demonstrate the feasibility of supplying functional factor VIII from an epidermal source in the absence of factor VIII expression in other tissues. Factor VIII expression from the dermal vasculature of transplanted skin is ruled out by the specificity of involucrin promoter activity for stratified squamous epithelia and the lack of factor VIII expression in the vascular endothelium of transgenic mouse skin (Figure 6).
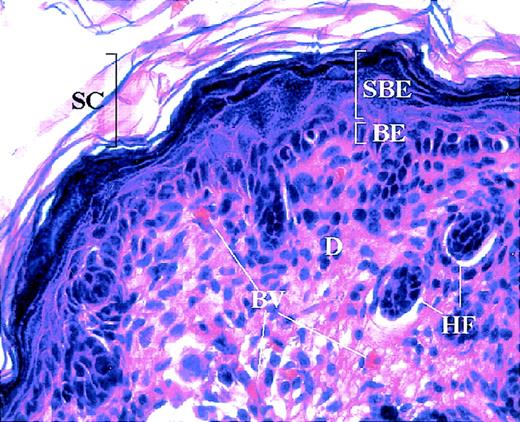
Mouse skin histology.
Hemotoxylin and eosin staining of normal mouse skin showing cutaneous compartments and structures. The basal epidermis (BE) consists of a single layer of proliferating keratinocytes, which give rise to the differentiating keratinocytes of the suprabasal epidermis (SBE). The latter ultimately form the stratum corneum (SC), the outermost barrier of the skin. The dermis (D) underlies the epidermis and houses several structures, including blood vessels (BV) and hair follicles (HF).
Mouse skin histology.
Hemotoxylin and eosin staining of normal mouse skin showing cutaneous compartments and structures. The basal epidermis (BE) consists of a single layer of proliferating keratinocytes, which give rise to the differentiating keratinocytes of the suprabasal epidermis (SBE). The latter ultimately form the stratum corneum (SC), the outermost barrier of the skin. The dermis (D) underlies the epidermis and houses several structures, including blood vessels (BV) and hair follicles (HF).
Our grafting studies model an ex vivo approach to cutaneous factor VIII gene therapy in which keratinocytes manipulated to express factor VIII in vitro are transplanted to patient skin. Employment of strategies to improve (1) factor VIII expression and secretion, (2) factor VIII accessibility to the circulation, and (3) factor VIII–specific activity would permit maximizing the efficiency of cutaneous factor VIII gene therapy. With optimization, it may be feasible to achieve correction of factor VIII deficiency using skin grafts significantly smaller than those (10%-15% of body surface area) maintained by the corrected mice in this study. Smaller graft sizes would also facilitate monitoring and graft excision in case of any potential adverse event.
Different times of onset and levels of factor VIII activity in recipients of factor VIII–expressing skin grafts may reflect differences in rapidity of graft take and revascularization as well as varying levels of expression of factor VIII in skin explants from different donor mice. Furthermore, we observed very high levels of factor VIII activity in 1 graft recipient, which may reflect some contribution of factor VIII from hair follicles of transplanted skin. Hair follicles in adult mice are generally in a resting phase (telogen). However, transplantation35 and hair depilation stimulate follicle cycling into the growing phase (anagen), which lasts 2 to 3 weeks. In contrast to telogen follicles, anagen follicles possess an epithelial inner root sheath, which expresses involucrin. Therefore, some factor VIII may be expressed under control of the involucrin promoter in the inner root sheath of anagen hair follicles in transgenic skin grafts, thus augmenting circulating levels of factor VIII. With time, however, diminished factor VIII activity was noted for both mice. The nature of this drop is unclear. It should be unrelated to an immune response because grafting studies were performed on immunodeficient factor VIII/RAG-1 double knockout mice.
In turn, analysis of potential immune responses directed against factor VIII and transplanted skin were not performed owing to the immunodeficient nature of the graft recipients. Nonetheless, the risk of humoral and cell-mediated immune responses limiting levels and duration of factor VIII expression through a cutaneous approach to gene therapy is significant given the presence of antigen-presenting cells in skin. Immunomodulation through systemic or possibly local, topical administration of immunosuppressive agents may help limit potential immune responses. Alternatively, irradiation of skin with ultraviolet light may induce systemic, antigen-specific tolerance in models for contact hypersensitivity36 and may have potential for application to cutaneous gene therapy. We are backcrossing both factor VIII transgenic and knockout mouse lines onto identical strains to perform skin grafting studies between immunocompetent, syngeneic mice. This will allow us to characterize the immunologic consequences of cutaneous factor VIII expression and potential approaches for their circumvention.
Previous studies have demonstrated systemic responses from a transgene product expressed in the epidermis. Wang et al24 generated transgenic mice that expressed human growth hormone (hGH) in the basal epidermis using the human keratin 14 promoter. These mice had high levels of circulating hGH and increased body mass. Hengge et al6 observed recruitment of neutrophils to areas of pig skin injected with an interleukin (IL)-8 plasmid. Similarly, Meng et al28 injected rat skin with a human IL-10 expression construct. Epidermal expression produced circulating IL-10 and suppression of contact hypersensitivity in sensitized rats.
Here we have demonstrated correction of an animal model with a heritable systemic disease by epidermal expression of a therapeutic transgene. Our results provide functional and phenotypic evidence for the feasibility of cutaneous gene therapy for treatment of systemic genetic diseases, such as the hemophilias and potentially many other disorders.
Acknowledgments
We thank George Cotsarelis, John Goodier, Michelle Kimberland, Eline Luning-Prak, John Moran, Eric Ostertag, John Stanley, Lorne Taichman, and Hong Wu for helpful discussions and technical advice. DNA microinjections for generation of transgenic mice were performed by Jean Richa of the Transgenic and Chimeric Mouse Facility of the University of Pennsylvania. We thank Denisa Wagner for kindly providing skin samples from vWF knockout mice.
Supported in part by the Judith Graham Pool Post-Doctoral Research Fellowship of the National Hemophilia Foundation (S.S.F.) and NIH grant RO1HL38165-13 (H.H.K.).
Reprints:Steven Fakharzadeh, Department of Dermatology, University of Pennsylvania School of Medicine, 415 Curie Blvd, Philadelphia, PA, 19104-6145; e-mail: ssf@mail.med.upenn.edu; or Haig H. Kazazian Jr, Department of Genetics, University of Pennsylvania School of Medicine, 415 Curie Blvd, Philadelphia, PA 19104-6145; e-mail: kazaziah@mail.med.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal