P-glycoprotein (pgp), which is the product of the MDR1(multidrug resistance-1) gene, has an established role as a mediator of cytotoxic drug resistance in acute myeloid leukemia (AML). To study the role of pgp in mediating apoptosis resistance in AML cells deprived of serum and growth factors, apoptosis was quantified by flow cytometry using uptake of the dye 7-amino-actinomycin D (7-AAD) alongside low forward scatter. In pgp+ve primary AML samples, there was a significant increase in apoptosis in the presence of the pgp-specific antibody UIC2 (mean increase: 58%; range: 11%-95%; P < .05). Likewise, apoptosis in growth factor–deprived TF1 cells cultured for 30 hours increased 2.5-fold in the presence of 25 μg/mL UIC2. The pgp reversal agent PSC-833 (1 μmol/L) augmented in vitro apoptosis by a median of 52% in pgp+ve patient samples and to a comparable degree in 6 pgp−ve samples. To determine whether the sphingomyelin-ceramide (SM-ceramide) pathway of apoptosis occurs in AML blasts in response to cytotoxic drugs, cells were incubated with daunorubicin at the patient-specific IC30 (the concentration of daunorubicin that caused apoptotic cell death in 30% of cells) in the presence of the ceramide synthase inhibitor fumonisin B1, which inhibited apoptosis by 18%-81% (median: 40%). Exogenous SM failed to augment apoptosis induced by growth factor withdrawal in pgp+ve TF1 cells and was significantly more effective at augmenting apoptosis in pgp−ve patient blasts (median increase in cell death: 33%; range: 19%-88%) than in pgp+ve samples (median: 7%; range: 0%-27%;P = .028). Cellular accumulation of exogenous SM was associated with apoptosis and also occurred in nonapoptotic patient cells treated with PSC-833. However, this effect was not seen following treatment with the UIC2 antibody. These results indicate that pgp is able to exert a protective effect on AML cell viability and that this is associated with a reduced effect of exogenous SM on apoptosis. The pgp reversal agent PSC-833 acts, at least in part, by a pgp independent mechanism to alter SM distribution and to augment apoptosis induced in AML cells by serum and growth factor withdrawal.
P-glycoprotein (pgp) is the product of theMDR-1 (multidrug resistance-1) gene and is responsible for transporting a variety of amphiphilic substances across plasma membranes.1 The term MDR-1 was conferred on the gene because of the ability of its product pgp to efflux a broad range of chemotherapeutic drugs such as anthracyclines and etoposide.2 Expression of pgp on leukemic cells has been measured in several series of patients with AML and has been established as a major indicator of poor prognosis.3-15Until recently, only 1 important role for pgp in tumor biology, namely its ability to efflux drugs, has been elucidated. However, a further role has now been demonstrated in a T-cell leukemia model: anti-fas, UV, and drugs induce caspase-mediated apoptosis, which is inhibited by pgp.16,17 In Chinese hamster ovary fibroblasts, pgp expression was found to delay apoptosis induced by growth factor withdrawal.18
The role of the sphingomyelin-ceramide (SM-ceramide) pathway has not previously been reported in primary AML cells, although a role for both SM hydrolysis19 and ceramide synthesis20 has been reported in cell line studies using daunorubicin. This pathway may be important in pgp-augmented cell survival because pgp has been reported to transport phospholipids across cell membranes.21 It has been hypothesized that pgp may act as a primary antiapoptotic molecule by reducing the pool of plasma membrane SM that can be hydrolyzed to ceramide.22Most plasma membrane SM is expressed in the outer leaflet, but a small signaling pool of internalized SM has been identified.23,24Sphingomyelin can be hydrolyzed to ceramide by neutral sphingomyelinase in response to exposure to proapoptotic stimuli such as tumor necrosis factor–alpha (TNF-α) or daunorubicin.25 The pgp modulator PSC-833 has been found to enhance SM redistribution from the outer to the inner plasma membrane and subsequent apoptosis of KG1a cells treated with TNF-α.22 It is not known whether PSC-833 was acting as a specific pgp reversal agent in this context.
The primary purpose of the current investigation was to establish whether there is a role for pgp in the apoptosis of primary leukemic cells independent of its role in mediating multidrug resistance. For this purpose we have used the pgp blocking antibody UIC226as well as the pgp reversal agent PSC-833. We also established the relevance of a SM-ceramide apoptotic pathway in the chemoresistance of leukemic cells using the specific ceramide synthase inhibitor fumonisin B1 (FB1).20 Finally, to investigate whether there may be a role for pgp-mediated SM compartmentalization in the apoptosis resistance of pgp+ve leukemic cells, we added exogenous fluorescent SM (C6-NBD-SM) to pgp+ve and pgp−ve leukemic cells and compared its effect on augmenting apoptosis in the 2 cell types as well as the effect of PSC-833 on cellular SM distribution.
Materials and methods
Reagents
The following products were used in this study: MRK-16 anti-pgp (Kamiya, Seattle, WA); 15D3 anti-pgp (Becton Dickinson, Cowley, England); control and secondary antibodies for pgp analysis (Dako, High Wycombe, England); rhodamine 123 (R123), 7-amino-actinomycin D (7-AAD), the annexin V apoptosis detection kit, propidium iodide, FB1, L-glutamine, and antibiotics (Sigma Aldrich, Poole, England); daunorubicin (Cerubidin, Rhone-Poulenc Rorer, West Malling, UK); C6-NBD-SM (Molecular Probes, Cambridge Bioscience, Cambridge, England); and granulocyte-macrophage–colony stimulating factor (GM-CSF) (Leucomax, Novartis, Basel, Switzerland).
Fumonisin B1 was dissolved in sterile water and stored at 4°C. PSC-833 (gift from Novartis) was stored at 1 mmol/L in ethanol. A 1 mmol/L stock solution of C6-NBDSM in ethanol was stored at −20°C. UIC2 anti-pgp blocking antibody (Immunotech, Beckman Coulter, High Wycombe, England) was stored in 200 μg/mL aliquots at −20°C, as were preservative-free control IgG2a antibodies (Calbiochem, Nottingham, England). Fetal calf serum (FCS) (First Link, Wolverhampton, England) was heat inactivated at 56°C for 60 minutes.
Cells
U937 and TF1 leukemic cell lines were grown in RPMI 1640 with 10% FCS, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. The medium of the growth factor dependent cell line TF1 was supplemented with 200 units/mL GM-CSF. Experiments were performed on cells in log phase. Fresh or cryopreserved blood or bone marrow samples from untreated patients with AML were used in this study. As several assays were to be performed on the same samples, all samples selected were from patients with high blast counts; 9 samples were collected at presentation, and the remaining 2 were from relapsed patients (RF and GM in Table 1). The viability of thawed cells was assessed using trypan blue dye exclusion, and only samples with more than 90% thawed cell viability were used.
MDR status of AML blasts and the TF1 and U937 cell lines
| . | Pgp D Value . | R123+/-PSC-833 Modulation Ratio . | Daunorubicin Cell:Bead Ratio . |
|---|---|---|---|
| pgp protein positive | |||
| GO | 0.34 | 3.2 | 1.5 |
| JD | 0.33 | 2.4 | 1.9 |
| RF | 0.47 | 1.3 | 2.0 |
| DS | 0.19 | 2.7 | 1.1 |
| BB | 0.15 | 3.0 | 2.2 |
| TF1 cell line | 0.93 ± 0.03 | 5.0 ± 0.47 | ND |
| pgp protein negative | |||
| CL | 0.02 | 1.5 | 1.8 |
| AJ | 0.05 | 1.1 | 2.9 |
| JK | 0.05 | 2.0 | 2.25 |
| AC | 0.08 | 1.4 | 2.1 |
| GM | 0.03 | 1.6 | 2.7 |
| LC | 0.02 | 1.9 | 1.4 |
| U937 cell line | 0.037 ± 0.02 | 1.12 ± 0.01 | ND |
| . | Pgp D Value . | R123+/-PSC-833 Modulation Ratio . | Daunorubicin Cell:Bead Ratio . |
|---|---|---|---|
| pgp protein positive | |||
| GO | 0.34 | 3.2 | 1.5 |
| JD | 0.33 | 2.4 | 1.9 |
| RF | 0.47 | 1.3 | 2.0 |
| DS | 0.19 | 2.7 | 1.1 |
| BB | 0.15 | 3.0 | 2.2 |
| TF1 cell line | 0.93 ± 0.03 | 5.0 ± 0.47 | ND |
| pgp protein negative | |||
| CL | 0.02 | 1.5 | 1.8 |
| AJ | 0.05 | 1.1 | 2.9 |
| JK | 0.05 | 2.0 | 2.25 |
| AC | 0.08 | 1.4 | 2.1 |
| GM | 0.03 | 1.6 | 2.7 |
| LC | 0.02 | 1.9 | 1.4 |
| U937 cell line | 0.037 ± 0.02 | 1.12 ± 0.01 | ND |
Values for TF1 and U937 cell lines are given as mean ±SD.
ND indicates not determined.
Determination of MDR status
The MDR status of patient blasts was determined by 3 previously described methods.15 First, pgp was measured using an unconjugated MRK-16 antibody and a fluorescein isothiocyanate–conjugated (FITC-conjugated) second layer. In 1 case there was high nonspecific binding of the control antibody, and the phycoerythrin-conjugated (PE-conjugated) 15D3 antibody gave a clear result. This antibody was also used to confirm protein results in 2 protein-negative, R123 modulation–positive samples. Pgp substrate efflux modulation by PSC-833 was determined in an accumulation assay using R123. Thawed or freshly isolated patient blasts were rested in culture medium containing 20% FCS for 60-90 minutes. They were then pelleted; resuspended at 106/mL; and incubated in medium containing 10% FCS at 37°C, in duplicate, with 200 ng/mL R123, with or without 2 μmol/L PSC-833. Control tubes with R123 incubated at 4°C were also set up. Following a 75-minute incubation, the cells were pelleted at 4°C, rinsed, and resuspended. They were analyzed using a FACScan or FACSCalibur flow cytometer (Becton Dickinson) as previously described.15 The mean fluorescence intensity (MFI) of each sample in the FL1 channel was recorded. Results were expressed as (MFI with PSC-833–cold control MFI)/(MFI with diluent control–cold control MFI). Third, results were validated using the standardized daunorubicin uptake assay. Rested leukemic blasts at 106/mL and carboxylate microspheres (Polysciences) were incubated in duplicate for 75 minutes with 2 × 10−6mol/L daunorubicin at 37°C. Control cells were also set up with daunorubicin at 4°C. Cells and beads were rinsed and analyzed as described.15 Results were expressed as (cell MFI–control cell MFI)/bead peak fluorescence.
Cell culture
Thawed or fresh mononuclear cell preparations from patients with AML were rested for 60-90 minutes in culture medium consisting of RPMI 1640 with 20% FCS and 1% L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were then counted, rinsed in 20 mL RPMI, and resuspended at 5 × 105/mL in culture medium with only 1% FCS. Only samples with a post-rest viability greater than 90% were used. For the C6-NBD-SM assay, aliquots were incubated for 30 minutes at 37°C at 2 × 106/mL with 4 μmol/L C6-NBD-SM in 1% FCS, pelleted, and resuspended in 1% FCS. Preliminary studies of pgp+ve cells had indicated that 30 minutes of preincubation with C6-NBD-SM was sufficient for saturation, and that 30 minute fluorescence at a concentration of 4 μmol/L C6-NBD-SM was approximately 1 log greater than autofluorescence in pgp+ve samples.
For chemosensitivity assays, cells were cultured for 48 hours in medium containing 10% FCS and dilutions of daunorubicin ranging from 0.01-1 μg/mL. The IC30 (ie, the concentration of daunorubicin that caused apoptotic cell death in 30% of cells) was determined for each patient and used for a follow-up assay in which patient cells were cultured with daunorubicin at the IC30 with and without 50 μmol/L FB1. Apoptotic cell death was quantified using 7-AAD as detailed below.
Determination of apoptotic cell death
7-AAD was diluted in PBS as a 50 μg/mL stock solution, which was stored at 4°C. This was mixed with cultured cells to give a final concentration of 12.5 μg/mL. Cultures were incubated for 20 minutes in the dark and analyzed using flow cytometry (Cellquest software, Becton Dickinson). Analysis was performed as illustrated by Philpott27 and Schmid28 to select cells undergoing apoptotic cell death, which was represented by 7-AAD–high cells with low forward scatter. The apoptotic nature of the cell death induced by growth factor withdrawal was examined in additional studies using MGM-stained cytospins for cells displaying morphological characteristics of apoptosis, ie, condensed nuclear chromatin and membrane blebbing, and by quantitative analysis using the annexin V/propidium iodide kit (Sigma Aldrich) according to manufacturer's instructions.
Statistical analysis
Statistical analyses were performed (software; SPSS, Chicago, IL). The differences in response to anti-pgp and control antibodies were analyzed using the Wilcoxon signed rank test. Differences in responses between pgp+ve and pgp−ve groups were analyzed using the Mann-Whitney U test.
The D value used to measure pgp is calculated in the Kolmogorov-Smirnov test by the flow cytometer's Cellquest software (Becton Dickinson) and is a measure, on a scale of 0 to 1, of the difference in distribution between 2 fluorescence curves. The D value is used to calculate the difference between test and control fluorescence when that difference is likely to be small.15 29 Values of less than 0.1 were considered to be negative.
Results
MDR status of patient cells and cell lines
P-glycoprotein protein and function are measured routinely on AML samples in our laboratory. Out of 11 samples, 5 protein positive (pgp D values: 0.15-0.47; mean: 0.30) and 6 protein negative samples were selected for further study (Table 1). PSC-833 increased R123 uptake more than twofold in 4/5 pgp+ve samples, but its effect was less marked in pgp−ve samples. Daunorubicin uptake was also characterized in these patients (Table 1). A previous study has shown that a daunorubicin cell to bead ratio of less than 2.1 is associated with MDR mediated by pgp, lung resistance protein (lrp), or multidrug resistance-associated protein (mrp).15Daunorubicin uptake was found to be low in 4/5 pgp+ve samples and 2/6 pgp−ve samples. TF1 cells are strongly pgp+ve (D = 0.93 ± 0.03), with a high PSC-833 modulation (5.0 ± 0.47), whereas U937 cells are pgp−ve phenotypically and have a low modulation ratio (1.12 ± 0.01).
Apoptosis in patient cells and TF1s augmented by UIC2 anti-pgp
To examine a role for pgp in apoptosis that could be separated from its role in drug uptake, a growth and serum factor withdrawal system was chosen, as this induces considerable apoptosis in most AML patient samples as well as in the pgp+ve growth factor–dependent TF1 cell line within 48 hours. Apoptotic cell death was quantified by flow cytometry using uptake of the dye 7-AAD in addition to low forward scatter. The quantitative use of 7-AAD to measure apoptosis was validated by comparison with the measurement of apoptotic cells identified by exposure of phosphatidyl serine using annexin V (Figure1). A close association was found, as has also been noted by others.30 Cultured leukemic patient cells do not undergo cell death at the same rate, and moreover they undergo considerable secondary necrosis, such that many of the nonviable cells observed after overnight incubation are necrotic rather than apoptotic. Figure 1 illustrates that the propidium iodide–positive (necrotic) cells are also annexin V positive, strongly indicating that necrosis was secondary to apoptosis. The apoptotic nature of cell death induced by growth factor withdrawal was examined in additional studies through MGM-stained cytospins, which showed cells displaying morphological characteristics of apoptosis, ie, condensed nuclear chromatin and membrane blebbing (data not shown).
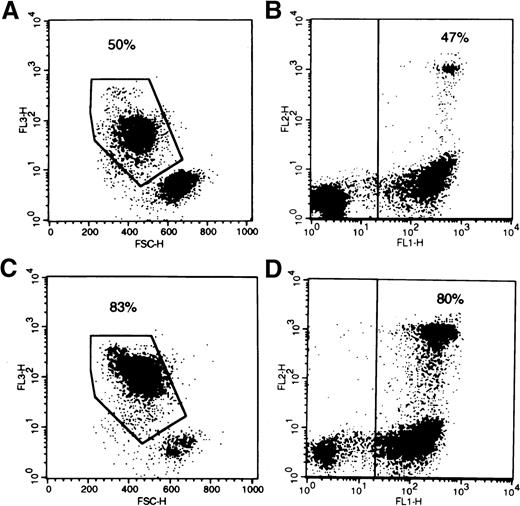
Comparison of 7-AAD and annexin V for the quantitation of apoptotic cell death.
AML patient cells were incubated overnight in 1% serum without growth factors and without (A, B) or with (C, D) 1 μmol/L PSC-833, the pgp reversal agent. We added 12.5 μg/mL 7-AAD to tubes (A) and (C). Tubes (B) and (D) were rinsed and stained with annexinV FITC and propidium iodide. Flow cytometer dot-plots illustrate nonviable cells: high 7-AAD in FL3, with decreased forward scatter in plots (A) and (C), positive annexin V in FL1 on plots (B) and (D). Secondary necrosis is also seen in plots (B) and (D) on the FL2 axis (propidium iodide incorporation). The percentage of cells determined by each method to be undergoing or to have undergone apoptosis is indicated on the plots.
Comparison of 7-AAD and annexin V for the quantitation of apoptotic cell death.
AML patient cells were incubated overnight in 1% serum without growth factors and without (A, B) or with (C, D) 1 μmol/L PSC-833, the pgp reversal agent. We added 12.5 μg/mL 7-AAD to tubes (A) and (C). Tubes (B) and (D) were rinsed and stained with annexinV FITC and propidium iodide. Flow cytometer dot-plots illustrate nonviable cells: high 7-AAD in FL3, with decreased forward scatter in plots (A) and (C), positive annexin V in FL1 on plots (B) and (D). Secondary necrosis is also seen in plots (B) and (D) on the FL2 axis (propidium iodide incorporation). The percentage of cells determined by each method to be undergoing or to have undergone apoptosis is indicated on the plots.
In pgp+ve primary AML samples cultured overnight in 1% FCS without growth factors, there was an increase in apoptotic cell death in all samples assayed in the presence of the pgp-specific antibody UIC2 compared with the isotype- and concentration-matched control antibody, with a mean increase of 58% (range: 11%-95%;P < .05 using the Wilcoxon signed rank test) (Table2 and Figure2). TF1 cells were also cultured in 1% FCS without growth factors in the presence of UIC2 and control IgG2a antibodies. At 30 hours, apoptosis had increased 2.5-fold compared with that of cells cultured with isotype- and concentration-matched control antibodies (Figure 3). In the presence of growth factors, UIC2-induced apoptosis was less than 1% in all experiments, demonstrating that the antibody was not toxic. As an additional negative control, apoptosis was also measured in U937 cells deprived of growth factors overnight in the presence of UIC2 or control IgG2a. We did not observe UIC2-dependent apoptosis.
The effect of UIC2 anti-pgp antibody and IgG2a control antibody on apoptotic cell death in pgp+ve leukemic samples
| Patient . | 7-AAD High (Nonviable) with IgG2a, % . | 7-AAD High (Nonviable) with UIC2, % . | Change, % . |
|---|---|---|---|
| GO | 23.3 | 25.8 | 11 |
| JD | 7.3 | 11.5 | 57 |
| RF | 27.2 | 53 | 95 |
| DS | 27.6 | 48 | 74 |
| BB | 30.8 | 45.2 | 47 |
| Patient . | 7-AAD High (Nonviable) with IgG2a, % . | 7-AAD High (Nonviable) with UIC2, % . | Change, % . |
|---|---|---|---|
| GO | 23.3 | 25.8 | 11 |
| JD | 7.3 | 11.5 | 57 |
| RF | 27.2 | 53 | 95 |
| DS | 27.6 | 48 | 74 |
| BB | 30.8 | 45.2 | 47 |
In samples from patients DS and BB, which had comparatively low D values for pgp expression (see Table 1), viability was decreased after 18 hours with 10 μg/mL UIC2. In the 3 remaining samples, there was little or no effect at this dose and time, and results obtained using 25 μg/mL UIC2 and control IgG2a for 28 hours are given.
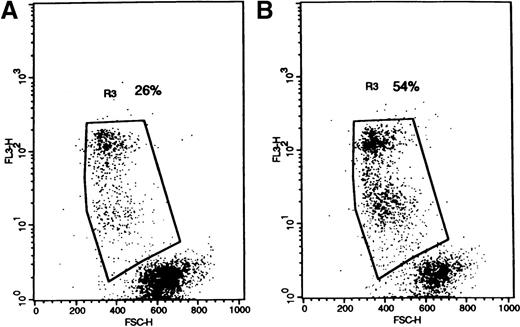
The effect of UIC2 on blast survival in pgp+ve and pgp−ve patient samples.
Primary AML cells were cultured in 1% FCS with UIC2 anti-pgp and isotype- and concentration-matched control antibodies. Flow cytometer dot-plots illustrate nonviable cells (high 7-AAD in FL3, decreased forward scatter) in (A) IgG2a and (B) UIC2-treated cells (patient RF). Numbers on plots are the percentages of nonviable cells.
The effect of UIC2 on blast survival in pgp+ve and pgp−ve patient samples.
Primary AML cells were cultured in 1% FCS with UIC2 anti-pgp and isotype- and concentration-matched control antibodies. Flow cytometer dot-plots illustrate nonviable cells (high 7-AAD in FL3, decreased forward scatter) in (A) IgG2a and (B) UIC2-treated cells (patient RF). Numbers on plots are the percentages of nonviable cells.
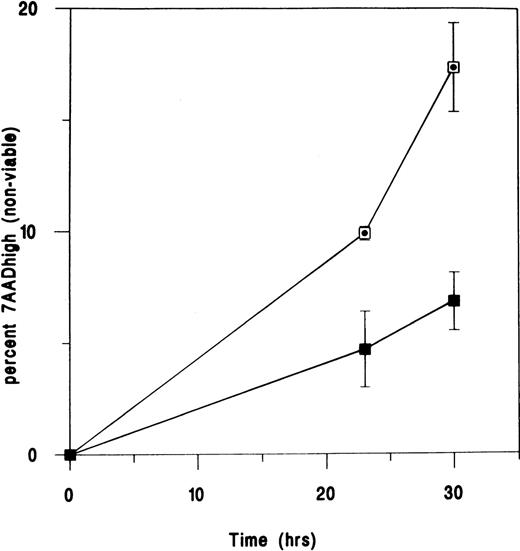
UIC2 augments apoptosis in TF1 cells deprived of growth factors.
Unfilled squares represent cells treated with 25 μg/mL UIC2. Filled squares represent IgG2a control-treated cells. Standard errors for 1 experiment are illustrated. Data points were plotted after the subtraction of control values from cells grown in the presence of GM-CSF with or without the appropriate antibody. Similar results were obtained in 2 further experiments.
UIC2 augments apoptosis in TF1 cells deprived of growth factors.
Unfilled squares represent cells treated with 25 μg/mL UIC2. Filled squares represent IgG2a control-treated cells. Standard errors for 1 experiment are illustrated. Data points were plotted after the subtraction of control values from cells grown in the presence of GM-CSF with or without the appropriate antibody. Similar results were obtained in 2 further experiments.
The pgp reversal agent PSC-833 also augments in vitro apoptosis in TF1s and patient cells cultured without growth factors
The effect of the pgp inhibitor PSC-833 on growth factor withdrawal in TF1 and primary AML blasts was also determined. TF1 cells were cultured with 0.5- and 1-μmol/L concentrations of PSC-833, which were found to enhance the cell death induced by growth factor withdrawal in a dose-dependent manner (Figure 4). In the presence of growth factors, PSC-833–induced apoptosis was less than 2% in all experiments, which demonstrates minimal toxicity for this compound.
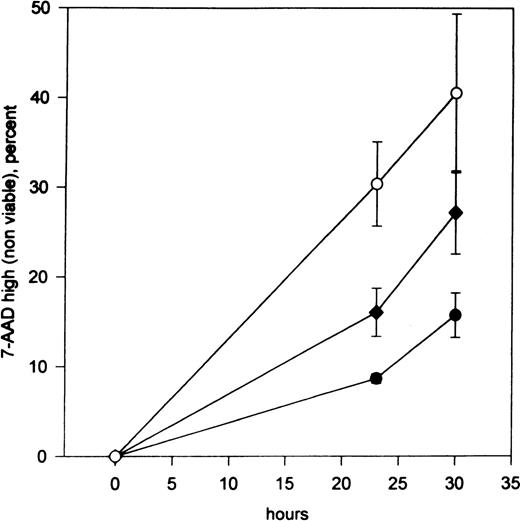
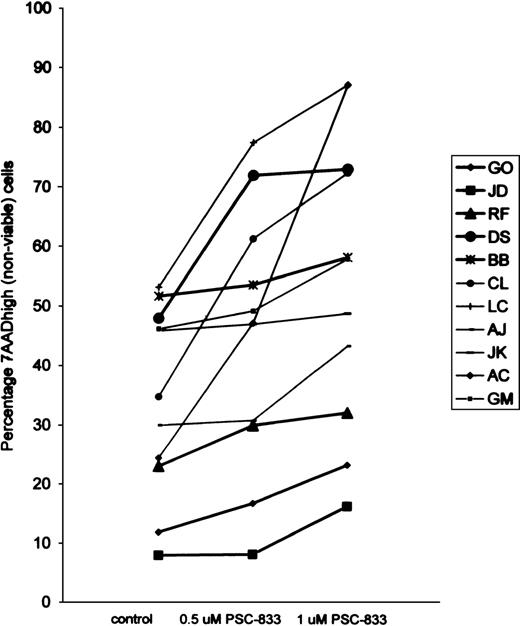
PSC-833 augments apoptosis in TF1 cells deprived of growth factors.
Percentage loss of TF1 viability after culture for 23 and 30 hours in 1% FCS without growth factors in the absence (filled circles) or presence of 0.5 μmol/L (filled diamonds) or 1 μmol/L (unfilled circles) PSC-833 (the mean plus or minus SE of 4 experiments). Data points were plotted after the subtraction of control values from cells grown in the presence of GM-CSF with or without the appropriate dose of PSC-833.
PSC-833 augments apoptosis in TF1 cells deprived of growth factors.
Percentage loss of TF1 viability after culture for 23 and 30 hours in 1% FCS without growth factors in the absence (filled circles) or presence of 0.5 μmol/L (filled diamonds) or 1 μmol/L (unfilled circles) PSC-833 (the mean plus or minus SE of 4 experiments). Data points were plotted after the subtraction of control values from cells grown in the presence of GM-CSF with or without the appropriate dose of PSC-833.
A pgp independent role for PSC-833 in interleukin-1 (IL-1) secretion and leukemic blast survival has recently been postulated in AML.31 To determine whether the apoptotic effect of PSC-833 on growth factor withdrawn cells was pgp specific, pgp+ve and pgp−ve patient cells were cultured in the presence of PSC-833, which was found to enhance cell death in all samples tested including 6 pgp−ve samples (Figures 1 and 5). At 1 μmol PSC-833, in pgp−ve samples, the increase was 6%-350%, and the median was 53%; in pgp+ve samples, the increase was 13%-204%, and the median was 52%. There was no significant difference between groups using the Mann-Whitney U test. These experiments indicated that the enhancement of cell death induced by growth and serum factor withdrawal by PSC-833 is not pgp specific.
The effect of PSC-833 on blast survival in pgp+ve and pgp−ve patient samples.
Leukemic patient sample viability after treatment with PSC-833 in 18-hour culture in 1% FCS without growth factors. Thick lines represent responses of pgp+ve blasts, and thin lines represent pgp−ve blasts.
The effect of PSC-833 on blast survival in pgp+ve and pgp−ve patient samples.
Leukemic patient sample viability after treatment with PSC-833 in 18-hour culture in 1% FCS without growth factors. Thick lines represent responses of pgp+ve blasts, and thin lines represent pgp−ve blasts.
Inhibition of daunorubicin-induced apoptosis by the ceramide synthase inhibitor FB1
The role of the SM-ceramide pathway in apoptosis is controversial. The controversy largely surrounds the lack of specificity of the lipid reagents used and the methodology for measuring ceramide up-regulation.32 Before investigating the possible role of pgp in SM distribution, it was thought advisable to establish a role for the SM-ceramide pathway in the apoptosis of primary AML cells. The natural fungal product FB1 is structurally similar to the ceramide precursor sphinganine and inhibits ceramide synthase activity, thus inhibiting de novo ceramide synthesis.20 To the best of our knowledge, the specific inhibitory role of FB1 in ceramide synthesis has not been challenged, and this compound has previously been used to demonstrate a role for the ceramide pathway in the daunorubicin-induced apoptosis of U937 cells.20 We therefore cultured primary leukemic samples with and without daunorubicin and FB1. The presence of FB1 was found to reduce daunorubicin-specific apoptosis by a median of 40% (range: 18%-81%) in the 7 samples studied (Table 3). This implicates a direct role for ceramide in daunorubicin-induced apoptosis in AML cells.
Effect of FB1 on daunorubicin-induced apoptosis
| . | Previously Determined IC30 Daunorubicin (μg/mL) . | 7-AAD High without FB1, % . | 7-AAD High with FB1, % . | Inhibition, % . |
|---|---|---|---|---|
| GO | 0.03 | 27.4 | 21.7 | 20.8 |
| JD | 0.1 | 39.7 | 24 | 39.5 |
| RF | 0.05 | 38.5 | 31.6 | 17.9 |
| DS | 0.05 | 31.4 | 22 | 29.9 |
| BB | 0.03 | 32 | 6.2 | 80.6 |
| CL | 0.005 | 21.4 | 12.9 | 39.7 |
| AJ | 0.01 | 21.9 | 4.4 | 79.9 |
| . | Previously Determined IC30 Daunorubicin (μg/mL) . | 7-AAD High without FB1, % . | 7-AAD High with FB1, % . | Inhibition, % . |
|---|---|---|---|---|
| GO | 0.03 | 27.4 | 21.7 | 20.8 |
| JD | 0.1 | 39.7 | 24 | 39.5 |
| RF | 0.05 | 38.5 | 31.6 | 17.9 |
| DS | 0.05 | 31.4 | 22 | 29.9 |
| BB | 0.03 | 32 | 6.2 | 80.6 |
| CL | 0.005 | 21.4 | 12.9 | 39.7 |
| AJ | 0.01 | 21.9 | 4.4 | 79.9 |
Patient cells were cultured for 48 hours in suspension culture with 10% FCS to determine the in vitro IC30 for daunorubicin. Assays were then repeated with and without daunorubicin at the previously determined IC30 for each patient, with and without 50 μmol/L FB1. Background values for cells cultured without daunorubicin have been subtracted.
SM in the apoptosis of pgp+ve and pgp−ve leukemic cells
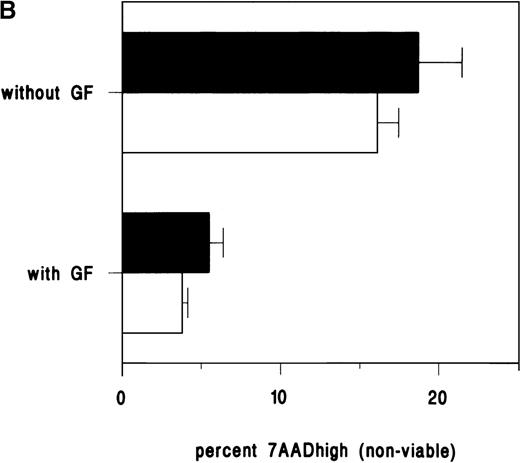
The low bioavailability of plasma membrane inner leaflet SM has been postulated as a limiting factor in TNF-induced apoptosis in a pgp+ve leukemic cell model.22 To expand this hypothesis we constructed models using a fluorescent SM, C6-NBD-SM, to label pgp+ve and pgp−ve leukemic cell membranes. If the hypothesis proposed by Bezombes et al22 were applicable, we would expect to observe that C6-NBD-SM, transported to the inner leaflet of stressed pgp−ve cells, would augment apoptosis; whereas in pgp+ve cells, the exogenous SM would be largely confined to the outer leaflet, and apoptosis would not be enhanced. We measured the effects of exogenous C6-NBD-SM on apoptosis. Preliminary experiments using flow cytometry to measure cell fluorescence enabled us to observe that C6-NBD-SM uptake reached a plateau within 30 minutes at 4 μmol/L in pgp+ve cells (data not shown), and this loading schedule was adopted in subsequent experiments. An experiment was performed on pgp−ve U937 cells to determine whether C6-NBD-SM induces apoptosis or whether it augments a preinduced apoptotic pathway. U937 cells survived for several days in 1% FCS; we therefore used daunorubicin (rather than serum and growth factor withdrawal) to induce apoptosis in these cells. Minimal apoptosis was induced by C6-NBD-SM alone in the absence of daunorubicin (Figure 6A). However, in combination with daunorubicin, C6-NBD-SM enhanced apoptosis. In contrast, C6-NBD-SM was ineffective at augmenting apoptosis induced by growth factor withdrawal in pgp+ve TF1 cells (Figure 6B).
The effect of SM on apoptosis in pgp+ve and pgp−ve cell lines.
(A) U937 cells were cultured in 10% FCS for 3 hours in the presence of increasing doses of daunorubicin. The cells were then rinsed, incubated for 30 minutes with or without C6-NBD-SM in 1% FCS, pelleted, and cultured for a further 18 hours. In the absence of daunorubicin, C6-NBD-SM made no difference to U937 viability, demonstrating that SM was not inherently toxic at this dose. However, daunorubicin-induced apoptosis was augmented in a dose-dependent manner by C6-NBD-SM. Error bars equal the standard error of the mean (SEM) of 3 experiments. (B) The lack of effect of C6-NBD-SM on apoptosis induced by growth factor withdrawal in pgp+ve TF1 cells is demonstrated. TF1 cells were loaded with C6-NBD-SM and cultured with and without GM-CSF. With growth factor withdrawal, 16% of cells were nonviable by 30 hours, but C6-NBD-SM did not enhance apoptosis. Error bars equal SEM of 3 experiments.
The effect of SM on apoptosis in pgp+ve and pgp−ve cell lines.
(A) U937 cells were cultured in 10% FCS for 3 hours in the presence of increasing doses of daunorubicin. The cells were then rinsed, incubated for 30 minutes with or without C6-NBD-SM in 1% FCS, pelleted, and cultured for a further 18 hours. In the absence of daunorubicin, C6-NBD-SM made no difference to U937 viability, demonstrating that SM was not inherently toxic at this dose. However, daunorubicin-induced apoptosis was augmented in a dose-dependent manner by C6-NBD-SM. Error bars equal the standard error of the mean (SEM) of 3 experiments. (B) The lack of effect of C6-NBD-SM on apoptosis induced by growth factor withdrawal in pgp+ve TF1 cells is demonstrated. TF1 cells were loaded with C6-NBD-SM and cultured with and without GM-CSF. With growth factor withdrawal, 16% of cells were nonviable by 30 hours, but C6-NBD-SM did not enhance apoptosis. Error bars equal SEM of 3 experiments.
The effect of C6-NBD-SM on the in vitro apoptosis of primary leukemic cells was then studied. Following a 30-minute incubation with C6-NBD-SM, blast samples were pelleted and incubated for a further 18 hours at 37°C in 1% FCS. In 6 pgp−ve cases, increases of 19%-88% in cell death, median 33%, were observed, whereas in the 5 pgp+ve cases, increases of 0%-27%, median 7%, were noted (Table 4 and Figure7). The Mann-Whitney U test demonstrated the difference between groups to be significant atP = .028.
The effect of C6-NBD-SM on augmenting apoptosis induced by growth factor and serum withdrawal in primary leukemic blasts
| . | pgp Status . | 7-AAD (Nonviable) without SM, % . | 7-AAD High (Nonviable) with SM, % . | Change, % . |
|---|---|---|---|---|
| GO | + | 11.9 | 14.4 | +21 |
| JD | + | 8 | 8.6 | +7 |
| RF | + | 23.1 | 22.9 | −0.9 |
| DS | + | 48 | 61 | +27 |
| BB | + | 51.7 | 54.6 | +5.6 |
| CL | − | 34.8 | 49.8 | +43.1 |
| LC | − | 53.2 | 63.4 | +19.2 |
| AJ | − | 30 | 41.6 | +38.7 |
| JK | − | 45.9 | 58.7 | +27.9 |
| AC | − | 24.5 | 46.1 | +88.2 |
| GM | − | 46.2 | 57 | +23.4 |
| . | pgp Status . | 7-AAD (Nonviable) without SM, % . | 7-AAD High (Nonviable) with SM, % . | Change, % . |
|---|---|---|---|---|
| GO | + | 11.9 | 14.4 | +21 |
| JD | + | 8 | 8.6 | +7 |
| RF | + | 23.1 | 22.9 | −0.9 |
| DS | + | 48 | 61 | +27 |
| BB | + | 51.7 | 54.6 | +5.6 |
| CL | − | 34.8 | 49.8 | +43.1 |
| LC | − | 53.2 | 63.4 | +19.2 |
| AJ | − | 30 | 41.6 | +38.7 |
| JK | − | 45.9 | 58.7 | +27.9 |
| AC | − | 24.5 | 46.1 | +88.2 |
| GM | − | 46.2 | 57 | +23.4 |
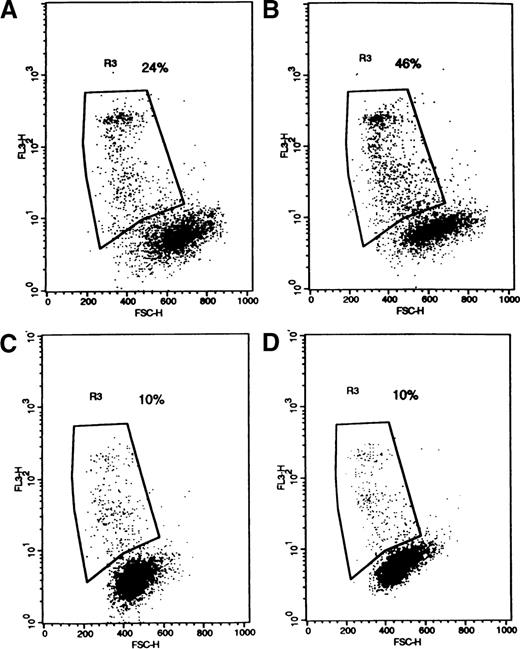
The effect of SM on apoptosis in pgp+ve and pgp−ve patient samples.
Flow cytometer dot-plots illustrating nonviable cells (high 7-AAD in FL3, decreased forward scatter) in a pgp−ve sample (AC) without (A) and with (B) C6-NBD-SM and in a pgp+ve sample (GO) without (C) and with (D) C6-NBD-SM.
The effect of SM on apoptosis in pgp+ve and pgp−ve patient samples.
Flow cytometer dot-plots illustrating nonviable cells (high 7-AAD in FL3, decreased forward scatter) in a pgp−ve sample (AC) without (A) and with (B) C6-NBD-SM and in a pgp+ve sample (GO) without (C) and with (D) C6-NBD-SM.
Accumulation of exogenous SM by AML blasts is enhanced by PSC-833
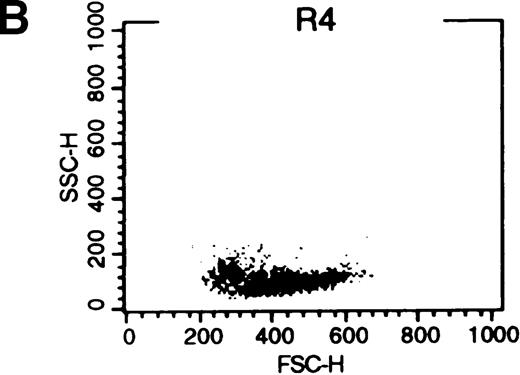
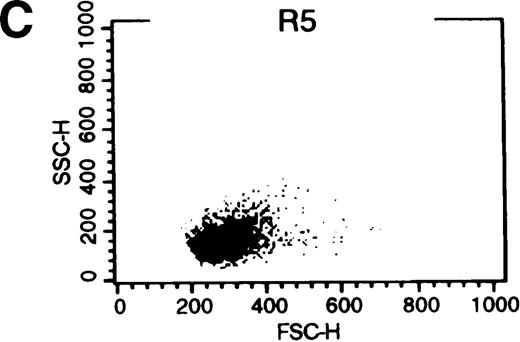
When exogenous phospholipids are incubated with cells in the presence of protein, the lipid retained in the membrane is affected by both uptake into the cell and back-exchange from the outer leaflet into the protein-containing medium.33 We therefore postulated that 18 hours after C6-NBD-SM loading, fluorescence in leukemic cells would be related to the amount of SM internalized. The studies by Bettaib et al34 documented low inner leaflet SM content in pgp+ve KG1a cells compared with pgp−ve U937 cells and subsequently determined that PSC-833 can enhance inner leaflet SM content in the presence of an apoptotic stimulus.22Therefore, we were interested in C6-NBD-SM fluorescence distribution in apoptotic and nonapoptotic cells. We examined cytofluorographs from samples that had been incubated overnight with C6-NBD-SM in 1% FCS without growth factors (Figure8). From these, we determined that high C6-NBD-SM fluorescence is largely associated with apoptotic cells. (Cells with reduced forward scatter and increased side scatter have been shown to be apoptotic in several models.35 36)
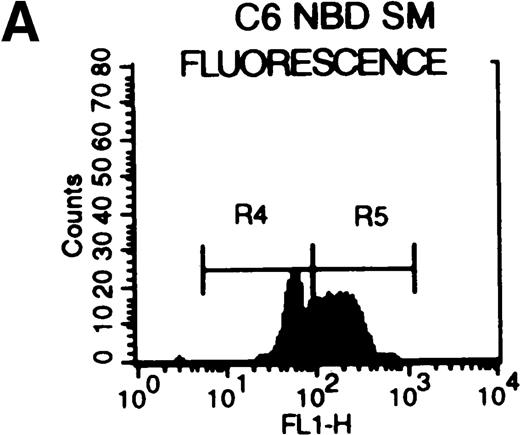
C6-NBD-SM fluorescence of leukemic blasts following 18-hour culture.
(A) Blasts from pgp−ve patient LC incubated overnight with C6-NBD-SM demonstrate intense fluorescence on the subset of cells defined by region 5 (R5). Backgating of the (B) R4 and (C) R5 subsets demonstrated that R5 C6-NBD-SM high cells were the smaller cells with higher side scatter, typical flow cytometric features of apoptosis.35 The figure is a representative plot of a consistent finding. (D) Histogram of the same sample incubated without (filled plot) and with (unfilled plot) 1 μmol/L PSC-833, which demonstrates an increase in C6-NBD-SM high cells. (E, F) C6-NBD-SM–loaded blasts from pgp+ve patients (E) GO and (F) RF demonstrate a fluorescence shift in the entire population as well as in the fluorescence-high subset when untreated samples (filled plots) are compared to samples treated with PSC-833 (unfilled plot, thick line). (F) The lowering effect of UIC2 (unfilled plot, dotted line) on C6-NBD-SM fluorescence is also shown.
C6-NBD-SM fluorescence of leukemic blasts following 18-hour culture.
(A) Blasts from pgp−ve patient LC incubated overnight with C6-NBD-SM demonstrate intense fluorescence on the subset of cells defined by region 5 (R5). Backgating of the (B) R4 and (C) R5 subsets demonstrated that R5 C6-NBD-SM high cells were the smaller cells with higher side scatter, typical flow cytometric features of apoptosis.35 The figure is a representative plot of a consistent finding. (D) Histogram of the same sample incubated without (filled plot) and with (unfilled plot) 1 μmol/L PSC-833, which demonstrates an increase in C6-NBD-SM high cells. (E, F) C6-NBD-SM–loaded blasts from pgp+ve patients (E) GO and (F) RF demonstrate a fluorescence shift in the entire population as well as in the fluorescence-high subset when untreated samples (filled plots) are compared to samples treated with PSC-833 (unfilled plot, thick line). (F) The lowering effect of UIC2 (unfilled plot, dotted line) on C6-NBD-SM fluorescence is also shown.
Patient cells were also treated with both C6-NBD-SM and 1 μmol/L PSC-833. C6-NBD-SM fluorescence was increased by PSC-833 in 10/11 patient samples (all except JD). Increased C6-NBD-SM may reflect an alteration in SM distribution preceding apoptosis or alternatively may be nonspecifically associated with a loss of cellular integrity in these samples. To distinguish between these possibilities, we examined cultures from pgp+ve samples that underwent only minimal apoptosis at 18 hours. An increase in C6-NBD-SM fluorescence was clearly distinguishable in the viable cells of samples treated with PSC-833 (Figure 8), supporting the finding that PSC-833 alters SM distribution.22 However, the pgp blocking antibody UIC2 was also used in conjunction with C6-NBD-SM in these assays and was found to cause a slight fall in the C6-NBD-SM fluorescence of viable pgp+ve cells (Figure 8). This suggests that PSC-833 is altering SM distribution, at least in part, by a pgp independent mechanism.
Discussion
Using the pgp-specific blocking antibody UIC2, we demonstrated a drug efflux independent role for pgp in augmenting the survival of primary leukemic blasts and TF1 cells in vitro. A similar role in apoptosis augmented by growth factor withdrawal was previously demonstrated in Chinese hamster ovary fibroblasts.18 When added to the findings of Smyth16 and Johnstone,17 who have demonstrated a role for pgp in the fas- and UV-induced caspase-mediated apoptosis of T cell lines, it is clear that a fundamental role for pgp in leukemic cell survival, which is much broader than its drug efflux role, has now been established. It is important to note in this context that only blocking antibodies can be considered as totally pgp specific. UIC2 is a well-documented antibody that recognizes an extracellular portion of pgp but not of the MDR 2/3 gene product.26,37,38 UIC2 reactivity is increased in the presence of pgp substrate drugs or in experiments in which adenosine 5′-triphosphate (ATP) is directly depleted, and it has therefore been suggested that the inhibitory function of the antibody may involve the trapping of a transient conformation of pgp associated with ATP hydrolysis.39 Many pharmaceutical agents that inhibit pgp function, including PSC-833, have broader roles,31 and in this study we have shown that PSC-833 enhances cell death when growth and survival factors are withdrawn from pgp−ve as well as pgp+ve patient samples. A direct cytotoxic effect of PSC-833, independent of initial blast pgp status, has also been noted in an in vitro AML blast chemosensitivity assay.40
Apart from pgp's function as a drug efflux pump, it has been suggested that pgp can transport phospholipids across cell membranes.21 In view of this, a potential role for pgp in reducing the bioavailability of SM has been postulated.22To determine whether reduced SM is relevant to the induction of apoptosis in AML blasts exposed to cytotoxic drugs, we initially used the ceramide synthase inhibitor FB1, which blocks the de novosynthesis of ceramide. In this system FB1 was found to reduce daunorubicin-specific apoptosis. In this respect our findings agree with those of Bose et al,20 who, like ourselves, cultured cells continuously with daunorubicin. In contrast, Jaffrezou et al19 found evidence for SM hydrolysis but not for de novo ceramide synthesis in the daunorubicin-induced apoptosis of U937 cells after a 1-hour pulse of daunorubicin. As ceramide is both a precursor and a breakdown product of SM,41,42the precise role of de novo ceramide synthesis vis-à-vis SM hydrolysis as contributors to apoptosis will be hard to establish and may partly depend on an experimental schedule. Furthermore, a direct drug-independent role for PSC-833 in enhancing ceramide synthesis has been shown in pgp+ve and pgp−ve epidermoid carcinoma cells.43 It also has been recently demonstrated that PSC-833 interacts with daunorubicin to induce SM hydrolysis and ceramide synthesis in drug-resistant HL60 leukemic cells.44
As further evidence of a role for the SM-ceramide pathway in the apoptosis of AML cells, we have shown that exogenous fluorescence-labeled SM enhances loss of viability induced by daunorubicin in the pgp−ve U937 cell line. Although SM was not toxic when used without drugs, the availability of exogenous SM enhanced daunorubicin-induced apoptosis. The availability of SM is therefore shown to be a factor limiting the degree of apoptosis in AML cells in response to cytotoxic drug exposure. The evidence for a proapoptotic role for SM is further supported by the demonstration that SM augments apoptosis in pgp−ve patient blasts. In contrast, SM failed to enhance apoptosis in the pgp+ve TF1 cell line and had a significantly decreased effect on pgp+ve patient blasts compared with that seen in the pgp−ve cases.
Many of the models used to study pgp have involved the selection of drug-resistant sublines in vitro, which tend to express pgp at much higher levels than those found in unselected leukemic cell lines.45 The unselected TF1 cell line has low pgp expression compared to other pgp+ve AML cell lines.45However, even this cell line expresses more pgp than any of the 100-plus clinical AML samples studied in our laboratory (data not shown). For this reason we consider that it is important to use patient data to help understand whether an in vitro pgp model is likely to be pathophysiologically relevant in AML. However, it is also important, when evaluating the data which we have presented, to realize that a pgp+ve patient sample may contain a mixture of pgp+ve and pgp−ve blasts. Hence the differences in SM metabolism between pgp+ve and pgp−ve cells may be even more clear-cut than our patient data reveals.
The choice of a fluorescence-labeled SM for this study enabled us to observe the uptake and retention of this compound on a single-cell basis. We found that the addition of PSC-833 enhanced the SM fluorescence in viable cells, compatible with the finding of Bezombes et al22 that PSC-833 enhanced the internalization of SM, potentially providing an internal pool of hydrolyzable SM to up-regulate ceramide-mediated apoptosis. However, we were unable to establish that PSC-833 was acting specifically on pgp in this context because the pgp-specific UIC2 antibody, while inducing a similar degree of apoptosis at 18 hours, did not increase C6-NBD-SM fluorescence. Cyclosporin A, which is closely related to PSC-833, is known to have a membrane fluidizing effect,46 which may account for an altered, pgp-independent distribution of phospholipids in the plasma membrane. In this context, cyclosporin A was also found to enhance C6-NBD-SM fluorescence in our system (data not shown).
The central finding of this study has been the demonstration that when pgp is blocked, apoptosis induced by growth factor withdrawal in acute myeloid leukemic cells is enhanced. We have also demonstrated for the first time that the SM-ceramide pathway is involved in cytotoxic drug-induced apoptosis in primary AML blasts. Furthermore we have shown that exogenous SM enhances apoptosis induced by growth factor withdrawal in pgp−ve AML blasts, but that pgp+ve cells are protected from SM enhancement of apoptosis. PSC-833 was found not only to enhance apoptosis but also to increase cellular SM accumulation. The fact that PSC-833 enhances cell death and SM internalization in both pgp+ve and pgp−ve cases is of particular clinical relevance, as this agent is currently undergoing clinical evaluation, and these findings suggest that it may be of benefit to a broader range of patients than just those with pgp+ve blasts.
The question of how pgp exerts its antiapoptotic effects remains open. Pgp-mediated alterations of the electrochemical gradient across the plasma membrane are well documented and may be relevant in this context.47,48 Additionally, a role for pgp has been documented in the transport of several membrane lipids.21,49,50 There is also a reported role for pgp in cytokine export.51 Which, if any, of these mechanisms is relevant to the survival of leukemic clones is currently a major focus of research in our laboratory.
Reprints:Monica Pallis, Academic Haematology, Clinical Sciences Building, Nottingham City Hospital, Nottingham NG5 1PB, UK; e-mail: m.pallis@nottingham.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal