High levels of nerve growth factor (NGF) are found in sera from individuals infected with human herpesvirus 8 (HHV-8). BC-1 and BCBL-1 cells are primary effusion lymphoma–derived B-cell lines; BC-1 cells are infected by HHV-8 and the Epstein-Barr virus (EBV), and BCBL-1 cells are infected only by HHV-8. Both cells express NGF receptors and produce NGF, whereas RAMOS cells (a B-cell line that is negative for HHV-8 and EBV) express NGF receptors but do not produce detectable NGF. Neutralization of endogenous NGF results in cell growth inhibition and apoptosis in BCBL-1 cells and, to a minor extent, in BC-1 cells. When the HHV-8 lytic cycle is induced in BCBL-1 cells by tetradecanoyl phorbol acetate (TPA), an initial reduction of endogenous NGF production is observed, and many cells undergo apoptosis. However, at 48 hours, TPA-treated cells produce significantly more NGF than untreated controls, and a subsequent recovery of cell viability is observed. Consistent with this finding, the addition of exogenous NGF or anti-NGF antibodies to TPA-treated cells reduces or increases, respectively, the rate of apoptosis in response to TPA. Finally, electron microscopy of TPA-treated BCBL-1 cells shows that the addition of exogenous NGF increases the number of cells producing and releasing complete virions as compared with the controls (25% versus 5%). On the contrary, NGF neutralization leads to the production of defective viral progeny in about 2% of cells. These data indicate that NGF is essential for both cell survival and virus maturation in HHV-8–infected cell lines.
Nerve growth factor (NGF) is a member of the family of neurotrophins, which are critical for the regulated development and survival of neuronal cells.1-3 Two cell surface receptors have been identified for NGF: p140trk-A, which binds NGF with high affinity, and p75NGFR, which binds this cytokine with low affinity.4 It is known that NGF subserves important roles also outside the nervous system.5 Several nonnervous cell types, such as keratinocytes,6 smooth muscle cells,7 and B memory lymphocytes,8produce NGF, and NGF receptors have been found in a number of normal epithelial, mesenchymal, and lymphoid tissues as well as in some human carcinomas and lymphomas.9-12
We have previously shown that high NGF serum levels are associated with seropositivity to human herpesvirus 8 (HHV-8) or Kaposi's sarcoma-associated herpesvirus (KSHV) both in patients with acquired immunodeficiency syndrome–related (AIDS- related) or AIDS-unrelated KS and in healthy individuals.13 We also reported that in vitro, NGF is mitogenic for spindle-shaped cells, which are obtained from KS lesions and express NGF receptors but do not produce the factor.13
HHV-8 is a recently discovered gamma herpesvirus14 that infects B cells in vivo as well as in vitro.15-17 HHV-8 has been associated with KS18; primary effusion lymphoma (PEL), which is a rare form of B-cell lymphoma growing in the serous body cavities as effusion without solid tumor formation19-22; and multicentric Castleman's disease (MCD).23-25 To date it has been difficult to culture HHV-8 efficiently, even though progress is being made in this field.26-28 However, latently infected B-cell lymphoma cell lines have been established from PEL29-34 and from the peripheral blood of PEL patients.35 PEL cells isolated from patients and most PEL cell lines are dually infected with HHV-8 and the Epstein-Barr virus (EBV). But some PEL cell lines, infected by only HHV-8,have been established31,32,35 and are commonly used in serological as well as virological studies.26 Although HHV-8 is primarily latent in most PEL-derived cell lines, viral lytic replication can be activated in these cells by treatment with phorbol esters or with sodium butyrate.32 36-38
PEL cells, exhibiting cytomorphologic features that bridge large cell immunoblastic and anaplastic lymphoma, usually lack surface immunoglobulins (Igs) and B-cell–associated antigens. However, they express CD45 and antigens, such as CD30, CD38, CD71, CD138, and epithelial membrane antigen (EMA),39 which are associated with the late stages of B-cell differentiation. The expression of several cytokines and their receptors on PEL cell lines has recently been investigated,40-43 but data on the NGF/NGF receptor system are still lacking.
This study aims to investigate whether HHV-8+ PEL cell lines are able to produce and/or respond to NGF and to examine the role, if any, of this factor in cell growth control mechanisms and in virus production during the HHV-8 lytic cycle. To this end, BC-1 cells,29 which are dually infected by HHV-8 and EBV, and BCBL-1 cells,32 which are infected only by HHV-8, have been examined. RAMOS cells, a B-lymphoma cell line that is negative for HHV-8 and EBV, have been included in the study as an uninfected control.
Materials and methods
Cell cultures
Cultural materials used included the following: BC-1 cells (American Type Culture Collection [ATCC, Rockville, MD]), BCBL-1 cells (gift from Prof C. F. Perno, University of Rome, Tor Vergata, Rome, Italy), and RAMOS cells (gift from Dr S. Grimaldi, Institute of Experimental Medicine, CNR, Rome, Italy); cell culture media and media supplements (Gibco BRL Life Technologies, Eragny, France); and plastic materials (Falcon, Becton Dickinson, Lincoln Park, NJ). The cells were maintained in RPMI 1640 supplemented with 10% or 20% fetal bovine serum (FBS), 200 mmol/L glutamine, 100 units/mL penicillin, and 50 μg/mL streptomycin at 37°C in a 5% carbon dioxide (CO2) humidified atmosphere and subcultured every 2-3 days. To induce the HHV-8 lytic cycle, BC-1 cells were exposed to 20 ng of tetradecanoyl phorbol acetate (TPA) (Sigma, Milan, Italy), and BCBL-1 cells were exposed to 3 mmol/L sodium butyrate (Sigma) for 2 days as previously reported.32 36
Quantification of NGF
In the experiments in which NGF was quantified, cells were harvested at different times of the culture, washed in phosphate-buffered saline (PBS) solution, and then incubated at 107 cells/mL in fresh medium for an additional 4 hours. NGF titration of cell supernatants was performed by a 2-site enzyme immunoassay (EIA) using a commercially available NGF mouse monoclonal antibody (mAb) 27/21 (Boehringer Mannheim, Mannheim, Germany) as previously described.44 The general specificity of mAb 27/21 to NGF is well documented, and there is no cross-reactivity with other growth factors such as epidermal growth factor, fibroblast growth factor, or insulin.44 The threshold of the sensitivity of our test was equal to 8 pg/mL, and values below this were considered negative.
Expression of NGF receptors
For Western blot analysis, 1 × 107 cells/sample were collected, washed twice in PBS (pH 7.4), and lysed in NP40 (0.25% in PBS) as previously reported.8An equal amount of protein for each sample (100 μg) was quantified (Biorad Protein Assay; Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions, separated on 10% sodium dodecyl sulfate–polyacrylamide gels, and electroblotted onto nitrocellulose membranes (Bio-Rad). The full range of rainbow recombinant protein molecular weight markers (Amersham Life Science, Arlington Heights, IL) was used to evaluate the relative molecular weight of the separated proteins. The blots were blocked with 5% nonfat milk in Tris-buffered saline-Tween (TBST), 20 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride, pH 7.5), 150 mmol/L sodium chloride (NaCl), and 0.005% Tween 20. They were then probed with goat polyclonal antibody anti-Trk A (Santa Cruz Biotechnology, Santa Cruz, CA) or mouse mAb anti-p75NGFR (NeoMarkers, Union City, CA) for 2 hours at room temperature according to the manufacturer's instructions. The antigen-antibody complexes were then visualized using appropriate alkaline phosphatase-conjugated secondary antibodies (Promega, Madison, WI).
Cell growth assay
BCBL-1, BC-1, and RAMOS cells were seeded into 25 cm2flasks at 2 or 5 × 105 cells/mL as appropriate, incubated at 37°C in a 5% CO2 humidified atmosphere, and counted daily for 3 days under a light microscope (in duplicate). Cell viability was evaluated using the trypan blue dye exclusion method.
For proliferation assays, BCBL-1 cells were cultured in 96-well plates at a concentration of 1 × 105 cells/mL for 24, 48, and 72 hours in humidified air with 5% CO2. Cells were pulsed with 3H-thymidine (0.037 MBq per well) during the final 12 hours of culture for each time point and counted in a β scintillation counter. Assays contained 30 μg/mL anti-NGF neutralizing antibodies (anti-NGF Abs) and 0.1-10 ng/mL human recombinant NGF (hr-NGF) (R&D Systems, Minneapolis, MN) and, as a control, 2 to 10 μmol/L each of commercially available human NGF phosphorothioate antisense oligonucleotides and randomized-sequence oligonucleotides (Biognostik, Gottingen, Germany).
Determination of apoptosis
Apoptosis was evaluated using 3 different techniques: flow cytometry, confocal laser scanning microscopy (CLSM), and light microscopy. For flow cytometric analysis, cell samples were collected and fixed in 1% formaldehyde in PBS on ice, washed once with PBS, incubated with 70% ethanol for 1 hour at −20°C, and then further incubated for 1 hour with a propidium iodide (Boehringer Mannheim) solution (0.1% sodium citrate, 20 μg/mL ribonuclease (RNase), 50 μg/mL propidium iodide, and 0.3% NP40). The stained cells were analyzed using a fluorescence-activated cell sorter (FACScan, Becton Dickinson) to detect relative DNA content based on red fluorescence levels. For each sample, 10 000 events were acquired, recorded, and analyzed (Lysis II software, Becton Dickinson).
For CLSM studies, cells were fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature and then applied to polylysine-coated coverslips. Nuclei were stained with 2 μg/mL propidium iodide and 0.1 mg/mL RNase for 5 minutes at room temperature, and coverslips were mounted on glass microscope slides. In the presence of glycerol and PBS, at a ratio of 4:1, the nuclei were observed under a confocal laser scanning microscope (LEICA TCS 4D; Leica, Heidelberg, Germany) equipped with an argon/krypton laser and oil immersion lenses (40 × 1.00-0.5 and 100 × 1.3-0.6). The excitation and emission wavelengths employed were 488 nm and 590 nm, respectively. Acquisitions were recorded employing a pseudo-color representation. A minimum of 300 cells per sample was examined.
For light microscopy studies, semithin sections of cells, which had been previously embedded in Spurr epoxy resin (Agar SL, Cambridge, UK) (see transmission electron microscopy, “Materials and methods”) and then stained with methylene blue, were examined. Cells showing aggregation of dense masses of chromatin beneath the nuclear membrane, nuclear fragmentation, and membrane blebbing were considered apoptotic. A minimum of 300 cells per sample was examined.
Transmission electron microscopy
The cells for electron microscopy were washed with PBS and then fixed at 4°C with 2.5% glutaraldehyde in Millonig's buffer with the addition of 2% sucrose. Postfixation was performed using 1% osmium tetroxide in the same buffer. Cells were then dehydrated in ethanol series and embedded in Spurr epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a transmission electron microscope (Philips CM12; Philips, Eindhoven, The Netherlands) at 80 kV.
Results
NGF receptor expression and NGF production
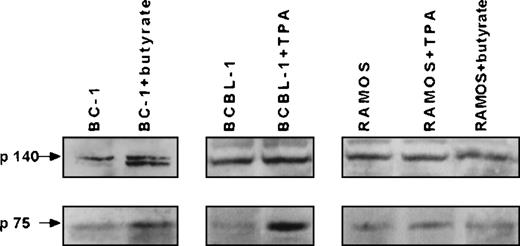
BC-1, BCBL-1, and RAMOS cells expressed both high-affinity (p140trk-A) and low-affinity (p75NGFR) NGF receptors, but only BC-1 and BCBL-1 cells, the HHV-8+ PEL cell lines, produced NGF (Figure 1 and Table 1). Increased expression of both NGF receptors was observed in BC-1 and BCBL-1 cells following the induction of the HHV-8 lytic cycle by sodium butyrate and TPA, respectively (Figure 1). BC-1 and BCBL-1 cells showed reduced NGF production after 24 hours of exposure to sodium butyrate and TPA, respectively. However, at 48 hours, treated cells produced significantly more NGF than untreated controls (Table 1). RAMOS cells showed unmodified NGF receptor expression and undetectable NGF production, even when exposed to sodium butyrate or TPA (Figure 1 and Table 1).
Expression of high-affinity and low-affinity NGF receptors by HHV-8+ and HHV-8− B-lymphoma cell lines.
The expression of high-affinity (p140trk-A) and low-affinity (p75NGFR) NGF receptors by B-lymphoma cell lines HHV-8+ (BC-1 and BCBL-1 cells) and HHV-8− (RAMOS cells) is shown. Western blot analysis was performed, and immunostaining was completed with anti-p140 and anti-p75 antibodies of proteins (100 μg/sample) from untreated cells and BC-1 cells treated with 3 mmol/L sodium butyrate and BCBL-1 cells treated with 20 ng/mL TPA at 48 hours of culture.
Expression of high-affinity and low-affinity NGF receptors by HHV-8+ and HHV-8− B-lymphoma cell lines.
The expression of high-affinity (p140trk-A) and low-affinity (p75NGFR) NGF receptors by B-lymphoma cell lines HHV-8+ (BC-1 and BCBL-1 cells) and HHV-8− (RAMOS cells) is shown. Western blot analysis was performed, and immunostaining was completed with anti-p140 and anti-p75 antibodies of proteins (100 μg/sample) from untreated cells and BC-1 cells treated with 3 mmol/L sodium butyrate and BCBL-1 cells treated with 20 ng/mL TPA at 48 hours of culture.
NGF production by HHV-8+ and HHV-8− B-lymphoma cells
| Cell Lines . | HHV-8 . | EBV . | NGF, pg/mL . | |
|---|---|---|---|---|
| 24 h . | 48 h . | |||
| BC-1 | + | + | 550 ± 36 | 480 ± 51 |
| BC-1 + Na butyrate | 186 ± 15 | 1500 ± 125 | ||
| BCBL-1 | + | − | 480 ± 55 | 336 ± 34 |
| BCBL-1 + TPA | 144 ± 15 | 1200 ± 86 | ||
| RAMOS | − | − | UD | UD |
| RAMOS + TPA | UD | UD | ||
| RAMOS + Na butyrate | UD | UD | ||
| Cell Lines . | HHV-8 . | EBV . | NGF, pg/mL . | |
|---|---|---|---|---|
| 24 h . | 48 h . | |||
| BC-1 | + | + | 550 ± 36 | 480 ± 51 |
| BC-1 + Na butyrate | 186 ± 15 | 1500 ± 125 | ||
| BCBL-1 | + | − | 480 ± 55 | 336 ± 34 |
| BCBL-1 + TPA | 144 ± 15 | 1200 ± 86 | ||
| RAMOS | − | − | UD | UD |
| RAMOS + TPA | UD | UD | ||
| RAMOS + Na butyrate | UD | UD | ||
Results represent the mean (± SD) of 3 independent experiments with 1 × 107 cells/mL; NGF was measured in the conditioned media as described in “Materials and methods.” UD indicates undetectable.
Effects of endogenous NGF neutralization on cell growth
The above data, indicating that HHV-8–infected cell lines produced NGF and expressed high- and low-affinity NGF receptors, supported the hypothesis of an autocrine circuit. To understand the function of this circuit, we neutralized endogenous NGF and assessed its impact on the properties of these cells. To this end, we employed either neutralizing antibodies to NGF or, in selected experiments, antisense oligonucleotides directed against NGF messenger RNA (mRNA).
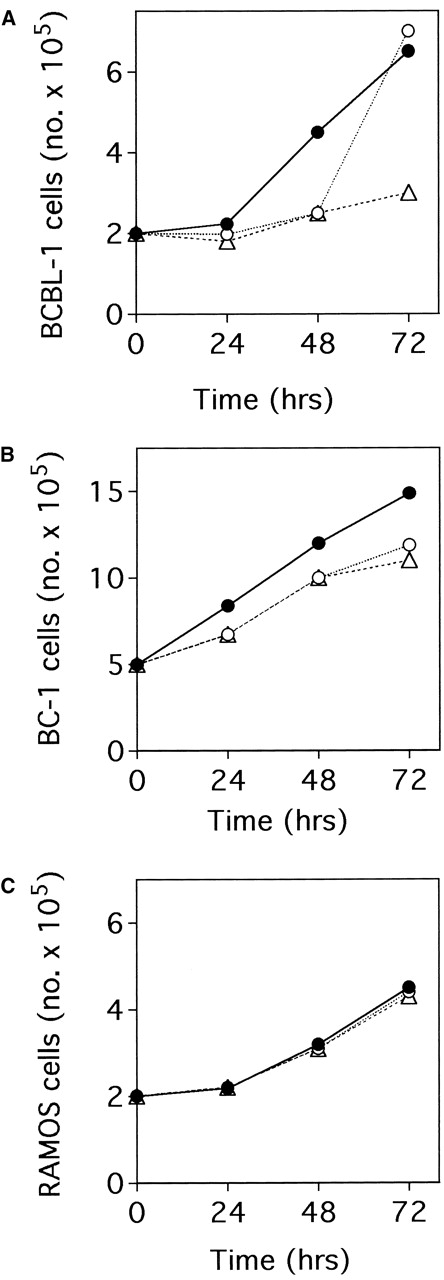
We first tested anti-NGF Abs in cell growth assays. In BCBL-1 cells, a single treatment with 30 μg/mL anti-NGF Ab at 0 hour completely prevented the increase in cell number that occurred in control cells for up to 48 hours, and a second treatment of 30 μg/mL at 48 hours prevented the increase for up to 72 hours (Figure2A). BC-1 cell growth was also found to be inhibited in the presence of anti-NGF Ab (Figure 2B). Specifically, after 72 hours of culture, the cell number was 20%-30% lower in BC-1 cells incubated with anti-NGF Ab when compared with control cells. On the contrary, RAMOS cell growth was not affected by the addition of anti-NGF Ab to the culture medium (Figure 2C).
Effects of endogenous NGF neutralization on the basal growth of HHV-8+ and HHV-8− B-lymphoma cell lines.
The figure depicts untreated cells (•), cells treated with 30 μg/mL anti-NGF Ab at 0 hour (○), and cells treated with 30 μg/mL anti-NGF Ab at 0 hour and 48 hours (▵). Cells were seeded in duplicate at (A, C) 2 × 105/mL or (B) 5 × 105/mL at 0 hour. The number of viable cells in each culture was determined daily using the trypan blue dye exclusion method. The values refer to the mean of each duplicate. In all cases, the SD was less than 10% of the mean. Similar results were obtained in 3 separate experiments.
Effects of endogenous NGF neutralization on the basal growth of HHV-8+ and HHV-8− B-lymphoma cell lines.
The figure depicts untreated cells (•), cells treated with 30 μg/mL anti-NGF Ab at 0 hour (○), and cells treated with 30 μg/mL anti-NGF Ab at 0 hour and 48 hours (▵). Cells were seeded in duplicate at (A, C) 2 × 105/mL or (B) 5 × 105/mL at 0 hour. The number of viable cells in each culture was determined daily using the trypan blue dye exclusion method. The values refer to the mean of each duplicate. In all cases, the SD was less than 10% of the mean. Similar results were obtained in 3 separate experiments.
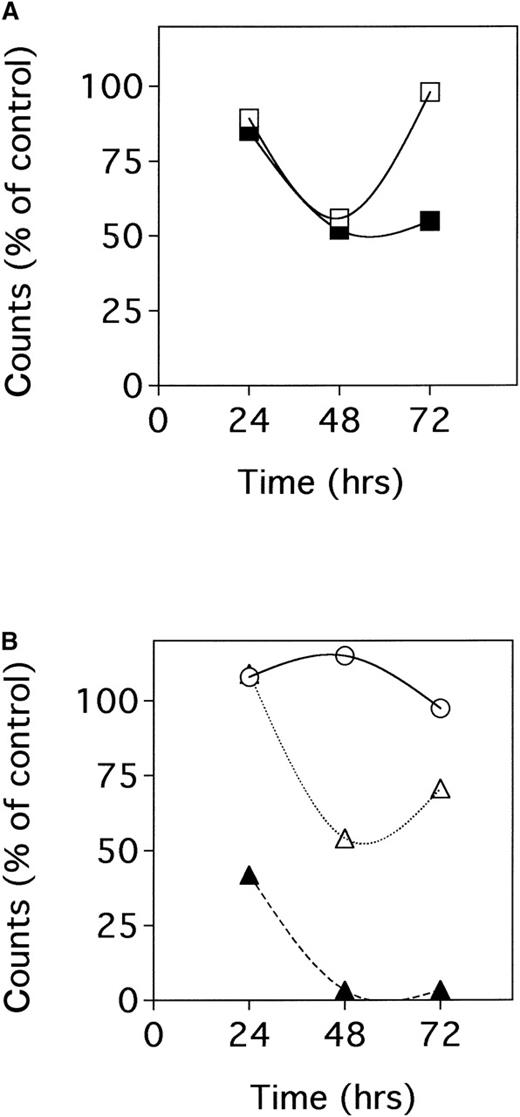
We then focused on BCBL-1 cells in which we tested either anti-NGF Abs or NGF antisense oligonucleotides in 3H-thymidine incorporation assays. We found that 3H-thymidine incorporation by BCBL-1 cells cultured in the presence of anti-NGF Abs was decreased with respect to that of untreated controls (Figure3A). Similarly, antisense oligonucleotides directed against NGF mRNA inhibited 3H-thymidine incorporation by BCBL-1 cells in a dose-dependent fashion (Figure 3B). Finally, in confirmation experiments on cell growth performed by the cell counting method, we observed that NGF antisense oligonucleotides, but not random-sequence oligonucleotides employed as the control of specificity, inhibited BCBL-1 cell growth. Specifically, at 48 hours of culture, BCBL-1 cell growth was decreased by 50% and 70% when NGF antisense oligonucleotides were employed at concentrations of 2 μmol/L and 10 μmol/L, respectively.
Effects of endogenous NGF neutralization by means of anti-NGF antibodies or NGF antisense oligonucleotides on3H-thymidine incorporation by BCBL-1 cells.
(A) Cells were treated with 30 μg/mL anti-NGF Ab at 0 hour (□) and with 30 μg/mL anti-NGF Ab at 0 and 48 hours (▪). (B) Cells were treated with 2 μmol/L (▵) or 10 μmol/L (▴) NGF antisense oligonucleotides and with 10 μmol/L (○) control random-sequence oligonucleotides. Cells were seeded in quadruplicate at 1 × 105 cells/mL in 96-well plates.3H-thymidine (0.037 MBq/well) was added in the final 12 hours of incubation for each time point. Results were calculated as the mean 3H-thymidine incorporation of quadruplicate cultures and expressed as the percentage of untreated controls. Similar results were obtained in 3 separate experiments.
Effects of endogenous NGF neutralization by means of anti-NGF antibodies or NGF antisense oligonucleotides on3H-thymidine incorporation by BCBL-1 cells.
(A) Cells were treated with 30 μg/mL anti-NGF Ab at 0 hour (□) and with 30 μg/mL anti-NGF Ab at 0 and 48 hours (▪). (B) Cells were treated with 2 μmol/L (▵) or 10 μmol/L (▴) NGF antisense oligonucleotides and with 10 μmol/L (○) control random-sequence oligonucleotides. Cells were seeded in quadruplicate at 1 × 105 cells/mL in 96-well plates.3H-thymidine (0.037 MBq/well) was added in the final 12 hours of incubation for each time point. Results were calculated as the mean 3H-thymidine incorporation of quadruplicate cultures and expressed as the percentage of untreated controls. Similar results were obtained in 3 separate experiments.
Effects of endogenous NGF neutralization on cell survival
These findings indicated that NGF was involved in the control of BCBL-1 cell growth. However, considering that the addition of recombinant NGF failed to increase 3H-thymidine incorporation by BCBL-1 cells (data not shown) and taking into account the fact that NGF is known to maintain survival of neurons as well as memory B lymphocytes,8 we considered whether NGF could support BCBL-1 cell survival. To this end, BCBL-1 cells were cultured in the presence or absence of anti-NGF Abs or NGF antisense oligonucleotides, and the percentage of apoptotic cells in each sample was measured by means of CLSM and FACS analysis. We found that endogenous NGF neutralization induced BCBL-1 cell apoptosis. Morphological evidence of apoptosis in cells exposed to anti-NGF Ab was assessed by CLSM (Figure 4). After 48 hours of culture the percentage of apoptotic cells was measured by FACS analysis, giving the following results: 12% for BCBL-1 cells grown in the absence of antibodies or oligonucleotides (controls); 30% for cells grown in the presence of 30 μg/mL anti-NGF Abs; 38% in cells cultured with 2 μmol/L NGF antisense oligonucleotides; 68% in cells cultured with 10 μmol/L NGF antisense oligonucleotides; and 10% in cells cultured with 10 μmol/L random oligonucleotides.
Confocal fluorescence micrographs of BCBL-1 cells cultured in the absence or presence of anti-NGF antibodies.
Untreated cells were observed at (A) 24 hours, (C) 48 hours, and (E) 72 hours. Cells were treated with 30 μg/mL anti-NGF Ab at 0 hour and observed at (B) 24 hours, (D) 48 hours, and (F) 72 hours. (G) Cells were treated with 30 μg/mL anti-NGF Ab at 0 and 48 hours and observed at 72 hours. To observe nuclei, cells were stained with propidium iodide. Apoptosis was evaluated using morphological parameters such as chromatin condensation and nuclear fragmentation (seen in dense black). (Original magnification ×40.)
Confocal fluorescence micrographs of BCBL-1 cells cultured in the absence or presence of anti-NGF antibodies.
Untreated cells were observed at (A) 24 hours, (C) 48 hours, and (E) 72 hours. Cells were treated with 30 μg/mL anti-NGF Ab at 0 hour and observed at (B) 24 hours, (D) 48 hours, and (F) 72 hours. (G) Cells were treated with 30 μg/mL anti-NGF Ab at 0 and 48 hours and observed at 72 hours. To observe nuclei, cells were stained with propidium iodide. Apoptosis was evaluated using morphological parameters such as chromatin condensation and nuclear fragmentation (seen in dense black). (Original magnification ×40.)
Effect of NGF on BCBL-1 cell survival during TPA-induced HHV-8 lytic cycle
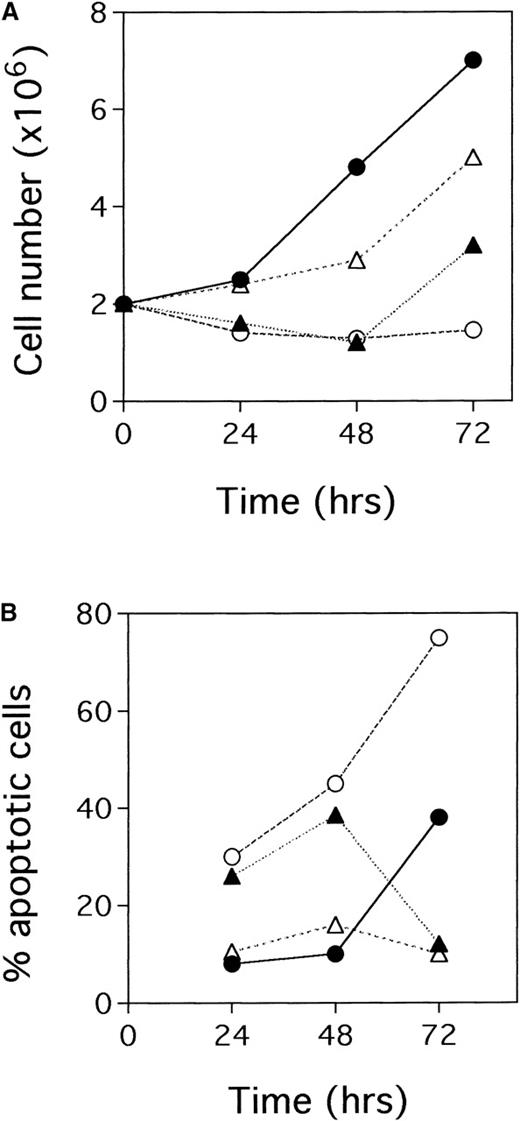
The addition of TPA to BCBL-1 cell cultures prevented cell growth over the ensuing 48 hours (Figure 5A). We found that this phenomenon was associated with marked apoptosis, which was already evident 24 hours after TPA addition and peaked at 48 hours (Figure 5B). Thereafter, the number of TPA-treated cells almost doubled, whereas the percentage of apoptotic cells was minimal (Figure5A and 5B). The initial drop in NGF production during TPA treatment and the subsequent complete recovery after 48 hours of culture again suggested the role of NGF as a survival factor for BCBL-1 cells. Therefore, experiments were performed in which we overcame the initial drop in NGF production by adding recombinant NGF to TPA-treated BCBL-1 cells and assessed its impact on cell growth and survival. As an internal control, TPA-treated cells were also cultured in the absence or presence of anti-NGF antibodies. We found that BCBL-1 cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour showed a decreased rate of apoptosis and faster recovery of cell growth compared with cells treated with TPA alone (Figure 5A and 5B). On the contrary, BCBL-1 cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours showed an increased rate of apoptosis and inhibited recovery of cell growth with respect to cells treated with TPA alone (Figure 5A and 5B). Morphological evidence of apoptosis, as assessed by light microscopy after 48 hours of culture, is shown in Figure6.
Effects of TPA plus hr-NGF or anti-NGF antibodies on BCBL-1.
(A) Cell growth curves and (B) the percentage of apoptotic cells is depicted. Cells were seeded in duplicate at 2 × 105/mL. The figure depicts untreated cells (•), cells treated with 20 ng/mL TPA at 0 hour (▴), cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour (▵), and cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours (○). The number of viable cells in each culture was determined daily using the trypan blue dye exclusion method. The values refer to the mean of each duplicate. In all cases, the SD was less than 10% of the mean. Similar results were obtained in 3 separate experiments.
Effects of TPA plus hr-NGF or anti-NGF antibodies on BCBL-1.
(A) Cell growth curves and (B) the percentage of apoptotic cells is depicted. Cells were seeded in duplicate at 2 × 105/mL. The figure depicts untreated cells (•), cells treated with 20 ng/mL TPA at 0 hour (▴), cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour (▵), and cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours (○). The number of viable cells in each culture was determined daily using the trypan blue dye exclusion method. The values refer to the mean of each duplicate. In all cases, the SD was less than 10% of the mean. Similar results were obtained in 3 separate experiments.
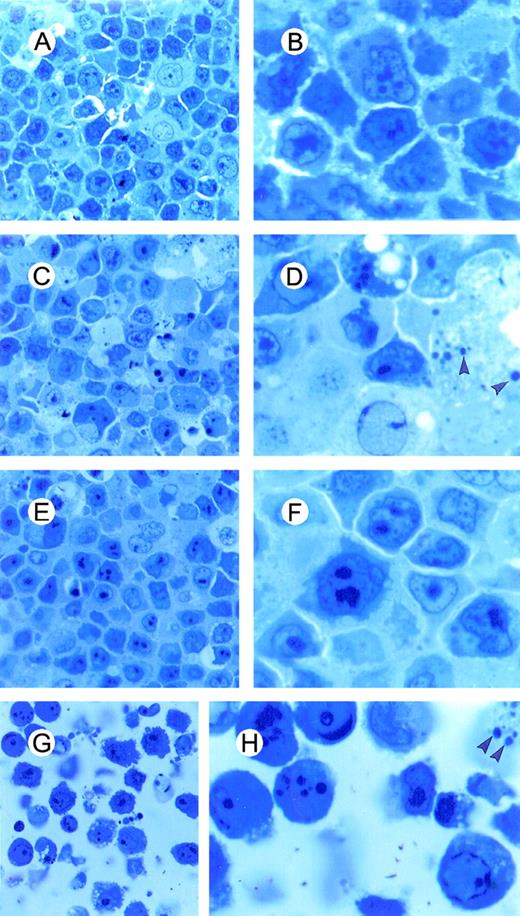
Light microscopy of BCBL-1 cells treated with TPA plus hr-NGF or anti-NGF Ab and observed at 48 hours of culture.
Semithin sections of cells were embedded in Spurr epoxy resin and stained with methylene blue as shown: (A, B) untreated cells, (C, D) cells treated with 20 ng/mL TPA at 0 hour, (E, F) cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour, and (G, H) cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours. (Original magnification ×40 in A, C, E, and G, and ×100 in B, D, F, and H. (C, D) Numerous cells showing aggregation of dense masses of chromatin beneath the nuclear membrane and nuclear fragmentation were observed. (E, F) Only a few cells showed morphological evidence of apoptosis, and (G, H) a high number of apoptotic cells were observed. Arrowheads indicate apoptotic bodies.
Light microscopy of BCBL-1 cells treated with TPA plus hr-NGF or anti-NGF Ab and observed at 48 hours of culture.
Semithin sections of cells were embedded in Spurr epoxy resin and stained with methylene blue as shown: (A, B) untreated cells, (C, D) cells treated with 20 ng/mL TPA at 0 hour, (E, F) cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour, and (G, H) cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours. (Original magnification ×40 in A, C, E, and G, and ×100 in B, D, F, and H. (C, D) Numerous cells showing aggregation of dense masses of chromatin beneath the nuclear membrane and nuclear fragmentation were observed. (E, F) Only a few cells showed morphological evidence of apoptosis, and (G, H) a high number of apoptotic cells were observed. Arrowheads indicate apoptotic bodies.
Effect of NGF on HHV-8 replication in TPA-treated BCBL-1 cells
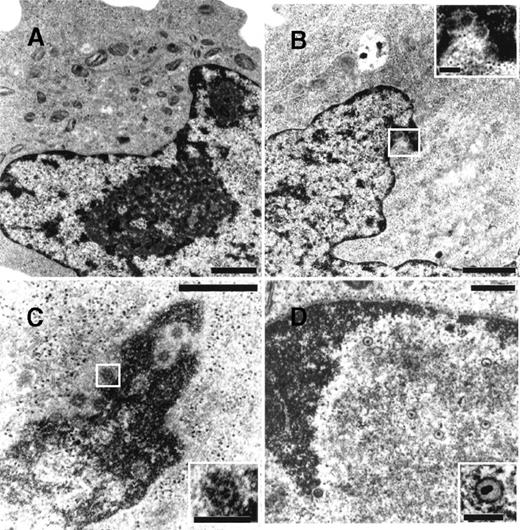
To determine whether NGF was able to affect viral maturation, electron microscopy studies were performed on BCBL-1 cells treated with TPA in the presence or absence of hr-NGF or anti-NGF Ab (Figure7). No HHV-8 viral particles were detected in untreated control cells (Figure 7A), whereas both complete and defective virions were detected in about 5% of TPA-treated cells (Figure 7B). Noticeably, treatment with TPA plus hr-NGF resulted in the production of complete virions that were visualized at a high number in approximately 25% of BCBL-1 cells (Figure 7C). On the contrary, the addition of anti-NGF Ab to TPA-treated cells reduced the percentage of virion-producing cells to about 2% and led to the production of predominately defective virions, which were visualized within nuclei of cells undergoing apoptosis (Figure 7D).
Transmission electron microscopy of HHV-8 virions in TPA-treated BCBL-1 cells at 48 hours of culture.
The following cells are depicted: (A) untreated cells, (B) cells treated with 20 ng/mL TPA at 0 hour, (C) cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour, and (D) cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours. (A) No HHV-8 viral particles were observed in control cells. (B) Mature enveloped virions originated from the nucleus of a TPA-treated cell. A detail, at higher magnification, is shown in the corner. (C) View of numerous mature HHV-8 virions, approximately 110 nm in diameter, in the nucleus of a cell treated with TPA plus hr-NGF. A detail, at higher magnification, is shown in the corner. (D) Nucleus of an apoptotic cell in a culture treated with TPA plus anti-NGF Ab. The condensed chromatin of the apoptotic cell can be seen along the nuclear membrane. Numerous defective virus particles, with internal electron dense structures possibly representing viral DNA, were observed. A detail of a defective virion can be seen at higher magnification in the corner. The bars in (A, B) equal 1 μm; in (C, D), 0.5 μm; and in the corners, 150 nm.
Transmission electron microscopy of HHV-8 virions in TPA-treated BCBL-1 cells at 48 hours of culture.
The following cells are depicted: (A) untreated cells, (B) cells treated with 20 ng/mL TPA at 0 hour, (C) cells treated with TPA plus 5 ng/mL hr-NGF at 0 hour, and (D) cells treated with TPA plus 30 μg/mL anti-NGF Ab at 0 and 24 hours. (A) No HHV-8 viral particles were observed in control cells. (B) Mature enveloped virions originated from the nucleus of a TPA-treated cell. A detail, at higher magnification, is shown in the corner. (C) View of numerous mature HHV-8 virions, approximately 110 nm in diameter, in the nucleus of a cell treated with TPA plus hr-NGF. A detail, at higher magnification, is shown in the corner. (D) Nucleus of an apoptotic cell in a culture treated with TPA plus anti-NGF Ab. The condensed chromatin of the apoptotic cell can be seen along the nuclear membrane. Numerous defective virus particles, with internal electron dense structures possibly representing viral DNA, were observed. A detail of a defective virion can be seen at higher magnification in the corner. The bars in (A, B) equal 1 μm; in (C, D), 0.5 μm; and in the corners, 150 nm.
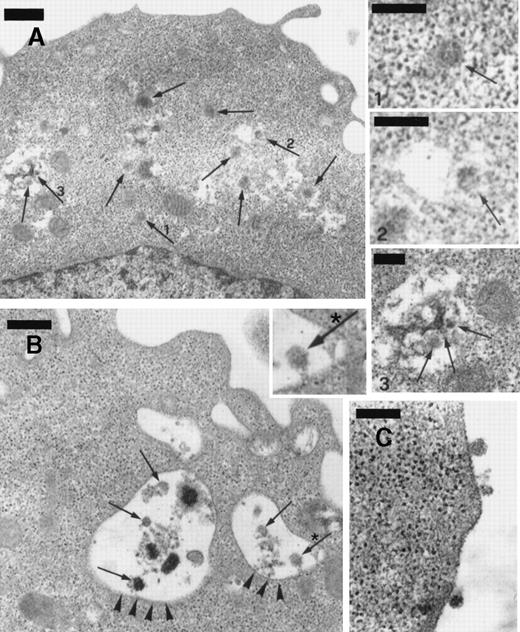
The numerous mature virions observed in BCBL-1 cells treated with TPA plus recombinant NGF were visualized within dilated cisternae of the endoplasmic reticulum, cytoplasmic vesicles, and the extracellular space (Figure 8). Although TEM alone does not establish replication competence of virus per se, our observations are highly suggestive of effective virus excretion by the cells in these conditions.
Transmission electron microscopy of BCBL-1 cells treated with TPA plus hr-NGF after 48 hours of culture.
(A) Numerous mature HHV-8 virions (arrows) were observed both in cytoplasm and in dilated cisternae of the endoplasmic reticulum (ER). The small pictures in the upper right-hand corner detail HHV-8 virions in the (1) cell cytoplasm and (2,3) dilated cisternae of ER. In some cases, (2) the outer membrane of the viral particles was connected to the ER membrane, suggesting a possible budding into ER. (B) Electron dense material and mature virions inside intracytoplasmic vesicles (arrowheads) were observed just beneath the cell membrane; a detail at higher magnification is inserted in *. (C) Morphologically mature viral particles are seen in the extracellular space of BCBL-1 cells. The bars in (A, B) equal 400 nm; in (C), 300 nm; and in (1, 2, 3), 200 nm.
Transmission electron microscopy of BCBL-1 cells treated with TPA plus hr-NGF after 48 hours of culture.
(A) Numerous mature HHV-8 virions (arrows) were observed both in cytoplasm and in dilated cisternae of the endoplasmic reticulum (ER). The small pictures in the upper right-hand corner detail HHV-8 virions in the (1) cell cytoplasm and (2,3) dilated cisternae of ER. In some cases, (2) the outer membrane of the viral particles was connected to the ER membrane, suggesting a possible budding into ER. (B) Electron dense material and mature virions inside intracytoplasmic vesicles (arrowheads) were observed just beneath the cell membrane; a detail at higher magnification is inserted in *. (C) Morphologically mature viral particles are seen in the extracellular space of BCBL-1 cells. The bars in (A, B) equal 400 nm; in (C), 300 nm; and in (1, 2, 3), 200 nm.
Discussion
The 2 HHV-8+ PEL cell lines tested, BC-1 and BCBL-1 cells, expressed both high- and low-affinity NGF receptors and produced NGF. RAMOS cells, the uninfected B lymphoma cell line used as a control, also expressed NGF receptors but did not produce NGF. In addition, while BC-1 and BCBL-1 cell proliferation was not significantly increased by exogenously added NGF (data not shown), neutralization of endogenous NGF induced apoptosis and cell growth inhibition in BC-1 and BCBL-1 cells but not in RAMOS cells. These findings suggest that NGF acts as an autocrine survival factor for HHV-8–infected cells, as has been shown for normal B memory lymphocytes.8 NGF neutralization inhibited BC-1 cell growth to a lesser extent than what was observed in BCBL-1 cells. This phenomenon could be due to BC-1 coinfection by HHV-8 and EBV. It is known that EBV transforms B cells45-47 and that previous EBV infection facilitates HHV-8 infection.47 Most PEL tumors are positive for both HHV-8 and EBV, implying that double infection could play a causal role in this entity. It is theoretically possible that either of the 2 viruses is the primary transforming agent, whereas the other virus provides an essential or nonessential cofactor.47 Thus, cells infected with both HHV-8 and EBV may possess a growth advantage with respect to cells infected by HHV-8 alone. It should be noted that BC-1 cells express NGF receptors, whereas it has been reported that EBV-transformed B lymphocytes do not.48
In further experiments, the HHV-8 lytic cycle was induced in BC-1 and BCBL-1 cells by sodium butyrate and TPA, respectively. TPA is a potent activator of the HHV-8 lytic cycle in BCBL-1 cells.32 In contrast, in BC-1 cells, even if HHV-8 early-gene expression is activated by TPA, sodium butyrate is required for HHV-8 to proceed to lytic viral DNA synthesis and late-gene expression.36
HHV-8+ PEL cell lines showed increased expression of both NGF receptors, particularly of p75NGFR, 48 hours after chemical induction of the HHV-8 lytic cycle. Although expression of p140trk-A alone is sufficient to achieve a cellular response to NGF, p75NGFR has been shown to up-regulate trk-A ligand interactions and signal transduction in some systems.49,50 In this regard, increased expression of both NGF receptors, observed during the HHV-8 lytic cycle, would indicate that under these experimental conditions, the cells are better equipped to use the cytokine. NGF production by HHV-8+ cells varied significantly during the chemically induced HHV-8 lytic cycle. With respect to untreated cells, NGF production was found to be reduced 24 hours after the addition of chemicals but increased at 48 hours. Exposure of RAMOS cells, the uninfected controls, to the same doses of sodium butyrate or TPA resulted in unmodified NGF receptor expression and undetectable NGF production. Therefore, the activation of the pathway “NGF receptor expression/NGF production” observed in HHV-8+ cell lines during the HHV-8 lytic cycle would appear more likely to be virus related rather than chemically related. Consistent with this hypothesis, the stimulation of BC-1 cells with TPA, which induces only HHV-8 early-gene transcription in this cell line, had the same effect on NGF receptor expression and NGF production as sodium butyrate, which activates the full HHV-8 lytic cycle (data not shown). Furthermore, it should be mentioned that the 2 chemicals act via different and independent metabolic pathways.36
The occurrence of BCBL-1 cell apoptosis following chemical induction of the HHV-8 lytic cycle has recently been reported by other investigators.38 In our study, BCBL-1 cells underwent apoptosis in the first 48 hours following TPA addition, whereas at 72 hours, a spontaneous recovery of cell growth took place. It has also been shown that exposure to TPA suppresses human interleukin-6 (IL-6) production by IL-6–dependent HHV-8–infected PEL cells for at least 48 hours.42 It is of interest that in TPA-treated BCBL-1 cells, the initial decrease in and the subsequent recovery of cell viability were preceded by a drop in and then a recovery of endogenous NGF production, respectively (see Table 1 and Figure 5). NGF maintains BCBL-1 cell survival during the TPA-induced HHV-8 lytic cycle. Consistent with this is the observation that BCBL-1 cells treated with TPA plus hr-NGF at the time of seeding showed reduced apoptosis and faster recovery of cell growth when compared with cells treated with TPA alone. Conversely, BCBL-1 cells treated with TPA plus anti-NGF Ab showed increased apoptosis and inhibited recovery of cell growth with respect to cells treated with TPA alone.
Finally, in TPA-stimulated BCBL-1 cells, the efficiency of viral production, evaluated both in terms of the number of cells containing virions and the presence of mature viral particles, was improved by the addition of exogenous NGF. Interestingly, this result was obtained by using the same NGF concentrations (5 ng/mL) as those found in sera from AIDS-KS patients in our previous studies.13 Because high HHV-8 replication26,51 and elevated NGF serum levels13 have been detected in AIDS-KS patients, our in vitro findings, showing that NGF improves survival of HHV-8 infected cells as well as virus maturation, could simulate what occurs during HHV-8 infection in vivo. On the contrary, neutralization of endogenous NGF by anti-NGF Ab resulted in the production of a predominantly defective virus progeny by TPA-treated BCBL-1 cells.
If NGF is capable of promoting cell survival and virus maturation in HHV-8–infected cell lines, it is conceivable that the activation of the pathway NGF receptor expression/NGF production can be triggered or in some way regulated by the virus itself in order to overcome apoptosis and to persist in infected cells. Alternatively, it may represent a virus-independent cell defense mechanism against TPA-induced apoptosis.
The data herein reported strengthen our previous observations concerning the existence of a close relationship among NGF, HHV-8 infection, and HHV-8–associated diseases.13 Moreover, they provide new insights for the understanding and treatment of HHV-8–related diseases. To fully elucidate the mechanism(s) involved in the observed phenomena, further in vivo and in vitro studies are currently ongoing.
Acknowledgments
We thank G. Barillari for helpful discussion; C. F. Perno and S. Grimaldi for providing us with BCBL-1 cells and RAMOS cells, respectively; and A. Inglis for her linguistic revision of the manuscript.
Supported by grant 50B.31 from the ISS-Ministero della Sanità, Project AIDS, Rome, Italy (F.P.), and by a grant from MURST, 40% 1998 (E.G.), Rome, Italy.
Submitted July 28, 1999; accepted December 28, 1999.
Reprints:Francesca Pica, Department of Experimental Medicine and Biochemical Sciences, University of Rome, Tor Vergata, Via di Tor Vergata 135, 00133 Rome, Italy; e-mail: pica@uniroma2.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal