Abstract
Reperfusion of tissues after interruption of their vascular supply causes free-radical generation that leads to tissue damage, a scenario referred to as “reperfusion injury.” Because sickle disease involves repeated transient ischemic episodes, we sought evidence for excessive free-radical generation in sickle transgenic mice. Compared with normal mice, sickle mice at ambient air had a higher ethane excretion (marker of lipid peroxidation) and greater conversion of salicylic acid to 2,3-dihydroxybenzoic acid (marker of hydroxyl radical generation). During hypoxia (11% O2), only sickle mice converted tissue xanthine dehydrogenase to oxidase. Only the sickle mice exhibited a further increase in ethane excretion during restitution of normal oxygen tension after 2 hours of hypoxia. Only the sickle mice showed abnormal activation of nuclear factor–κB after exposure to hypoxia-reoxygenation. Allopurinol, a potential therapeutic agent, decreased ethane excretion in the sickle mice. Thus, sickle transgenic mice exhibit biochemical footprints consistent with excessive free-radical generation even at ambient air and following a transient induction of enhanced sickling. We suggest that reperfusion injury physiology may contribute to the evolution of the chronic organ damage characteristic of sickle cell disease. If so, novel therapeutic approaches might be of value.
A significant proportion of the tissue damage resulting from interruption of vascular flow can be attributed to oxygen free radicals that are generated after restitution of blood flow and, with it, oxygen supply. This concept is referred to as “reperfusion injury.” The occurrence of oxidant-mediated damage resulting from such ischemia-reperfusion cycles has been demonstrated in several different animal organ and tissue models.1-3
Among the biochemical changes that occur under low oxygen tension after tissues lose their blood supply are catabolism of adenosine triphosphage to hypoxanthine and conversion of xanthine dehydrogenase (XD) to xanthine oxidase (XO).2-4 Unlike XD, in the presence of oxygen XO generates superoxide when it catalyzes the conversion of xanthine or hypoxanthine to uric acid. Therefore, restitution of blood flow and oxygen supply allows XO to rapidly generate superoxide and ultimately, through iron-dependent chemistry, generate the highly reactive and damaging hydroxyl radical.5,6 In some reperfusion injury models, other oxidant-generating mechanisms seem to predominate in the free-radical generation that is common to all reperfusion injury situations. Examples include oxidants derived from activated inflammatory cells,7 the mitochondrial electron transport chain,8 and peroxynitrite metabolism.9-11 The many consequences of this initial free-radical generation include peroxidation of polyunsaturated membrane fatty acids1-11and activation of nuclear factor–κB (NF-κB).12,13Induction of cytokines during this process is an additional mechanism that activates NF-κB.8,9,14,15 This transcription factor up-regulates expression of a number of proinflammatory molecules such as endothelial cell adhesion molecules. Its activation thereby promotes neutrophil recruitment and a generalized endothelial prothrombotic state.14-16 Thus, reperfusion injury states are characterized by vigorous inflammatory responses.8 9
Considering these characteristics of reperfusion injury physiology, we hypothesized that sickle cell anemia represents an archetypal reperfusion injury scenario. Insofar as the vasoocclusive character of sickle cell disease is caused by reversible red blood cell (RBC) sickling, perfect opportunity should be provided for transient ischemia or hypoxia, followed by reperfusion. In fact, no other human disease is characterized by lifelong, and perhaps near-constant,17 18development of transient vasoocclusion. While no direct evidence for reperfusion injury in sickle cell disease has been reported yet, several aspects of the disease are consistent with it (see “Discussion”). Therefore, as a partial test of our hypothesis that reperfusion injury physiology contributes to the vascular pathobiology of sickle disease, in particular to chronic organ deterioration, we investigated several markers of reperfusion injury in sickle transgenic mice.
Materials and methods
Mice
We established a colony of sickle transgenic mice using founders kindly provided by Dr Mary Fabry. These mice have linked human α and βS globin transgenes on the C57Bl/6J background homozygous for absence of murine βmajorglobin.19 Hematologic characteristics, the organ damage that these mice undergo at ambient air, their response to hypoxia, and other features of this mouse model have been reported elsewhere.19-21 Normal control mice were C57Bl/6J, the same genetic background as the sickle mice.
Study protocol
We studied mice subjected to a sequential hypoxia-reoxygenation (H/R) protocol and mice at ambient air. The H/R protocol involved a 30-minute prehypoxia period for baseline measurements at ambient air, followed by 120 minutes of hypoxia, and then a 50-minute reoxygenation period during which ambient air conditions were restored. We used 2 different methods to induce the hypoxia.
For studies that required collection of expired gas, the mice were anesthetized with intraperitoneal pentobarbital (10 mL/kg of a 0.5% solution) and intubated with a Silastic tracheostomy tube that was connected to a low-resistance unidirectional flow valve (provided by Hans Rudolph, Kansas City, MO). Inflow gases (ultrapure oxygen and nitrogen) passed through a flow meter, mixing chamber, and humidifier before passing into the mouse, which breathed the regulated, nonpressurized mixture spontaneously on demand. Outflow gases passed into a collection tube (see “Measurement of expired ethane”). To induce hypoxia, the flow of gases was adjusted to supply 11% ± 1% oxygen, which we found to correspond to a PaO2 of 70 ± 2 mmHg, a level at which the sickle mice developed a significant increase in proportion of sickled cells. During the reoxygenation period, the flow of gases was adjusted to restore 21% oxygen.
For studies not requiring collection of expired gas, unanesthetized mice were placed in a closed chamber, and the mixture of gas flow was adjusted to supply 11% ± 1% oxygen for the duration of the hypoxia period. For reoxygenation and for the ambient air controls, mice were left to breathe room air. Thus, this protocol entailed neither surgical manipulation nor anesthesia.
Measurement of induced sickling
To measure red cell sickling, we drew blood via a carotid artery catheter directly into a syringe containing 10% glutaraldehyde. A drop of blood was viewed by light microscopy to assess the proportion of sickled cells out of 500 randomly counted cells. Sickled cells were defined as having a length-to-width ratio of at least 3:1.
Measurement of expired ethane
As an index of whole-body lipid peroxidation, we measured ethane in expired gas,22 a method that has previously been applied successfully to study of oxidant stress in the mouse.23-28Expired gas was passed through a Carbotrap 200 desorption tube (Supelco Inc, Bellefonte, PA), which adsorbs short-chain hydrocarbons at room temperature.29 Expired gas was thus collected during the 30-minute prehypoxia baseline period and the 50-minute reoxygenation period. After collection, the Carbotrap tube was immediately transferred to a ballistic thermal desorber (Thermal Tube Desorber Model 890; Dynatherm Analytical Instruments, Kelton, PA), which transferred the trapped hydrocarbons to a gas chromatograph (Hewlett-Packard 5890A; Hewlett Packard, Palo Alto, CA) with a flame ionization detector. The amount of expired ethane was calculated by comparison of a sample signal with that from known standard gases.
To evaluate the effect of allopurinol on ethane excretion, we studied animals at ambient air. A 30-minute gas collection at baseline was followed by intraperitoneal injection of 40 mg/kg of a 0.4% (wt/vol) solution of allopurinol (Sigma, St Louis, MO) or vehicle control. Ambient air conditions were continued for 4 hours, during the last 30 minutes of which another postallopurinol gas collection was made.
Measurement of 2,3-dihydroxybenzoic acid
To indirectly detect hydroxyl radical generation, we measured hydroxylation of salicylic acid (SA) (Sigma) to form 2,3-dihydroxybenzoic acid (2,3-DHBA)30,31 using a modification of the method of Kim and Wells.32 This method has been previously applied successfully to the study of oxidant stress in the mouse.33,34 Thirty minutes after intraperitoneal injection of 0.1 g/kg of a 10-mg/mL solution of SA, 100 to 200 μL of blood was drawn and centrifuged for 10 minutes. Then, 50 μL of plasma was extracted and vortexed with 50 μL of 8-μM 3,4-DHBA (added as an internal standard), 50 μL of 12-N HCl, and 500 μL of ethyl acetate. This was centrifuged for 2 minutes at 5000 rpm, and 200 to 400 μL of supernatant was extracted. The ethyl acetate was evaporated under nitrogen gas, and the residue was dissolved in 50μL of the mobile phase solution, centrifuged for 10 minutes, and injected in aliquots of 10 μL into a high-performance liquid chromatographer (Hewlett-Packard Series II 1080; Vydac 201HS RP-18C analytical column; flow rate 0.8 mL/minute, running time 60 minutes; mobile phase solution: 0.03-M sodium citrate and 0.03-M sodium acetate, pH 4.0). Peaks for 2,3-DHBA, 3,4-DHBA, and SA were recorded on Chemstation software (Hewlett-Packard) and the molar amounts calculated using standard curves recorded daily. Levels of 2,3-DHBA (μM) and SA (mM) were corrected using the 3,4-DHBA internal standard and the final level of 2,3-DHBA expressed as a ratio to level of SA.30
The differing half-lives of SA and 2,3-DHBA, coupled with the wide variation in rate of clearance of SA from animal to animal, made it impossible to apply this assay to animals run through our H/R protocol, which would have required more than 1 injection of SA. Therefore, this assay was applied only to animals breathing ambient air.
Measurement of NF-κB activation
Nuclear protein extraction.35
From mice rendered unconscious by a 20-second exposure to CO2, liver and kidney were rapidly harvested and immediately frozen in liquid nitrogen.
A total of 500 mg of tissue was ground in a mortar while still immersed in liquid nitrogen, crushed with the tight pestle of a Dounce homogenizer in 5 mL of a cell lysis buffer (0.6% Nonidet P-40 [NP-40]; 150-mmol/L NaCl; 10-mmol/L HEPES, pH 7.9; 0.5-mmol/L phenylmethylsulfonyl fluoride [PMSF]) and centrifuged for 30 seconds at 2000 rpm. The supernatant was incubated on ice for 5 minutes and recentrifuged at 5000 rpm for 5 minutes. The nuclear pellet was resuspended in 500 μL of a nuclear extraction buffer (25% glycerol; 20-mmol/L HEPES, pH 7.9; 420-mmol/L NaCl; 1.2-mmol/L MgCl2; 0.2-mmol/L ethylenediaminetetraacetic acid [EDTA]; 0.5-mmol/L dithiothreitol (DTT); 0.5-mmol/L PMSF; 2-mmol/L benzamidine, and 0.5 μg/mL each of pepstatin, leupeptin and aprotinin), incubated on ice for 20 minutes, and centrifuged for 20 seconds at 14 000 rpm. Aliquots of the supernatant were stored at −70°C until use. Sample protein content was assayed using a BCA Protein Assay kit (Pierce, Rockford, IL); after incubation (37°C for 30 minutes), sample absorbance at 600 nm was compared with an albumin standard.
NF-κB probes.36
A 35–base-long oligonucleotide probe, designed from the NF-κB binding site (5′-TCTCAACAGAGGGGACTTTCCGAGAGGCCATCTGG-3′), was radioactively labeled randomly using 32P-labeled deoxycytidine triphosphate and deoxyguanosine triphosphate (Amersham Corp, Buckinghamshire, England) and Klenow fragment.
The probe was centrifuged for 5 minutes at 2300 rpm in a Sephadex G-50 Quick Spin Column (Boehringer Mannheim, Indianapolis, IN).
Manipulations
To verify specificity of bands for NF-κB, we used an excess of unlabeled probe to compete for binding (“cold competition” assay). To obtain positive control cells for NF-κB assays, we used microvascular endothelial cells treated with tissue necrosis factor in vitro, and we used mice given Escherichia colilipopolysaccharide and D-galactosamine.37To identify which NF-κB subunits were being visualized, we conducted supershift assays in which antibodies to p50, p52, p65, or cRel were included. Activity of each of these antibodies had previously been verified in other systems.
Polyacrylamide gel
A 4% polyacrylamide gel was pre-run for 1 hour in at 4°C prior to loading samples. Each gel lane was loaded with a mixture of 10 μg of nuclear protein extract, 5 μL of Markman's buffer (50-mM KCl; 20-mM HEPES, pH 7.9; 0.5-mmol/L EDTA; 5% glycerol, 1-mmol/L DTT; 0.5-mmol/L PMSF, 1-mg/mL bovine serum albumin, 0.1% NP-40), 1.5 μL of labeled probe, 1 μL of poly(dIdC), 1 μL of bromphenol blue (if needed, 1 μL of unlabeled probe or antibody), and distilled water to a total volume of 26 μL. Electrophoresis was done for 1.25 hours in a cold room.
Tissue XD-XO conversion
Hypoxia-induced conversion of tissue XD to XO was measured by comparing XO activity with total XD-plus-XO activity38 in hypoxic and nonhypoxic tissue. This method has previously been used successfully in mice.39 40 Hypoxic tissue was harvested after 2 hours of hypoxia with no reoxygenation period. Nonhypoxic tissue was harvested at ambient air.
From mice anesthetized with diethyl ether, liver and kidney were harvested and immediately frozen in liquid nitrogen; 0.3 to 1 g of tissue was crushed to a powder with a mortar and pestle while continuously immersed in liquid nitrogen. The crushed tissue was then transferred to a Dounce homogenizer containing 10 mL of “XD-XO buffer” (0.25-mol/L sucrose, 100-mmol/L DTT, 100-mmol/L PMSF, 50-mmol/L EDTA, 50-mmol/L KH2PO4, pH 7.4) per gram of tissue, homogenized with 10 strokes of the tight pestle, and centrifuged for 30 minutes at 40 000g. The supernatant was frozen in liquid nitrogen and stored at −70°C until use. A total of 5 μL of the supernatant was diluted in 2 mL of a “standard buffer” (50-mmol/L KH2PO4; 0.1-mmol/L EDTA, pH 7.4) in a glass cuvette at 37°C and read in an LS-5B Luminescence Spectrophotometer (Perkin-Elmer Corp, Norwalk, CT) set to 345-nm excitation, 390-nm emission, and 5-nm bandwidth slit. The rate of pterin oxidation to isoxanthopterin, indicating XO activity, was determined by adding 20 μL of 1-mM pterin and measuring the fluorescence change over 5 minutes. Then, 20 μL of 1-mM methylene blue was added as an electron acceptor to measure combined XD and XO activity and the fluorescence change measured over 5 minutes. After inhibiting the reaction with 20 μL of 10-mmol/L allopurinol, 5 μL of 1-mM isoxanthopterine was added and the immediate increase in fluorescence measured as an internal standard for calculating enzyme activity using the formula: U = {ΔF × [IXPT]/FIXPT} × 0.001 × Vc/(Vs × T), where U is the enzyme activity in micromolars per minute per grams of tissue; ΔF is the change in fluorescence intensity observed per minute; [IXPT] is the concentration of isoxanthopterin added at the end of assay; FIXPT is the immediate increase in fluorescence produced by addition of 5 μL of 1-mM isoxanthopterin; Vcis the cuvette volume in milliliters; Vs is the volume of sample added to the cuvette in milliliters; and T is the amount of tissue, in grams per milliliter of homogenate.
Statistical analysis
Results are expressed as mean ± SD. ANOVA was done, and study groups were compared using the Student t test. Instat software (GraphPad Software, San Diego, CA) was used for all statistical analyses.
Results
Reversible sickling
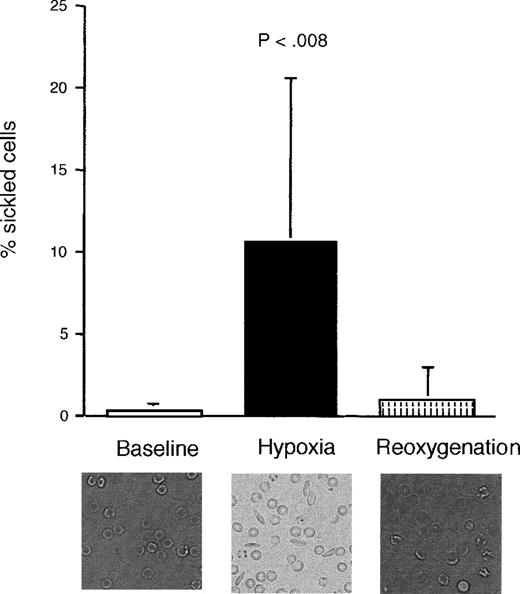
Blood obtained from sickle mice that had been exposed to 2 hours of hypoxia (11% ± 1% oxygen, reducing PaO2 from 98 ± 2 to 70 ± 2 mmHg) developed an increased proportion of sickled RBCs (Figure 1). This was reversed during the reoxygenation period, although there was some persistence of misshapen, but not sickled, forms. There was no significant difference in proportion of sickled cells between the prehypoxia and the reoxygenation periods. Normal mice, as expected, did not show any evidence of sickling with hypoxia (data not shown).
Proportion of sickled cells in sickle transgenic mice before, during, and after induction of hypoxia.
The photomicrographs show representative sections of blood smears taken from a single mouse during each period. There was no significant difference between the prehypoxia and reoxygenation periods. Data shown as mean ± SD (n = 9).
Proportion of sickled cells in sickle transgenic mice before, during, and after induction of hypoxia.
The photomicrographs show representative sections of blood smears taken from a single mouse during each period. There was no significant difference between the prehypoxia and reoxygenation periods. Data shown as mean ± SD (n = 9).
Unlike previously published models of reperfusion injury that entailed induction of complete ischemia by completely occluding the blood supply to a specific organ, our experimental design used exposure to moderate systemic hypoxia to increase the likelihood that sickling-induced occlusion would occur. Thus, we used the reversible RBC sickling as our method of temporarily obstructing blood flow in the whole animal. The disadvantage of this approach is that the degree to which hypoxia occurs undoubtedly varies from organ to organ and from animal to animal, thus increasing variability in the data. The advantage, however, is that this manipulation simulates what happens pathophysiologically in sickle disease. Moreover, this allows normal mice subjected to the same conditions to be used as hypoxia-only controls. Normal mice, which cannot show RBC sickling, would be exposed only to transient hypoxia, while the sickle mice would possibly develop reversible vasoocclusion and thereby develop actual ischemia and reperfusion injury physiology.
Ethane excretion
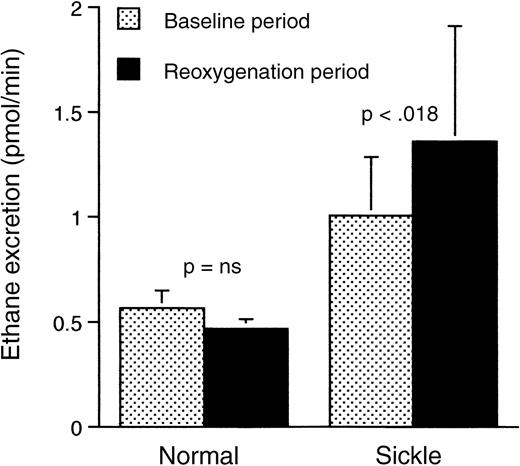
Peroxidation of polyunsaturated membrane fatty acids during periods of oxidant stress leads to formation of the volatile alkanes, which are then excreted through the lungs. Thus, measurement of the levels of ethane in the expired gases can identify a state of increased oxidant stress.22 As shown in Figure2, the sickle mice subjected to hypoxia-reoxygenation had a significantly higher rate of ethane excretion during the reoxygenation period than during the baseline, prehypoxia period (P < .018). Normal mice, on the other hand, exhibited no significant difference between the 2 periods. Of additional interest was the fact that, compared with normal mice, the sickle mice had a significantly (P < .03) higher rate of ethane excretion, even under ambient air, prior to induction of hypoxia. This was shown in separate experiments (not shown) but is also evident in Figure 2.
Rate of ethane excretion in respiratory gases from normal and transgenic mice studied prehypoxia and posthypoxia.
There was a significant difference between the baseline and reoxygenation periods for the sickle mice but not for the normal mice. Data shown as mean ± SD (n = 5 for the normal mice, n = 13 for the sickle mice).
Rate of ethane excretion in respiratory gases from normal and transgenic mice studied prehypoxia and posthypoxia.
There was a significant difference between the baseline and reoxygenation periods for the sickle mice but not for the normal mice. Data shown as mean ± SD (n = 5 for the normal mice, n = 13 for the sickle mice).
Activation of NF-κB
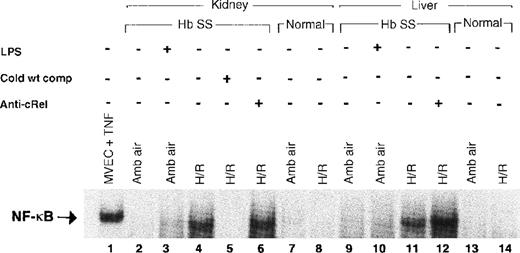
When activated, NF-κB moves from cytoplasm to the nucleus in response to multiple potentially injurious environmental stimuli, including various cytokines and oxygen-free radicals.12,13 15 Examining nuclear proteins extracted from liver and kidney, we compared levels of nuclear translocation of NF-κB in sickle and normal mice, with and without exposure to H/R. Figure 3 shows a gel representative of our findings. There was no consistent evidence of NF-κB activation at ambient air in kidneys (lane 2) or liver (lane 9) of sickle mice. However, after H/R, there was intense activation of NF-κB in both the kidneys (lane 4) and the liver (lane 11) of the sickle mice. In contrast, there was no activation of NF-κB in the normal mice either at ambient air (lanes 7 and 13) or after H/R (lanes 8 and 14).
Electrophoretic mobility shift assay for NF-κB.
Lane 1, positive control with TNF-treated microvascular endothelial cells. Lanes 3 and 10 show a weak NF-κB band in mice pretreated withEscherichia coli LPS and D-galactosamine, a combination that stimulates murine NF-κB activation. The sickle mouse kidney reveals a strong NF-κB band after H/R (lane 4), which is not present in the sickle mouse at ambient air (lane 2). The band on lane 4 is identified as NF-κB by the ability to competitively inhibit it with unlabeled NF-κB consensus oligonucleotide (lane 5). Sickle mouse liver at ambient air shows a faint NF-κB band (lane 9) that is much more prominent in the mouse liver after H/R (lane 11). This band is competitively inhibited by unlabeled NF-κB consensus oligonucleotide (data not shown). Antibody to the cRel subunit of NF-κB does not cause a supershift in sickle mouse liver or kidney. There is no NF-κB band demonstrable in the normal mouse liver or kidney with or without H/R. Hb SS indicates sickle mouse; LPS, Escherichia colilipopolysaccharide plus D-galactosamine; cold wt comp, unlabeled NF-κB consensus oligonucleotide probe; anti-cRel, antibody to cRel subunit of NF-κB; MVEC, microvascular endothelial cell; TNF, tumor necrosis factor; amb air, ambient air; H/R, hypoxia and reoxygenation.
Electrophoretic mobility shift assay for NF-κB.
Lane 1, positive control with TNF-treated microvascular endothelial cells. Lanes 3 and 10 show a weak NF-κB band in mice pretreated withEscherichia coli LPS and D-galactosamine, a combination that stimulates murine NF-κB activation. The sickle mouse kidney reveals a strong NF-κB band after H/R (lane 4), which is not present in the sickle mouse at ambient air (lane 2). The band on lane 4 is identified as NF-κB by the ability to competitively inhibit it with unlabeled NF-κB consensus oligonucleotide (lane 5). Sickle mouse liver at ambient air shows a faint NF-κB band (lane 9) that is much more prominent in the mouse liver after H/R (lane 11). This band is competitively inhibited by unlabeled NF-κB consensus oligonucleotide (data not shown). Antibody to the cRel subunit of NF-κB does not cause a supershift in sickle mouse liver or kidney. There is no NF-κB band demonstrable in the normal mouse liver or kidney with or without H/R. Hb SS indicates sickle mouse; LPS, Escherichia colilipopolysaccharide plus D-galactosamine; cold wt comp, unlabeled NF-κB consensus oligonucleotide probe; anti-cRel, antibody to cRel subunit of NF-κB; MVEC, microvascular endothelial cell; TNF, tumor necrosis factor; amb air, ambient air; H/R, hypoxia and reoxygenation.
To confirm band specificity for NF-κB, we conducted supershift experiments. As shown in Figure 4, the activated NF-κB in the sickle mice consists of the p65 (lanes 6 and 11) and the p50 (lanes 8 and 13) subunits. There was no gel retardation in response to antibodies to either the cRel subunit (lanes 6 and 12 in Figure 3 and lanes 7 and 12 in Figure 4) or the p52 subunit (lanes 9 and 14 in Figure 4) of NF-κB.
Electrophoretic mobility shift assay with antibody supershift to identify the subunits of NF-κB in the kidney and liver of sickle mice exposed to H/R. Lanes 1 to 4, TNF-treated MVEC controls; lanes 5 to 9, sickle mouse kidneys after H/R; lanes 10 to 14, sickle mouse liver after H/R. The supershift assay shows the presence of the p65 (lanes 6 and 11) and p50 (lanes 8 and 13) subunits of NF-κB in the kidney and liver of the sickle mice after H/R. There is no retardation with antibodies to p52 and cRel, suggesting that these subunits are not involved in the H/R-induced activation of NF-κB. This experiment also confirms that the identified bands are indeed caused by the presence of NF-κB. MVEC indicates microvascular endothelial cells; TNF, tumor necrosis factor; H/R, hypoxia and reoxygenation; anti-p65/50/52/cRel, antibodies to these NF-κB subunits.
Electrophoretic mobility shift assay with antibody supershift to identify the subunits of NF-κB in the kidney and liver of sickle mice exposed to H/R. Lanes 1 to 4, TNF-treated MVEC controls; lanes 5 to 9, sickle mouse kidneys after H/R; lanes 10 to 14, sickle mouse liver after H/R. The supershift assay shows the presence of the p65 (lanes 6 and 11) and p50 (lanes 8 and 13) subunits of NF-κB in the kidney and liver of the sickle mice after H/R. There is no retardation with antibodies to p52 and cRel, suggesting that these subunits are not involved in the H/R-induced activation of NF-κB. This experiment also confirms that the identified bands are indeed caused by the presence of NF-κB. MVEC indicates microvascular endothelial cells; TNF, tumor necrosis factor; H/R, hypoxia and reoxygenation; anti-p65/50/52/cRel, antibodies to these NF-κB subunits.
Therapeutic effect of allopurinol
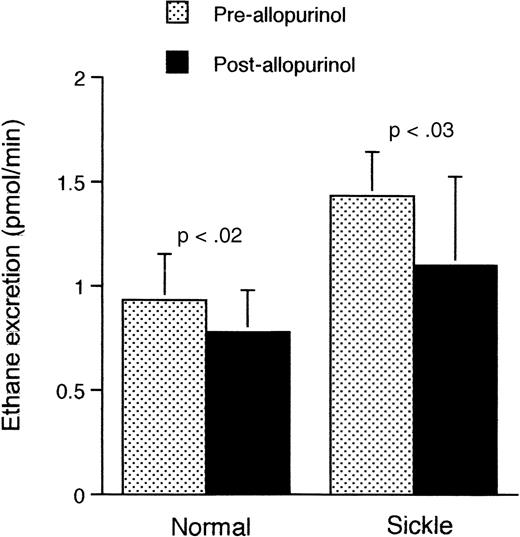
Because excessive ethane generation (Figure 2) was evident in the sickle mouse even at ambient air, we shifted our focus to the ambient situation. To test the involvement of the XO pathway in the increased ethane excretion, we administered the XO inhibitor allopurinol to sickle and normal mice under ambient air conditions. As shown in Figure5, preadministration of allopurinol significantly reduced the rate of ethane excretion in both sickle (P < .03) and normal mice (P < .02).
Rate of ethane excretion at ambient air in normal and transgenic mice before and after administration of allopurinol.
Data shown as mean ± SD (n = 5 for both groups).
Rate of ethane excretion at ambient air in normal and transgenic mice before and after administration of allopurinol.
Data shown as mean ± SD (n = 5 for both groups).
Salicylic acid hydroxylation
Hydroxyl radical attacks SA, generating the salicylate hydroxylation products 2,5-DHBA and 2,3-DHBA. Unlike 2,5-DHBA, the only known route of in vivo production of 2,3-DHBA is by hydroxyl radical attack on SA.30 31 Therefore, we measured the level of 2,3-DHBA production from SA in both sickle and normal mice at ambient air. As shown in Figure 6, we observed significantly higher levels of 2,3-DHBA in sickle mice than normal mice (P = .0018), indicating a higher level of hydroxyl radical generation at ambient air in the sickle mice. As noted previously, technical constraints precluded reliable application of this test to mice run through our H/R protocol.
Generation of hydroxyl radical in normal and transgenic mice at ambient air, as measured by production of 2,3-DHBA from SA.
Data shown as mean ± SD (n = 9 for both groups).
Generation of hydroxyl radical in normal and transgenic mice at ambient air, as measured by production of 2,3-DHBA from SA.
Data shown as mean ± SD (n = 9 for both groups).
Conversion of XD to XO
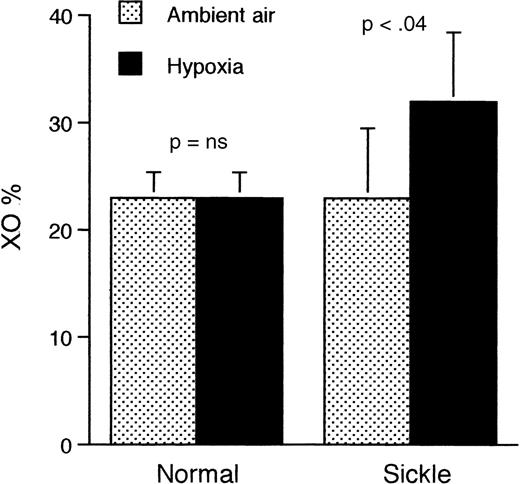
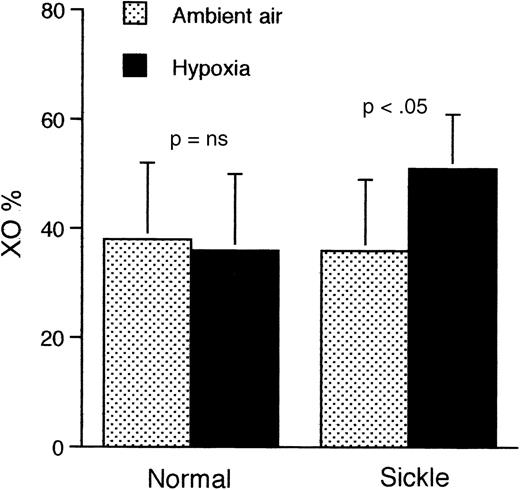
Because one mechanism of generation of free radicals involves conversion of XD to XO during periods of interruption of tissue oxygen supply, we compared the levels of XD in the liver and kidneys of mice at ambient air and at the end of a 2-hour period of hypoxia. The liver of sickle mice that were subjected to hypoxia (Figure7) showed a significantly higher proportion of XO than either hypoxic normal mice (P < .03) or sickle mice at ambient air (P < .04). Results were similar in kidney tissue (Figure 8).
Proportion of XO relative to XD in the liver of sickle and normal mice at ambient air and after 2 hours of hypoxia only (without reoxygenation).
Only the sickle mice show a significantly higher proportion of XO after exposure to hypoxia. Data shown as mean ± SD (n = 4 for both groups).
Proportion of XO relative to XD in the liver of sickle and normal mice at ambient air and after 2 hours of hypoxia only (without reoxygenation).
Only the sickle mice show a significantly higher proportion of XO after exposure to hypoxia. Data shown as mean ± SD (n = 4 for both groups).
Proportion of XO relative to XD in the kidneys of sickle and normal mice at ambient air and after 2 hours of hypoxia only (without reoxygenation).
Only the sickle mice show a significantly higher proportion of XO after exposure to hypoxia. Data shown as mean ± SD (n = 6) for both groups.
Proportion of XO relative to XD in the kidneys of sickle and normal mice at ambient air and after 2 hours of hypoxia only (without reoxygenation).
Only the sickle mice show a significantly higher proportion of XO after exposure to hypoxia. Data shown as mean ± SD (n = 6) for both groups.
Discussion
Sickle cell disease is characterized by such remarkable clinical and hematologic heterogeneity, even within specific genotypes, that there is a great deal of interest in the influence of acquired and environmental factors in modulating the phenotypic appearance of disease.41-43 Studies showing discordance between levels of intracellular hemoglobin polymer formation and the clinical course of sickle cell disease44 argue that the wide variability in morbidity in patients with sickle cell anemia cannot be explained solely by the physical sickling of their red blood cells. Therefore, the chronic organ damage that occurs in these patients irrespective of the frequency of vasoocclusive crises45 perhaps suggests the existence of a more subtle, yet inexorable, mechanism of injury that continues beyond the dramatic bouts of clinically evident severe vasoocclusion. Mechanisms underlying the progression of this chronic organ injury need to be identified.
Notably, sickle cell anemia patients manifest many features that are consistent with the presence of ongoing reperfusion injury physiology (described in “Introduction”). These include, for example, transient vascular occlusion, the iron-replete condition of patients, and an apparent influence of white blood cell counts on mortality.46 Sickle patients tend to have elevated levels of cytokines and other biological modifiers.17 Indeed, they have elevated levels of various acute phase reactants even when not experiencing acute painful episodes of sufficient severity to draw clinical attention.17,47 This is believed to reflect occurrence of subclinical ischemic episodes. It is also known that endothelial cells react to reperfusion injury with a repertoire of responses, including up-regulation of expression of cell adhesion molecules, interleukins, von Willebrand factor, tissue factor, and neutrophil recruitment.8,9,48,49 These features are also believed to be part of the endothelial biology of sickle disease.50-54 Indeed, it recently has been suggested that sickle cell patients are in a near-constant inflammatorystate,51 which could result from ongoing reperfusion injury.
Thus, we propose that the repeated transient interruption of vascular luminal patency, which occurs life-long in sickle patients, comprises a state of ongoing ischemia-reperfusion injury physiology. In the present report, we have conducted a partial test of this hypothesis by taking advantage of the availability of the sickle transgenic mouse. We used a model that has a phenotypically mild form of disease yet slowly develops evidence of organ damage at ambient air,19-21potentially allowing us to demonstrate the influence of subtle and insidious contributors to chronic organ damage. We tested for the presence of several typical footprints of reperfusion injury: conversion of XD to XO, expired ethane to indicate lipid peroxidation, conversion of plasma SA to 2,3-DHBA to indicate hydroxyl radical generation, and activation of NF-κB in liver and kidney as a downstream effect of oxidant stress or evolution of cytokines. While none of these individual markers is specific for reperfusion injury per se, their concurrent development should be expected if reperfusion injury is occurring and, therefore, provides evidence for presence of this unique physiology.
Our results demonstrate an increase in these markers of reperfusion injury in sickle mice subjected to hypoxia followed by reoxygenation. The absence of such changes in normal mice subjected to the same protocol clearly indicates that something different occurs in the sickle mice. This is supported, in particular, by the fact that only sickle mice converted XD to XO during hypoxia (without subsequent reoxygenation). Because only the sickle mice could develop RBC sickling and vascular occlusion during hypoxia, these results provide evidence for occurrence of reperfusion injury physiology in the sickle mouse. Of great additional interest, we found that the markers of oxidative stress (expired ethane and salicylate hydroxylation) also were expressed to a significantly higher level in sickle mice even under ambient air conditions. This suggests a state of chronic reperfusion injury physiology in the unmanipulated mouse at ambient air that could explain the slow development of chronic organ damage.21These observations also reveal why we could not provide “hypoxia-only” control measurements in the sickle model. Unlike traditional reperfusion injury models in which blood supply is completely cut off for a defined period, the very nature of sickle disease dictates that a period of modest hypoxia would itself include some degree of concurrent reoxygenation, as the transient and unpredictable occlusions develop and resolve.
Extant questions pertain to how directly these observations can be extrapolated to humans with sickle disease. Four issues are particularly important.
1. It is not yet known how to gauge the magnitude of an oxidant stress measured in this manner in terms of whether it truly represents risk for developing chronic organ damage, the predicted consequence of ongoing reperfusion injury physiology. Neither our results nor the literature are helpful in this regard. We are hopeful that future application of preventive strategies to the sickle mouse model may be able to answer this question.
2. Even though the oxidation detection systems we employed have been validated previously in mice (per “Materials and methods”), there is no way for us to know if our model generates a great deal of oxidant stress or only a modest amount. The reason is that the endpoints (expired ethane and salicylate hydroxylation) are systemic detectors that, therefore, report averaged (diluted) oxidation from the whole mouse. Yet the relevant damaging stress is likely to be taking place only in certain organs or even suborgan regions. There can be no “positive control” for our specific experimental model, because our preliminary exploration of this model showed that more severe hypoxia resulted in animal death, presumably from irreversible vascular occlusion. We do note, however, that the -fold increases in our measured endpoints (comparing sickle with normal animals at ambient air and comparing sickle animals with versus without hypoxia-reoxygenation) are consistent with those seen in previously described models of oxidant stress. For example, we earlier described a 2-fold increase in ethane excretion in the rat subjected to complete unilateral renal ischemia followed by restoration of blood flow.55 Others have similarly observed 2-fold ethane changes in mice that are acutely23 or chronically25 iron-loaded, or that are subjected to acute ethanol toxicity,24 or that develop paracetamol26 or paraquat27 toxicity. For salicylate hydroxylation, no models as subtle as ours have been reported in mice, but severe concussive injury33 and bilateral carotid occlusion followed by reperfusion34 are reported to produce, respectively, 4-fold and 1.5-fold increases in salicylate hydroxylation within the brain tissue itself. Severe systemic oxidant stress in mice caused by paraquat induced a 10-fold increase in plasma hydroxylation products.32 In terms of XD/XO, the degree of XD-to-XO conversion reported in the reperfusion injury literature varies widely from tissue to tissue and model to model.
3. The extent to which sickle mouse physiology parallels human sickle disease physiology remains to be defined.
4. Rodents seem to have higher levels of tissue XD/XO than other animals,56 perhaps increasing the likelihood that we would detect an effect of XD/XO in this model. Therefore, the apparent therapeutic benefit of allopurinol seen here might not hold up in sickle humans if other mechanisms of oxidant generation predominate.
These issues will require future study. Nevertheless, our demonstration that sickle mice exhibit biochemical footprints that are typical of well-defined models of reperfusion injury physiology supports the hypothesis that this is a feature of sickle disease. In separate studies, we have preliminarily used this model to also observe abnormal leukocyte endothelial attachment and emigration in sickle mice.57 Thus, our findings could help explain the chronic organ damage, the vascular injury and endothelial activation,51,52 and the apparent inflammatory state that are characteristic of sickle cell anemia. Indeed, there are striking parallels between the vascular pathobiologies of reperfusion injury58 and of sickle disease. Additionally, we have furnished preliminary evidence for a potential therapy in sickle cell disease: allopurinol. Various antioxidants and antiinflammatory strategies would be expected to have therapeutic efficacy in this model but remain to be tested. Our results argue for a general exploration of the role of antioxidants and inhibitors of the various ischemia and reperfusion mechanisms in the therapy of sickle cell disease. If our results can be corroborated in human studies, it will provide the rationale for initiation of completely novel therapy for patients with sickle cell disease in an attempt to prevent chronic organ damage.
Acknowledgments
We thank Kuan Sheng, Marsha Patten, and Troy Decker for technical assistance.
Supported by the National Institutes of Health (PO1-HL55552).
Reprints:Robert P. Hebbel, Box 480 UMHC, 420 Delaware St, SE, Minneapolis, MN 55455.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal