Abstract
Chronic transfusion therapy is being used more frequently to prevent and treat the complications of sickle cell disease. Previous studies have shown that the iron overload that results from such therapy in other patient populations is associated with significant morbidity and mortality. In this study we examined the extent of iron overload as well as the presence of liver injury and the predictive value of ferritin in estimating iron overload in children with sickle cell disease who receive chronic red blood cell transfusions. A poor correlation was observed between serum ferritin and the quantitative iron on liver biopsy (mean 13.68 ± 6.64 mg/g dry weight;R = 0.350, P = .142). Quantitative iron was highly correlated with the months of transfusion (R = 0.795, P < .001), but serum ferritin at biopsy did not correlate with months of transfusion (R = 0.308, P = .200). Sixteen patients had abnormal biopsies showing mild to moderate changes on evaluation of inflammation or fibrosis. Liver iron was correlated with fibrosis score (R = 0.50, P = .042). No complications were associated with the liver biopsy. Our data suggest that, in patients with sickle cell disease, ferritin is a poor marker for accurately assessing iron overload and should not be used to direct long-term chelation therapy. Despite high levels of liver iron, the associated liver injury was not severe.
Red blood cell (RBC) transfusions have been actively used for many years to treat acute illnesses in sickle cell disease (SCD). The recent demonstration by Adams and colleagues1that chronic RBC transfusions are effective in preventing stroke has lead to a rapid increase in patients who are receiving chronic transfusions and concern about the development of iron overload and possible injury. The Stroke Prevention Trial in Sickle Cell Anemia (STOP) study has in fact already reported an increase in serum ferritin in study participants, suggesting the development of iron overload in these patients.2 Disease complications and early death have been associated with increased iron overload in patients with hereditary hemochromatosis and β thalassemia,3 4 raising concern about the effects of iron overload on patients with SCD.
The goals of this study were to determine the severity of iron overload in patients who receive chronic RBC transfusions, to assess whether serum ferritin is a useful clinical marker of iron overload, and to determine whether significant liver injury occurs in patients with SCD who are chronically transfused.
Patients, materials, and methods
In this study, patients participating in the Northern California Comprehensive Sickle Cell Center (NCCSCC) and receiving chronic RBC transfusions agreed to percutaneous liver biopsy to assess their iron overload. These patients were participants in a chronic transfusion protocol, initiated because of the recent increased frequency of RBC transfusions in SCD. One of the aims of the protocol was to monitor the degree of iron overload and liver tissue injury. According to protocol criteria, patients were immunized to hepatitis A and B, serum ferritin was monitored monthly and at biopsy, and liver functions (alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase) were monitored bimonthly. Hepatitis serologies were obtained yearly. Serum ferritin was analyzed by heterogeneous sandwich magnetic separation assay (MSA), with a range of 0.3 to 1000 μg/L and standardized by the World Health Organization (WHO) 1st International Standard (IS 80/802) (Unilab, San Jose, CA). Serum ferritin at initiation of chronic transfusion therapy and peak serum ferritin were single values measured at the respective time points. Serum ferritin at biopsy was calculated as the average of all measurements obtained in the 6 months preceding the biopsy. A similar schema for determining ALT values was used. To be eligible for liver biopsy, patients with sickle cell hemoglobinopathy must have enrolled in a chronic monthly transfusion program, completed monthly transfusions for a minimum of 1 year, had normal coagulation studies, and provided informed consent.
The goal of the transfusion protocol for all patients was to maintain their hemoglobin S (HbS) percentage at or below 30% and the prehemoglobin and posthemoglobin greater than 9 g/dL and less than 12 g/dL, respectively. All patients received leukodepleted, phenotypically matched red cell units. A detailed deferoxamine protocol, which included education and child life home visits, was used. In brief, after 1 year of chronic transfusion therapy, deferoxamine therapy was initiated. The protocol was initiated at 25 mg/kg deferoxamine over 8 to 10 hours subcutaneously 5 days a week and increased gradually to 30 to 50 mg/kg per day. All patients received 100 mg vitamin C on the days deferoxamine was used.
Prebiopsy evaluation included assessment of complete blood count (CBC), prothrombin time, activated partial thromboplastin time, type and screen with blood units available, and completion of an abdominal ultrasound on the morning of the procedure to outline the liver and screen for abnormalities. The procedure was completed in the operating room using intravenous and inhaled agents (propofol and sevoflurane orisoflurane) for sedation to minimize pain and control breathing during the biopsy. Patients were admitted overnight for observation and repeat CBC was obtained at 4 and 24 hours after the biopsy. Percutaneous liver biopsy was completed with the Bard Monopty 16-gauge needle (Bard Interventional Products Division, Billerica, MA). Biopsies were immediately fixed in formalin. After paraffin imbedding, sections were obtained for hemotoxylin and eosin and reticulin to stain connective tissue, and Perl's to stain iron. The remainder of the block or, in earlier cases, the specimen in a trace element-free container was sent for quantitative iron by ICP-ES or ICP-MS (Mayo, Rochester, MN). The liver iron was reported as milligram per gram (mg/g) dry weight of liver (normal range 0.4-2.2 mg/g).
The histologic sections were routinely evaluated by 2 pathologists blinded to the subject's identity. Iron deposition was evaluated using the scale developed by Deugnier et al5 that includes an assessment of iron deposits in hepatocytes, in Kupffer cells, and in main structures of portal tracts. In this scale, hepatocyte score ranges from 0 to 36, and Kupffer cell and portal tract scores each range from 0 to 12, for a possible total iron score of 0 to 60. The large possible score in the hepatocyte compartment reflects the 3:1 ratio of hepatocyte to Kupffer cell number in the liver. Inflammatory activity was graded according to the modified Scheuer system,6 which includes assessment of portal and lobular activity, graded from 0 to 4, with zero representing none and 4 representing the most severe. Finally, fibrosis was scored from 0 to 4 with 4 representing cirrhosis.7
Results
Subjects
The study included 20 patients with hemoglobin SS who were on chronic transfusion therapy (12 with history of stroke, 5 on the STOP study with increased flow velocity with transcranial doppler, 2 with severe vasocclusive crisis, and 1 with a history of pulmonary complications of prematurity). These patients ranged from 2 to 31 years of age, with a mean age of 13.5 years. No patient had a hepatitis B or C infection. Nineteen of the patients were receiving deferoxamine; the youngest patient had not yet started on chelation therapy. Six patients had previously undergone splenectomy. Patients had mean ALT 22 ± 8 U/L at the initiation of chronic transfusion therapy. Peak ALT 170 ± 277 U/L, was reached in 38 ± 36 months; however, the ALT at the time of biopsy had decreased to 27 ± 15 U/L. Only 1 patient had an abnormal ALT at the time of biopsy and that value was within 2 times the upper limit of normal.
Iron overload
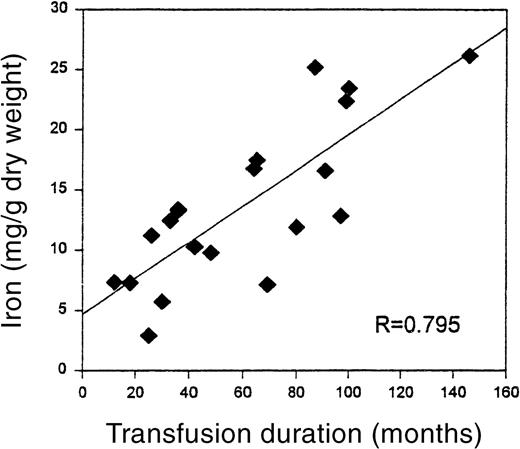
The mean duration of the chronic transfusion therapy until biopsy in this group was 57 ± 35 months (mean ± SD, range 12 to 146 months). Quantitative iron was highly positively correlated with the months of transfusion (R = 0.795, P < .001), despite aggressive use of deferoxamine chelation (Figure1). Mean liver iron was 13.68 mg/g dry weight, with a range of 2.9 to 26.19 mg/g dry weight.
Relationship of liver iron to duration of transfusion therapy.
The significant correlation (P < .001) between transfusion duration, in months, and liver iron on biopsy specimen.
Relationship of liver iron to duration of transfusion therapy.
The significant correlation (P < .001) between transfusion duration, in months, and liver iron on biopsy specimen.
Ferritin
The mean ferritin at the initiation of chronic transfusion therapy was 420 ± 154 ng/mL. Peak ferritin of 4614 ± 1989 ng/mL (mean, SD) was reached in 42 ± 24 months. Subjects had a range of 2 to 11 serum ferritin measurements in the 6 months before biopsy. This average serum ferritin at the time of biopsy was 2686 ± 1039 ng/nL. A poor correlation (R = 0.350, P = .142) was observed between average serum ferritin and the quantitative iron on liver biopsy. Average serum ferritin also did not correlate with months of transfusion (R = 0.308, P = .200), age of the patient, or splenectomy.
Liver injury
Injury on liver biopsy was assessed independently by the 2 pathologists. Again, there was a strong correlation between pathologists' scores on fibrosis (R = 0.904,P < .001) and inflammation (R = 0.782,P < .001) with 84% agreement on individual scores for fibrosis and 89% agreement on individual scores for inflammation. Sixteen of the 20 samples had mild-to-moderate abnormalities on assessment of inflammation or fibrosis. The mean scores were less than 1 on both parameters, suggesting that the extent of liver injury was mild, despite the very high iron overload. Severity of liver iron overload did not correlate with inflammation (R = −0.196, P = .380), but did correlate with fibrosis (R = 0.480, P = .042).
Iron staining pattern
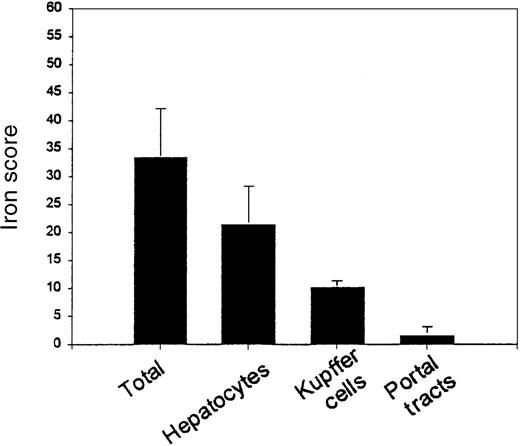
The 2 pathologists assessed iron intensity and distribution. There was 92% agreement between pathologists on individual scores in each location and a strong correlation between pathologists on total iron score (R = 0.988, P < .001). The mean total iron score was 33.474 ± 8.72 (possible range 0-60) (Figure2), with mean hepatocyte score of 21.45 ± 6.832 (possible range 0-36), mean Kupffer cell score of 10.2 ± 1.196 (possible range 0-12), and mean portal tract score of 1.579 ± 1.539 (possible range 0-12). The mean hepatocyte iron score was 58% of the maximum possible score; the mean Kupffer cell score was 83% of the maximum possible score. This suggests that the intensity of staining in Kupffer cells is greater than in hepatocytes (P < .001). Although less intense iron staining occurs in hepatocytes, the hepatocyte compartment is larger than the Kupffer cell compartment and accounts for three- fifths of the total iron score. Of note, the total iron score by histologic assessment was correlated with the biochemical liver iron (R = 0.602, P = .011).
Distribution of iron in the liver.
The proportion of total iron score accounted for by hepatocytes (64%), Kupffer cells (31%), and portal tracts (5%) is shown.
Distribution of iron in the liver.
The proportion of total iron score accounted for by hepatocytes (64%), Kupffer cells (31%), and portal tracts (5%) is shown.
Splenectomy and liver iron
The mean iron level in patients who had previously undergone splenectomy was 20.6 ± 6.25 mg/g, whereas the mean iron level in patients without previous splenectomy was 10.72 ± 4.18 mg/g (P < .01). There was a positive correlation between splenectomy and months of transfusion (R = 0.664,P < .01). In multivariate analysis, splenectomy did not appear to be an independent predictor of liver iron (P = .106), whereas months of transfusion was an independent predictor (P < .01). No significant association was noted between splenectomy and age of the patient (R = 0.296,P = .205) or ferritin at biopsy (R = 0.050,P = .840).
Complications of biopsy
No complications were associated with the liver biopsy. No patient developed infection related to the liver biopsy or suffered organ injury or bleeding. Hemoglobin did not decrease after liver biopsy (pre-Hb 10.9 ± 1.4 g/dL vs post-Hb 10.5 ± 1.0 g/dL) and no transfusions were required in relation to the biopsy.
Discussion
Iron overload
In this study, transfusion-dependent children with sickle cell disease demonstrated increased iron deposition with duration of transfusion, despite chelation therapy. From Cohen et al,8this would imply ineffective chelation therapy or poor compliance in the entire population. Our patients were receiving 30 to 50 mg/kg per dose of deferoxamine and 14 of the 19 patients on chelation began this therapy within 2 years of starting transfusion therapy (mean lag time 13.5 ± 8.8 months, range 2-30 months).
Seventeen of our 20 patients had liver iron above the range of 3 to 7 mg/g dry weight, suggested by Olivieri and Brittenham9 as a conservative goal to prevent complications, based on the lack of adverse effects of this iron level on heterozygous hereditary hemochromatosis patients. Ten patients (50%) had levels above 15 mg/g dry weight. Patients with β thalassemia and iron burden greater than 15 mg/g dry weight have greatly increased risk of cardiac disease and early death.4 At this time, it is unclear whether patients with sickle cell disease have the same risks as observed for hereditary hemochromatosis and thalassemia.10
Ferritin
Plasma ferritin is correlated significantly with other measures of body storage iron in most studies but clinically is an imprecise estimate of body iron burden. In this study, plasma ferritin concentration did not correlate with liver iron. This finding is consistent with recent reports from the thalassemia literature that suggest serum ferritin may in fact be a poor and possibly misleading measure in the heavily overloaded patient.11,12 The relationship between plasma ferritin and body iron stores is distorted by ascorbate deficiency, fever, infection, inflammation, and hepatic dysfunction, all of which occur in patients with sickle cell disease.11 Brittenham et al11 found that only 57% of the variability in ferritin could be explained by the variation in hepatic iron and, therefore the usefulness of ferritin in predicting hepatic iron is limited. One means of improving the value of ferritin measurements would be the application of a logarithmic quantitation model that uses individual transfused iron values. This model has been shown to yield a median correlation between serum ferritin and transfused iron of 0.92 in patients with sickle cell disease.13
Liver injury
As expected, this study demonstrated a high level of iron overload. This iron overload is of concern as it may result in portal fibrosis.9 In the hemochromatosis population, increased liver iron has also been associated with inflammation5 and cancer.14
In this study, the severity of inflammation and fibrosis was low. Liver iron score did not correlate with inflammation but did correlate with fibrosis. These findings suggest that patients with SCD may have a different response to iron overload in comparison to patients with thalassemia or hereditary hemochromatosis.
Iron staining pattern
The cell distribution of iron shows different patterns in hereditary hemochromatosis and transfusional overload. In hereditary hemochromatosis, the hepatocyte contains high amounts of iron, whereas the Kupffer cell or sinusoidal cell does not contain iron, except in late-stage disease, while in transfusional overload, the reverse pattern is reported.15 In this study, the intensity of iron staining in the hepatocyte and Kupffer cell, relative to the total possible score for each, was 0.58 versus 0.85, respectively, demonstrating higher relative deposition of iron in the Kupffer cell as expected in transfusional overload. However, overall, the hepatocyte accounted for a large proportion (0.6) of the total iron score. This may reflect overflow from the Kupffer cell to the heptocyte in patients who are very heavily iron overloaded as previously observed by Bothwell and colleagues.16
Splenectomy and liver iron
Splenectomy has been used in thalassemia and sickle cell disease to treat hypersplenism and reduce transfusion requirement. Although we were not able to demonstrate a statistically significant effect of splenectomy on liver iron in multivariate analysis, this was a preliminary study that included only 6 subjects with splenectomy, and it is possible that splenectomy will prove to have a significant effect in increasing liver iron in a larger sample. A similar increase in liver iron has been reported in splenectomized patients with E/beta thalassemia, and has been associated with an increase in intestinal iron uptake.17 Similar findings have not been described in sickle cell disease. Further studies are needed to characterize this phenomenon.
Complications of biopsy
It is well known that percutaneous liver biopsy is associated with a small risk of bleeding, infection, and puncture of an abdominal organ other than the liver.18 In our study, the risks were minimized by obtaining ultrasound guidance for the procedure; arresting the breathing during the biopsy, preventing laceration by the needle; completing the procedure on patients with normal coagulation studies; and providing close observation after the procedure with blood available, if needed. In the group reported in this study, no medical complications were noted. Hemoglobin did not decrease in relation to biopsy. These findings are consistent with those of Angelucci et al18 who found a complication rate of 0.2% in patients with iron overload.
Conclusion
In conclusion, chronic transfusion produced high levels of iron overload, ferritin was shown to be a poor clinical marker for this population, iron overload increased with duration of transfusion despite aggressive chelation, liver injury was mild, splenectomy was associated with increased liver iron, and percutaneous liver biopsy proved a safe procedure in this group of patients.
Acknowledgment
We would like to thank Ekua Hackney-Stephens for her expert clinical assistance.
Supported by NIH grants nos. M01RR01271-16 and HL-20985.
Reprints:Paul Harmatz, Gastroenterology and Nutrition, Children's Hospital Oakland, 747 52nd St, Oakland, CA 94609; e-mail:pharmatz@mail.cho.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal