It has been reported that clinically significant heart involvement affected 5% to 10% of patients undergoing bone marrow transplantation (BMT) after pretreatment with CY and TBI1 and that life-threatening or fatal cardiac toxicity occurred in about 1%-5% of patients receiving CY-containing preparative regimens.2.3 But ischemic heart disease has not been a common event in the setting of BMT.4 Tacrolimus, developed by Fujisawa Pharmaceutical Company, has a potent immunosuppressive activity in vitro and in vivo. Nowadays, tacrolimus is widely used for prophylaxis and treatment of graft versus host disease (GVHD) after BMT, but some adverse effects have been reported: renal impairment, hyperlipidemia, hyperglycemia, hypertension, and cardiac event.5 Concerning cardiac event, sinus arrest, bradycardia, Q-T prolongation, and hypertrophic cardiomyopathy have been reported.6-9 Reports from kidney transplantation showed cardiac symptoms suggesting ischemic heart disease,10 but there is no report of myocardial ischemia related to tacrolimus in the BMT setting. Here we report a case of myocardial ischemia after BMT, which we suspect was caused by tacrolimus overdose.
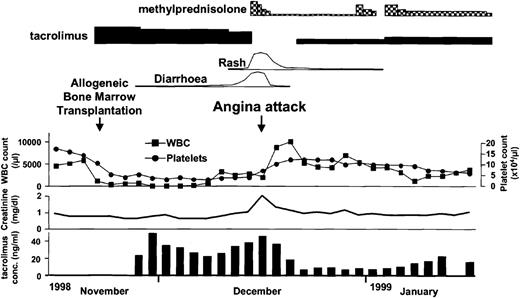
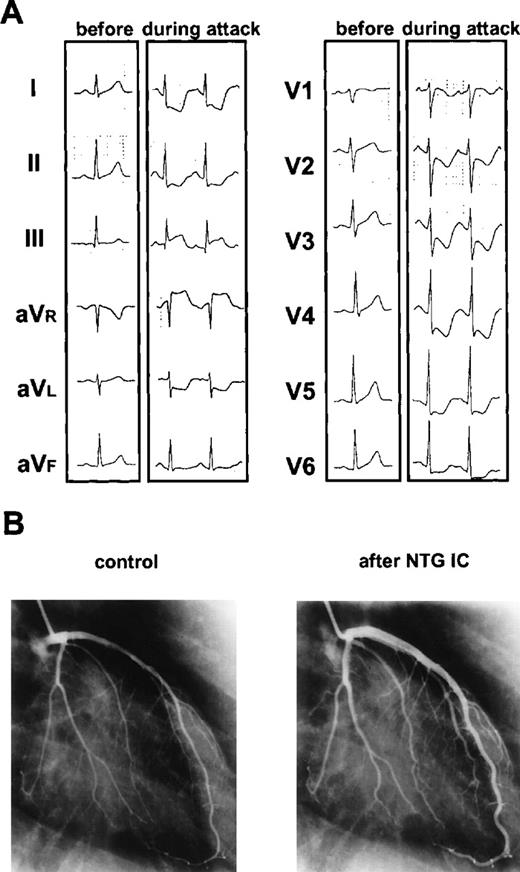
A 20-year-old patient was found to have chronic myelocytic leukemia in the first chronic phase when she presented with leukocytosis. Her bone marrow cells were all Ph1 positive. She was treated only with hydroxycarbamide until conditioning was started for BMT. She underwent an allogeneic BMT from a human lymphocyte antigen (HLA) genotypically matched and ABO minor mismatched unrelated donor, with hyperfractionated total body irradiation and a cyclophosphamide preparative regimen. Tacrolimus was used for GVHD prophylaxis started on day −1 as a continuous intravenous infusion at a dose of 0.05 mg/kg per day. The initial posttransplantation course was uneventful, and hematopoietic recovery was smooth with granulocyte colony stimulating factor administration from day 9 to day 17; the patient became independent of blood transfusions on day 12, and the granulocyte count exceeded 500/μL on day 17 (Figure1). The patient began having diarrhea on day 17 and on day 21 presented with a rash that spread over 25% of her body surface area by day 24. Diagnosis of acute GVHD grade II was made, and she was treated with methylprednisolone. At the same time, the blood concentration of tacrolimus was gradually increased, and the serum creatinine level rose to 2.0 mg/dL. We discontinued tacrolimus on day 25, when its concentration rose to 45.4 ng/mL, and on that day the patient manifested severe chest pain and dyspnea (Figure 1). Electrocardiography (ECG) showed ST depression in the chest leads from V2 to V6 (Figure 2). We started nitroglycerin and diltiazem immediately after the attack of pain, and it soon disappeared. Troponin T rose to 0.5 ng/mL on the next day. Coagulation studies showed no remarkable abnormality except a low antithrombin III (ATIII) activity (59.3%). The serum thrombomodulin was elevated to 6.0 ng/mL, which suggested endothelial damage. Thallium scintigraphy showed no definite perfusion defect on day 30, and the patient's ECG reverted to normal by day 32. For GVHD we treated the patient only with methylprednisolone from day 25 to day 31, and we carefully readministered tacrolimus by oral intake on day 32. Her rash and diarrhea were successfully controlled whereas the concentration of tacrolimus never exceeded 15 ng/mL. We performed coronary angiography on day 47. It revealed spastic coronary arteries without any significant organic lesions, which widened more than twice the width of controls shortly after intracoronary injection of isosorbide dinitrate (Figure 2). We continued oral diltiazem and nitroglycerin patches through day 130, and the patient was free from attack after that. Her ATIII activity normalized on day 53 after BMT.
Patient's clinical course after BMT.
Depicted are the white blood cell and platelet counts in peripheral blood, serum creatinine level, tacrolimus concentration in whole blood, and medication administration.
Patient's clinical course after BMT.
Depicted are the white blood cell and platelet counts in peripheral blood, serum creatinine level, tacrolimus concentration in whole blood, and medication administration.
Ischemic changes on electrocardiography and on diameter of coronary artery.
(A) Ischemic changes on electrocardiography before and during angina attack. During the attack, ST level was depressed significantly in the chest leads from V2 to V6. (B) Changes of diameter of coronary artery before and after intracoronary injection (IC) of isosorbide dinitrate (NTG). Coronary arteries were spastic without any significant organic lesions and widened more than twice the width of controls shortly after intracoronary injection of isosorbide dinitrate.
Ischemic changes on electrocardiography and on diameter of coronary artery.
(A) Ischemic changes on electrocardiography before and during angina attack. During the attack, ST level was depressed significantly in the chest leads from V2 to V6. (B) Changes of diameter of coronary artery before and after intracoronary injection (IC) of isosorbide dinitrate (NTG). Coronary arteries were spastic without any significant organic lesions and widened more than twice the width of controls shortly after intracoronary injection of isosorbide dinitrate.
Tacrolimus is widely used for prophylaxis and treatment of GVHD after bone marrow transplantation but is not without some adverse effects.5 Reports from kidney transplanters have shown an 18.6% incidence of cardiac symptoms, including chest pain and abnormal ECGs.10 The mechanism by which tacrolimus causes myocardial ischemia is still unknown. Because blood tacrolimus concentrations tended to be higher in patients with cardiac symptoms as shown in this case,10 we propose that tacrolimus could be the causative agent of this patient's myocardial ischemia, and clinicians should be aware of this potential toxic manifestation. The patient's ATIII activity was decreased below normal when the attack occurred. There are reports that the ATIII level decreases during BMT, which could cause a hypercoagulable state and result in a wide spectrum of thrombotic complications.11 But in this case, because coronary angiography revealed no organic stenosis, microthrombi formation due to low ATIII activity would be unlikely as the cause of this event.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal