Abstract
Blood stem cell transplantation (BSCT) results in rapid hematopoietic recovery in both the allogeneic and autologous transplant settings. Because of the large numbers of progenitor cells in mobilized blood, the administration of growth factors after transplantation may not provide further acceleration of hematopoietic recovery. A randomized, double-blind, placebo-controlled study was performed to determine the effects of filgrastim (granulocyte colony-stimulating factor; G-CSF) administration on hematopoietic recovery after allogeneic BSCT. Fifty-four patients with hematologic malignancies undergoing a related, HLA-matched allogeneic BSCT were randomly assigned to receive daily filgrastim at 10 μg/kg or placebo starting on the day of transplantation. A minimum of 3 × 106 CD34+ cells/kg in the allograft was required for transplantation. All patients received a standard preparative regimen and a standard regimen for the prevention of graft-versus-host disease (GVHD). The median time to achieve an absolute neutrophil count greater than 0.5 × 109/L was 11 days (range, 9-20 days) for patients who received filgrastim compared with 15 days (range, 10-22 days) for patients who received placebo (P = .0082). The median time to achieve a platelet count greater than 20 × 109/L was 13 days (range, 8-35 days) for patients who received filgrastim compared with 15.5 days (range, 8-42 days) for patients who received placebo (P = .79). There were no significant differences for red blood cell transfusion independence, the incidence of acute GVHD, or 100-day mortality between the groups. The administration of filgrastim appears to be a safe and effective supportive-care measure following allogeneic BSCT.
Blood, compared with bone marrow, is increasingly being used as the source of hematopoietic stem cells for allogeneic stem cell transplantation (alloSCT).1 Blood stem cells, mobilized with hematopoietic growth factors and collected from HLA-matched sibling donors, have been transplanted successfully in the allogeneic setting.2-4 Transplantation of mobilized allogeneic blood stem cells occurred without a significant increase in graft-versus-host disease (GVHD), despite a 1- to 2-log increase in T-cell numbers in the blood allograft compared with an allogeneic bone marrow harvest.5 Blood is used as the primary hematopoietic stem cell source in more than 30% of allogeneic transplants performed today.1 6
The major advantage of mobilized blood for alloSCT is rapid hematopoietic recovery.7-9 After mobilization with hematopoietic growth factors, large numbers of stem and progenitor cells can be obtained from the blood.10-15 The number of stem and progenitor cells, as measured by CD34 expression, correlates with hematopoietic recovery in both the autologous and allogeneic transplantation settings.16,17 However, improved hematopoietic recovery may also be achieved with the administration of hematopoietic growth factors, such as granulocyte colony-stimulating factor (G-CSF; filgrastim) and granulocyte–macrophage colony-stimulating factor (GM-CSF; sargramostim), after allogeneic and autologous transplantation regardless of the stem cell source.18-23 Some controversy exists regarding the use of growth factors after alloSCT, especially in unrelated bone marrow transplantation, because of observations of an increased incidence of GVHD.19-21 In addition, the use of hematopoietic growth factors after transplantation of mobilized blood stem cells may not improve the recovery of neutrophils if large numbers of stem or progenitor cells are infused.8,16 23
Previous phase II studies at the University of Nebraska Medical Center (UNMC) have shown that both the administration of growth factors after allogeneic bone marrow transplantation and the transplantation of mobilized blood improved the rate of hematopoietic recovery following alloSCT.9 23 To determine the effects of growth factor administration following alloSCT using mobilized blood as the sole allogeneic stem cell source, we performed a randomized, double-blind trial of filgrastim versus placebo following alloSCT. The primary end point of this trial was the rate of hematopoietic recovery, and the secondary end points were the incidence and severity of acute GVHD and survival at 100 days after transplantation.
Patients and methods
Eligibility criteria
Patients between the ages of 16 and 65 years with a diagnosis of acute myelogenous leukemia (AML) in first or subsequent remission, acute lymphocytic leukemia in first remission with high-risk features or second or subsequent remission, chronic lymphocytic leukemia (CLL) having failed primary therapy, multiple myeloma (MM), Hodgkin disease or non-Hodgkin lymphoma (NHL) having failed primary therapy, myelodysplastic syndrome (MDS), or chronic myelogenous leukemia (CML) in chronic or accelerated phase were eligible for participation in this study. A Karnofsky score greater than 80%, lung diffusion capacity greater than 50%, serum creatinine level less than 1.5 mg/dL or creatinine clearance level greater than 65 mL/min, total bilirubin level less than 2.0 mg/dL, and aspartate aminotransferase level less than 2 times normal were additional eligibility criteria. All patients had an acceptable family donor matched for 5 or 6 HLA antigens who consented to serve as an allogeneic blood stem cell donor. Patients were enrolled at UNMC and the Saint Luke's Hospital of Kansas City (SLH) Blood and Marrow Transplant Program. This study was approved by the UNMC institutional review board (IRB) and the SLH IRB. Written informed consent was obtained from each patient and donor before transplantation and stem cell mobilization, respectively.
Blood stem cell mobilization and collection
Hematopoietic progenitor and stem cells were mobilized in donors with filgrastim (granulocyte colony-stimulating factor, G-CSF; Amgen, Thousand Oaks, CA) at 10 μg · kg−1 · d−1 by subcutaneous (SC) administration for 5 to 6 days.15Apheresis was performed using the Cobe Spectra (COBE BCT, Lakewood, CO) apheresis device. Twelve liters of blood was processed during each daily apheresis. Apheresis began on day 5 of filgrastim administration. A minimum of 3.0 × 106 CD34+/kg recipient weight and 6.5 × 108 mononuclear cells/kg recipient weight were collected from each donor.8 15 Cells were cryopreserved in 5% dimethylsulfoxide plus 6% hydroxyethylstarch. Cells were thawed and infused on day 0.
Preparatory regimen
All patients received an identical preparative regimen consisting of cyclophosphamide (60 mg · kg−1 · d−1), which was administered on days −5 and −4, and total-body irradiation (1200 cGy), which was administered in 6 fractions of 200 cGy twice a day on days −3, −2, and −1. Mesna 60 mg · kg−1 · d−1 was started 1 hour before the first dose of cyclophosphamide and continued until 24 hours after completion of the second dose of cyclophosphamide.
GVHD prophylaxis
All patients received identical GVHD prophylaxis consisting of cyclosporine A (CsA) and “mini”-methotrexate.24Patients received CsA 2 mg/kg intravenously (IV) over 2 hours every 12 hours, starting on day −3. Methotrexate (5 mg/m2) IV was given on days +1, +3, +6, and +11. Patients could receive oral CsA after engraftment if they were able to take medications by mouth. CsA was continued for approximately 6 months unless there was clinical evidence of active chronic GVHD. All patients were evaluated for acute GVHD daily during hospitalization and at least twice weekly after discharge until 100 days after transplantation.
Supportive care
All patients received irradiated blood products. Red blood cells (RBCs) were transfused if hemoglobin was less than 8 g/dL or if patients had symptoms that were thought to be related to anemia. Platelets were transfused if platelets were less than 20 × 109/L or if there was evidence of active bleeding. Patients who were cytomegalovirus (CMV) negative by serology and received an allograft from CMV-negative donors received CMV-negative blood products. All CMV-positive or CMV-negative patients receiving stem cells from CMV-positive donors were monitored for expression of CMV antigenemia. If CMV was detected, patients were treated with preemptive ganciclovir.25 Intravenous broad-spectrum antibiotics were initiated when the absolute neutrophil count (ANC) dropped below 0.5 × 109/L and were continued until neutrophil recovery. Amphotericin 0.5 to 1.0 mg/kg was administered if either positive fungal culture or persistent fever with negative blood cultures occurred despite adequate antibacterial coverage. All patients were hospitalized in reverse isolation rooms with HEPA filters.
Randomization and end points
Patients were randomly assigned to receive filgrastim or placebo following transplantation of allogeneic stem cells. The investigator, the patient, and all treating personnel were blinded to the product the patient received. Filgrastim and placebo were dispensed at a central pharmacy in numbered lots. Patients received filgrastim (10 μg/kg) or placebo by daily SC administration starting on day 0 (day of transplantation) until the ANC was greater than 1.0 × 109/L for 3 consecutive days or for a total of 28 days, whichever came first. If the patient had not achieved an ANC greater than 1.0 × 109/L by day 28 or in the event of a culture-confirmed bacterial or fungal infection or sepsis before day 28, the study drug was discontinued and the patient received open-label filgrastim at 10 μg · kg−1 · d−1 by SC injection until the ANC was greater than 1.0 × 109/L for 3 consecutive days.
Statistical analysis
This study was designed to assess the effect of the use of filgrastim on hematopoietic recovery following alloSCT. Hematopoietic recovery was defined as the time from stem cell infusion until the first day of an ANC of 0.5 × 109/L for 3 consecutive days and the times to platelet and RBC transfusion independence. Platelet transfusion independence was defined as the day after transplantation that the platelet number was greater than (or equal to) 20 × 109/L without the necessity of platelet transfusions for at least 30 days. RBC transfusion independence was defined as the day after transplantation that the hemoglobin level was greater than (or equal to) 8 g/dL without the necessity of RBC transfusions for at least 30 days. The primary end point of the study was neutrophil recovery, and platelet and RBC recovery were secondary end points. Additional end points were the incidence and severity of acute GVHD and 100-day survival. The study was designed to enroll a total of 100 “events” (patients observed to attain an ANC of 0.5 × 109/L, 50 per arm) to provide 80% power to detect a median increase of 3 days in the time to an ANC of 0.5 × 109/L (from 12 days to 15 days), testing at the 5% level of statistical significance (2-sided). If the null hypothesis was not rejected, then one could be reasonably certain that the true difference in the “time to an ANC of 500” distributions was such that the true medians would be no more different than about 3 days. Cox proportional hazards regression was used to assess the relation of treatment to ANC recovery after adjustment for CD34 dose.
Distributions of time-to-event data were estimated using the Kaplan-Meier method. Patients who died before the event of interest were censored at their time of death. Time-to-event data were analyzed using the log-rank test. Comparisons of proportions were done using Fisher's exact test, and the difference between distributions was tested using the Wilcoxon rank-sum test.
The study was monitored by an external monitoring committee consisting of 2 statisticians, 1 of whom was a member of the UNMC IRB, and 1 clinician familiar with transplantation, but who was not directly involved in the conduct of the study or the enrollment of patients participating in this study. An interim analysis was scheduled and performed when the study had accrued 50 eligible and evaluable patients, and the data were reported to the external monitoring committee. The external monitoring committee was also blinded to the specific products the patients received, and the data were presented to them as product “A” or “B.”
Results
Fifty-four patients with hematologic malignancies were enrolled in this trial and treated consecutively in the UNMC and the SLH transplant programs between August 1996 and October 1998. Four patients were determined to be ineligible and were excluded from analysis. The protocol statistician (J.C.L.) reported the data of the analysis of the first 50 evaluable patients to the external monitoring committee. After their review of the data, the committee recommended that accrual to the protocol be terminated and that unblinded study results be reported to the principal investigator.
Patient characteristics
The median patient age was 44 years (range, 20-60 years) for patients who received filgrastim and 41 years (range, 25-58 years) for patients who received placebo (Table1). Twenty-seven patients were male and 23 were female. Diagnoses included AML (n = 6), CML (n = 19), NHL (n = 16), MDS (n = 3), CLL (n = 3), and MM (n = 3). The 2 treatment groups were well balanced relative to disease distribution with the exception of CML, which was more common in the patients who received filgrastim (n = 13) than in the patients who received placebo (n = 6). No statistically significant differences were found when sex, age, diagnosis, CMV status, ABO compatibility, and disease status at transplantation were compared between the groups.
Patient characteristics
| Characteristic . | Total . | Filgrastim . | Placebo . | P Value . |
|---|---|---|---|---|
| Number | 50 | 26 | 24 | |
| Sex | .27 | |||
| Males | 27 | 12 | 15 | |
| Females | 23 | 14 | 9 | |
| Age, y | .50 | |||
| Median | 44 | 44 | 41 | |
| Range | 20-60 | 20-60 | 25-58 | |
| Diagnosis | .54 | |||
| CML | 19 | 13 | 6 | .41 |
| First chronic phase | 10 | 5 | ||
| First accelerated phase | 2 | 0 | ||
| Unknown | 1 | 1 | ||
| AML | 6 | 2 | 4 | .40 |
| CR1 | 2 | 1 | ||
| CR2 | 0 | 3 | ||
| NHL | 16 | 8 | 8 | 1.0 |
| PIF-resistant | 1 | 1 | ||
| PIF-sensitive | 2 | 3 | ||
| PIF-untreated | 1 | 0 | ||
| CR1 | 0 | 1 | ||
| CR2 | 1 | 0 | ||
| First relapse—untreated | 1 | 0 | ||
| First relapse—sensitive | 0 | 1 | ||
| Second relapse—untreated | 1 | 0 | ||
| Unknown | 1 | 2 | ||
| MDS | 3 | 1 | 2 | 1.0 |
| CR1 | 0 | 1 | ||
| Untreated | 1 | 0 | ||
| Unknown | 0 | 1 | ||
| CLL | 3 | 1 | 2 | 1.0 |
| Rai stage II | 0 | 1 | ||
| Rai stage IV | 1 | 1 | ||
| MM | 3 | 1 | 2 | 1.0 |
| CR | 0 | 1 | ||
| Unknown | 2 | 0 | ||
| HLA matching | 1.0 | |||
| 6/6 | 48 | 25 | 23 | |
| 5/6 | 2 | 1 | 1 | |
| ABO compatibility | .61 | |||
| Compatible | 16 | 14 | ||
| Minor incompatibility | 3 | 5 | ||
| Major incompatibility | 7 | 4 | ||
| Unknown | — | 1 | ||
| CMV serology (donor and/or recipient) | .13 | |||
| Negative | 6 | 10 | ||
| Positive | 20 | 12 | ||
| Unknown | 0 | 2 |
| Characteristic . | Total . | Filgrastim . | Placebo . | P Value . |
|---|---|---|---|---|
| Number | 50 | 26 | 24 | |
| Sex | .27 | |||
| Males | 27 | 12 | 15 | |
| Females | 23 | 14 | 9 | |
| Age, y | .50 | |||
| Median | 44 | 44 | 41 | |
| Range | 20-60 | 20-60 | 25-58 | |
| Diagnosis | .54 | |||
| CML | 19 | 13 | 6 | .41 |
| First chronic phase | 10 | 5 | ||
| First accelerated phase | 2 | 0 | ||
| Unknown | 1 | 1 | ||
| AML | 6 | 2 | 4 | .40 |
| CR1 | 2 | 1 | ||
| CR2 | 0 | 3 | ||
| NHL | 16 | 8 | 8 | 1.0 |
| PIF-resistant | 1 | 1 | ||
| PIF-sensitive | 2 | 3 | ||
| PIF-untreated | 1 | 0 | ||
| CR1 | 0 | 1 | ||
| CR2 | 1 | 0 | ||
| First relapse—untreated | 1 | 0 | ||
| First relapse—sensitive | 0 | 1 | ||
| Second relapse—untreated | 1 | 0 | ||
| Unknown | 1 | 2 | ||
| MDS | 3 | 1 | 2 | 1.0 |
| CR1 | 0 | 1 | ||
| Untreated | 1 | 0 | ||
| Unknown | 0 | 1 | ||
| CLL | 3 | 1 | 2 | 1.0 |
| Rai stage II | 0 | 1 | ||
| Rai stage IV | 1 | 1 | ||
| MM | 3 | 1 | 2 | 1.0 |
| CR | 0 | 1 | ||
| Unknown | 2 | 0 | ||
| HLA matching | 1.0 | |||
| 6/6 | 48 | 25 | 23 | |
| 5/6 | 2 | 1 | 1 | |
| ABO compatibility | .61 | |||
| Compatible | 16 | 14 | ||
| Minor incompatibility | 3 | 5 | ||
| Major incompatibility | 7 | 4 | ||
| Unknown | — | 1 | ||
| CMV serology (donor and/or recipient) | .13 | |||
| Negative | 6 | 10 | ||
| Positive | 20 | 12 | ||
| Unknown | 0 | 2 |
CML indicates chronic myelogenous leukemia; AML, acute myelogenous leukemia; CR, complete remission; NHL, non-Hodgkin lymphoma; PIF, primary induction failure; MDS, myelodysplastic syndrome; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; CMV, cytomegalovirus.
Compliance
Among the patients randomized to receive placebo, 7 did not complete their assigned therapy: 1 because of early death, 1 at the physician's request, and 5 because of documented infection or suspected sepsis. The latter 6 patients were subsequently started on filgrastim. Among the patients who were assigned to receive filgrastim, only 1 did not complete the assigned therapy because of documented infection.
Stem cell product
Patients randomized to receive placebo received a median of 8.1 (range, 2.3-26.2) × 106 CD34+ cells/kg and 52.8 (range, 13.2-178.4) × 104 colony-forming units of granulocyte-macrophage (CFU-GM)/kg (Table2). Patients randomized to receive filgrastim received a median of 10.4 (range, 4.0-20.3) × 106 CD34+ cells/kg and 63.8 (range, 20.3-165.8) × 104 CFU-GM/kg.
Allograft characteristics
| Characteristic . | Filgrastim (n = 23) . | Placebo (n = 21) . | PValue . |
|---|---|---|---|
| CD34+ infused (×106/kg) | |||
| Median | 10.36 | 8.12 | .44 |
| Range | 4.02-20.32 | 2.25-26.17 | |
| CFU-GM infused (×104/kg) | |||
| Median | 63.79 | 52.84 | .47 |
| Range | 20.33-165.77 | 13.21-178.40 | |
| MNC infused (×108/kg) | |||
| Median | 8.09 | 8.05 | .50 |
| Range | 2.95-15.77 | 5.38-14.94 |
| Characteristic . | Filgrastim (n = 23) . | Placebo (n = 21) . | PValue . |
|---|---|---|---|
| CD34+ infused (×106/kg) | |||
| Median | 10.36 | 8.12 | .44 |
| Range | 4.02-20.32 | 2.25-26.17 | |
| CFU-GM infused (×104/kg) | |||
| Median | 63.79 | 52.84 | .47 |
| Range | 20.33-165.77 | 13.21-178.40 | |
| MNC infused (×108/kg) | |||
| Median | 8.09 | 8.05 | .50 |
| Range | 2.95-15.77 | 5.38-14.94 |
CFU-GM indicates colony-forming units of granulocyte-macrophage; MNC, mononuclear cells.
Hematologic recovery
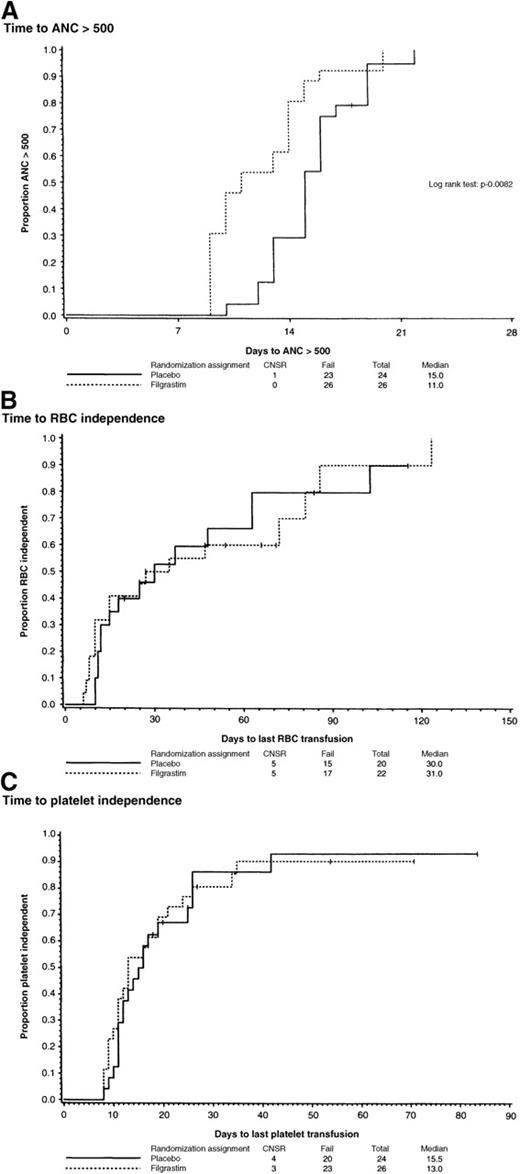
All patients recovered an ANC level greater than 0.5 × 109/L within 22 days after alloSCT (Figure1). The median time to an ANC of greater than 0.5 × 109/L was 11 days (range, 9-20 days) for patients who received filgrastim compared with 15 days (range, 10-22 days) for patients receiving placebo (P = .0082). The median time to a platelet count greater than 20 × 106/mL and transfusion independence was 13 days (range, 8-35 days) for patients who received filgrastim compared with 15.5 days (range, 8-42 days) for patients receiving placebo (P = .79). There was no significant difference (P = .84) in platelet transfusion requirements between the groups (Table 3). The median time to RBC transfusion independence was 35 days (range, 6-124 days) for patients who received filgrastim compared with 30 days (range, 10-103 days) for patients receiving placebo (P = .93). There was no significant difference (P = .94) in RBC transfusion requirements between the groups (Table 3).
Patient outcomes
| Outcome . | Filgrastim . | Placebo . | P Value . |
|---|---|---|---|
| RBC transfusions | |||
| Median | 6 | 6 | .94 |
| Range | 0-52 | 0-50 | |
| Platelet transfusions | |||
| Median | 7.5 | 8.0 | .84 |
| Range | 1-78 | 3-50 | |
| 100-d survival | 73% | 83% | .50 |
| GVHD | |||
| Acute | (n = 25) | (n = 23) | |
| Overall | 68% | 78% | .58 |
| Grade II-IV | 48% | 61% | .41 |
| Grade III-IV | 30% | 12% | .11 |
| Chronic | (n = 20) | (n = 19) | |
| Overall | 58% | 45% | .53 |
| Extensive | 21% | 35% | .48 |
| Outcome . | Filgrastim . | Placebo . | P Value . |
|---|---|---|---|
| RBC transfusions | |||
| Median | 6 | 6 | .94 |
| Range | 0-52 | 0-50 | |
| Platelet transfusions | |||
| Median | 7.5 | 8.0 | .84 |
| Range | 1-78 | 3-50 | |
| 100-d survival | 73% | 83% | .50 |
| GVHD | |||
| Acute | (n = 25) | (n = 23) | |
| Overall | 68% | 78% | .58 |
| Grade II-IV | 48% | 61% | .41 |
| Grade III-IV | 30% | 12% | .11 |
| Chronic | (n = 20) | (n = 19) | |
| Overall | 58% | 45% | .53 |
| Extensive | 21% | 35% | .48 |
RBC indicates red blood cell; GVHD, graft-versus-host disease.
Although the differences in the numbers of CD34+ cells and CFU-GM did not differ statistically between the groups, the patient outcomes were compared relative to the number of CD34+cells they received. After adjustment for CD34 dose, patients who received filgrastim had faster recovery of an ANC greater than 0.5 × 109/L than patients who received placebo (P = .016).
GVHD
Forty-eight patients were evaluable for acute GVHD (Table 3). The overall incidence of acute GVHD was 68% for patients who received filgrastim compared with 78% for patients receiving placebo (P = .58). The incidence of grade II-IV acute GVHD was 48% and 61%, respectively, for patients receiving filgrastim compared with placebo (P = .41). The incidence of grade III-IV acute GVHD was 30% and 12%, respectively, for patients receiving filgrastim compared with placebo (P = .11).
Thirty-nine patients were evaluable for chronic GVHD (Table3). The median follow-up of these patients was 15.3 months. The overall incidence of chronic GVHD was 58% for patients who received filgrastim compared with 45% for patients receiving placebo (P = .53). There was no discernible difference in the incidence of extensive chronic GVHD between the groups (P = .48).
One-hundred-day and overall survival
The 100-day survival for patients who received filgrastim was 73%, compared with 83% for patients receiving placebo. The primary causes of death within the first 100 days for the patients who received filgrastim included multiorgan failure in 3 patients, andStaphylococcus infection, Pseudomonas infection, acute respiratory distress syndrome, and pulmonary failure in the other 4 patients, respectively. The primary causes of death within the first 100 days for the patients who received placebo were Aspergilluspneumonia, cerebrovascular bleeding, liver failure, and sepsis.
At a median follow-up of 15.3 months, the overall survival rates for patients who received filgrastim and patients who received placebo were 65% and 58%, respectively (P = .58). Only 1 relapse has been observed in the 2 groups.
Discussion
The administration of hematopoietic growth factors following either autologous or allogeneic bone marrow transplantation is known to improve the rate of neutrophil recovery.18-20,26 However, some investigators have hypothesized that the administration of growth factors following transplantation of mobilized blood stem cells may be unnecessary and even detrimental to platelet recovery, depending on the number of stem and progenitor cells infused.8,16,22,27 In addition, there have been concerns that the administration of growth factors following allogeneic bone marrow transplantation may increase the incidence of acute GVHD.20 21
The results of this prospective, randomized trial demonstrated that administration of filgrastim following alloSCT significantly improved the recovery rate of neutrophils. This beneficial effect was observed even though 5 patients in the placebo group eventually received filgrastim. Filgrastim administration did not have a detrimental effect on the rate of platelet recovery. Platelet recovery was more rapid in the patients who received filgrastim, but the difference did not reach statistical significance. The improvement in neutrophil recovery remained when analyzed relative to the dose of progenitor cells the patient received. Bensinger et al16 have suggested that administration of growth factors after transplantation has little to no effect on hematopoietic recovery of either neutrophils or platelets in the autologous SCT setting with progenitor cell doses in excess of 5 × 106 CD34+/kg. When the progenitor cell dose was analyzed in this trial, positive effects of growth factor administration were seen for neutrophils at all dose levels, but the beneficial effects declined at higher progenitor cell doses. Progenitor cell dose did not appear to have a significant effect on platelet recovery.
In this trial, filgrastim administration was initiated on the same day as stem cell infusion. Whether this is the optimal timing for growth factor administration in the allogeneic setting is uncertain.28,29 There has been concern regarding administration of growth factors during the same period that patients are receiving methotrexate for the prevention of GVHD. Growth factor–mediated proliferation of stem and progenitor cells during methotrexate administration could delay hematopoietic recovery or result in graft failure. This trial found no evidence of adverse effects of growth factor administration on the rate of hematopoietic recovery, engraftment, or the incidence or severity of acute GVHD. A beneficial effect of growth factor administration on hematopoietic recovery may not be observed in patients who do not receive methotrexate as part of their GVHD prophylaxis. Conversely, the initiation of filgrastim on the day of stem cell infusion may have beneficial immunomodulatory effects on acute GVHD.30Whether delaying filgrastim administration until a later time after transplantation for either economic or biologic reasons would have beneficial or detrimental effects requires another randomized trial.
Five of the 6 patients whose treatment assignments were unblinded for either documented or suspected infection were receiving placebo. These data may suggest that the administration of filgrastim could have delayed or prevented infection, but infection was not a primary end point of this trial, and the numbers are too small to make definitive conclusions on this observation.
Despite previous reports of an increase in acute GVHD in patients undergoing allogeneic bone marrow transplantation who received hematopoietic growth factors after transplantation, this was not observed in this trial. However, in this trial all patients received mobilized blood as their sole stem cell source, and the trial was not adequately powered to address this issue specifically. In other published trials on alloSCT that used mobilized blood as the stem cell source and used growth factors after transplantation, an increase in acute GVHD has not been observed.9,31,32 This may reflect the observation that T cells in blood mobilized with filgrastim have a bias toward a T2 rather than a T1 phenotype.33 The transplantation of larger numbers and percentages of T2 cells has been associated with decreased acute GVHD in both murine models and human studies.34-36 Whether the administration of filgrastim after transplantation has an effect on T-cell phenotype and on GVHD has yet to be determined.
The administration of filgrastim after transplantation had minimal effect on treatment-related morbidity and mortality within the first 100 days of transplantation. Although most patients recovered 0.5 × 109/L neutrophils within 2 weeks of transplantation, discharge was delayed until resolution of mucositis and the ability to take medications and adequate nutrition by mouth. Similarly, although the median follow-up was only 15 months, long-term survival was not significantly different between the groups. Relapse rates, which had been a concern with the use of growth factors in patients with hematologic malignancies, particularly leukemias, were extremely low in both groups.
The administration of filgrastim following allogeneic blood SCT resulted in more rapid recovery of neutrophils than placebo. This treatment was well tolerated without significant effects on platelet recovery or acute GVHD. The precise and optimal timing of filgrastim administration after transplantation remains an important question, as well as the effect of filgrastim on chronic GVHD and immune reconstitution. These data suggest that filgrastim can be administered safely after transplantation and can be considered as an effective supportive-care measure for allogeneic SCT.
Acknowledgment
We thank Ms MaryAnn Foote for her valuable editorial assistance.
Reprints:Michael R. Bishop, Department of Experimental Transplantation and Immunology, National Cancer Institute, Medicine Branch, Bethesda, MD 20892; e-mail: mbishopmail.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal