Abstract
Tissue factor (TF) is the cellular receptor for factor FVIIa (FVIIa), and the complex is the principal initiator of blood coagulation. The effects of FVIIa binding to TF on cell migration and signal transduction of human fibroblasts, which express high amounts of TF, were studied. Fibroblasts incubated with FVIIa migrated toward a concentration gradient of PDGF-BB at approximately 100 times lower concentration than do fibroblasts not ligated with FVIIa. Anti-TF antibodies inhibited the increase in chemotaxis induced by FVIIa/TF. Moreover, a pronounced suppression of chemotaxis induced by PDGF-BB was observed with active site-inhibited FVIIa (FFR-FVIIa). The possibility that hyperchemotaxis was induced by a putative generation of FXa and thrombin activity was excluded. FVIIa/TF did not induce increased levels of PDGF β-receptors on the cell surface. Thus, the hyperchemotaxis was not a result of this mechanism. FVIIa induced the production of inositol-1,4,5-trisphosphate to the same extent as PDGF-BB; the effects of FVIIa and PDGF-BB were additive. FFR-FVIIa did not induce any release of inositol-1,4,5,-trisphosphate. Thus, binding of catalytically active FVIIa to TF can, independent of coagulation, modulate cellular responses, such as chemotaxis.
Introduction
Tissue factor (TF) is a transmembrane glycoprotein with sequence homology to the class II cytokine/hematopoietic growth factor receptor family that includes receptors for interferon-α, -β, and -γ and interleukin-10.1,2 It can mediate the activation of coagulation factor VII to its activated form, FVIIa.3 The FVIIa/TF complex is the principal initiator of the blood coagulation cascade through the activation of factor IX and factor X.3,4 Aberrant expression also suggests a central role for TF in thrombosis and inflammation in septicemia, cancer, metastasis, and atherosclerosis (reviewed in Østerud5 and Semeraro and Colucci6). Recently, a role of TF as a true receptor involved in signal transduction was identified. Rottinger et al7 showed that 200 nmol/L FVIIa induced Ca++oscillations in approximately 30% of human umbilical vein endothelial cells pretreated with IL-1-β to express TF and in almost 100% of MDCK cells with high constitutive expression of TF. In a subsequent study, FVIIa and FXa induced oscillations in the concentration of intracellular free calcium.8 Moreover, FVIIa/TF was shown to induce the phosphorylation of mitogen-activated protein kinases (MAPK) in cells transfected with TF, and the active site of FVIIa was found to be mandatory for the response.9,10 Up-regulation of the egr-1 gene was shown also to be induced by FVIIa and FXa.10,11 Other studies have provided evidence that TF functions in tumor cell metastasis by not yet well-defined mechanisms.12,13 However, Ott et al14recently identified actin-binding protein 280 (ABP-280) as a ligand for the TF cytoplasmic domain, providing a molecular pathway by which TF may support tumor cell metastasis. The molecular signals and the biologic functions transduced by FVIIa/TF are, however, still poorly understood.
Human fibroblasts have a constitutive expression of TF.1These cells also express receptors for platelet-derived growth factor (PDGF).15,16 PDGF induces in its target cells mitogenicity, actin reorganization, and directed cell migration (chemotaxis) (for review, see Heldin and Westermark15). We have previously shown that PDGF-BB is an efficient chemotactic factor for human fibroblasts and that the chemotactic response is mediated by the β-receptor class.17 Therefore, these cells were chosen to study putative signal transduction and cell migration induced by binding FVIIa to TF.
In this article we show for the first time a clear connection between the signaling induced by FVIIa binding to TF and the cellular response to a growth factor. We present data that in human fibroblasts the FVIIa/TF complex leads to a hyperchemotactic response to PDGF-BB.
Materials and methods
Cell cultures
Human foreskin fibroblasts AG1518 and AG1523 were grown to confluence in Eagle minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS). Before use, the cells were detached by trypsinization (2.5 mg/mL for 10 minutes at 37°C), washed in Hanks balanced salt solution, and resuspended in EMEM with 10% FBS or in Ham's medium supplemented with 0.1% FBS. Two human vascular smooth muscle cell-lines, aortic A-5720 and coronary artery CA-5050 (Clonetics; Bio Whittaker, Walkersville, MD), were grown to confluence in SmGM-2 supplemented with 5% FBS. The cells were treated as the fibroblasts but resuspended in SmGM-2 with 5% FBS for chemotaxis. For analysis of TF antigen expression, subconfluent and confluent cultures of AG 1518, AG 1523, A-5720, and CA-5050 were also serum-starved overnight (approximately 20 hours) in medium containing 0.1% FBS.
Proteins
Human FVIIa (NovoNordisk A/S, Gentofte, Denmark), was expressed and purified as described.18 FFR-FVIIa (NovoNordisk) was obtained by blocking of FVIIa in the active site with D-Phe-L-Phe-L-Arg chloroethyl ketone.19 Recombinant Tick anticoagulant peptide (TAP) was kindly provided by Dr P. Vlasuk, Corvas (San Diego, CA). Hirudin was purchased from Sigma (St. Louis, MO). Human thrombin and human FXa were from Enzyme Research Laboratory (South Bend, IN). Anti-TF monoclonal antibodies, TF8-5G9, TF9-5B7, and MTFH-1,20 were a kind gift of Dr James H. Morrissey, Oklahoma Medical Research Foundation. The anti-phosphotyrosine antibody, PY99, was from Santa Cruz Technology (Santa Cruz, CA).
Flow cytometry
The surface expression of TF was analyzed by immunofluorescence with a flow cytometer (Coulter Epics XL-MCL; Beckman Coulter, Fullerton, CA). The instrument was calibrated daily with Flow Check calibration beads (Coulter). For indirect immunofluorescence experiments, AG1518 or AG1523 fibroblasts and human vascular smooth muscle cells were washed twice with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin, incubated for 30 minutes on ice with a fluorescein isothiocyanate (FITC)-labeled anti-human TF monoclonal antibody (4508CJ; American Diagnostica, Greenwich, CT). The anti-Aspergillus niger glucose oxidase monoclonal IgG1 (Dakopatts) was used as a negative control. Mean channel fluorescence intensity and percentage of positive cells were determined for each sample.
Determination of TF activity
The procoagulant activity of TF was determined as described by Lindmark et al.21 Briefly, aliquots containing 0.2 × 105 AG1518 or AG1523 fibroblasts were washed twice with PBS and placed in the wells of a 96-well microtiter plate (Nunc, Roskilde, Denmark). The procoagulant activity was measured in a 2-stage amidolytic assay in which a chromogenic substrate, S-2222 (Chromogenix, Mölndal, Sweden), is cleaved by FXa, which in turn is activated from FX by the FVIIa/TF complex. A reaction mixture containing final concentrations of 0.6 mmol/L S-2222, 2 mmol/L CaCl2, and coagulation factors from the factor concentrate Prothromplex-T TIM4 (Baxter, Vienna, Austria) at a final concentration of 1 U/mL FVII and 1.2 U/mL FX was added to the wells, and change in absorbance at 405 nm after a 30-minute incubation at 37°C was determined. Measurements were performed in triplicate.
Chemotaxis assay
The migration response of fibroblasts and vascular smooth muscle cells (SMCs) was assayed by means of the leading front technique in a modified Boyden chamber, as previously described.17 22 Nitrocellulose filters (pore size, 8 μm) were coated with a solution of type 1 collagen at room temperature overnight. The filters were air-dried for 30 minutes immediately before use. Human foreskin fibroblasts AG1523 were grown to confluence in EMEM supplemented with 10% FBS. The cells were detached by trypsinization (2.5 mg/mL for 10 minutes at 37°C) and suspended in EMEM with 10% FBS. The fibroblasts were incubated for 10 minutes with or without FVIIa, FFR-FVIIa, Fxa, or thrombin before assay. SMC were grown in SmGM-2 with 5% FBS, resuspended in the same medium, and incubated for 10 minutes with FVIIa or FFR-FVIIa before assay. One hundred microliters of the cell suspension (2 × 105 cells/mL) was added above the filter of the Boyden chamber. PDGF-BB was diluted in assay media (EMEM with 10% FBS or SmGM-2 with 5% FBS) and added below the filter in the chamber. The cells were incubated for 6 hours at 37°C in a humidified chamber containing 95% air/5% CO2. FVIIa, FFR-FVIIa, FXa, or thrombin was present during the entire experiment. The filters were then removed, fixed in ethanol, stained with Mayer hemalum, and mounted. Migration was measured as the distance of the 2 farthest migrating fibroblast nuclei of one high-power field (12.5 × 24) in focus. The migration distance in each filter was calculated as the mean of the readings of at least 3 different parts of the filter. Experiments were performed with 2 to 4 separate filters for each concentration of chemoattractant. For each set of experiments, the migration of cells toward the assay media served as control.
When anti-TF monoclonal antibodies or inhibitors to coagulation factors TAP and Hirudin, were used, cells were preincubated for 10 minutes with these agents, then with or without FVIIa, FFR-FVIIa, FXa, or thrombin before the chemotaxis assay was performed. Antibodies, TAP, or Hirudin were also present during the entire chemotaxis experiment.
Assay for release of inositol triphosphate
Six-well plates with subconfluent cultures of AG1518 human fibroblasts were incubated overnight (approximately 20 hours) with 2 μCi of myo-[3H]-inositol (Amersham, Buckinghamshire, UK) in 2 mL Ham F12 with 0.1% FBS. Medium was changed to Ham F12 with 0.1% FBS (containing 2 mmol/L CaCl2) and 20 mmol/L LiCl, and the cells were incubated for 15 minutes at 37°C. Cells were then incubated in the absence or presence of 100 nmol/L FVIIa or 100 nmol/L FFR-FVIIa for 1 hour. PDGF-BB (0, 10, or 100 ng/mL) was added, and the incubation was continued for 10 minutes at 37°C. The IP3 assay was performed as previously described by Eriksson et al.23
Assay for determination of PDGF β-receptors
Twelve-well plates with subconfluent cultures of AG 1518 were treated as described for IP3 release. After incubation for 6 hours in the absence or the presence of 100 nmol/L FVIIa, binding of sodium iodide 125I-PDGF-BB was measured according to Heldin et al.24
Assay for agonist-induced PLC-γ1 phosphorylation
Subconfluent cultures of AG1518 were serum-starved overnight (approximately 20 hours) in medium containing 0.1% FBS and then incubated in the absence or presence of 100 nmol/L FVIIa or FFR-FVIIa for 1 hour, followed by incubation with 0, 2, 10, or 100 ng/mL PDGF-BB for 5 minutes at 37°C. Cells were lysed, and PLC-γ1 was precipitated, essentially as previously described,16 with anti-PLC-γ1 antiserum generated by immunizing rabbits with a peptide corresponding to the carboxy terminus of bovine PLC-γ1.25 Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with the anti-phosphotyrosine antibody PY99.
Statistical analysis
Data were analyzed using the Statistica for Windows package (StatSoft, Tulsa, OK). The Student t test for dependent samples was used to determine statistical significance between different data sets. P < .05 was considered statistically significant.
Results
Effects of FVIIa and FFR-FVIIa on the chemotactic response of fibroblasts and vascular smooth muscle cells to PDGF-BB
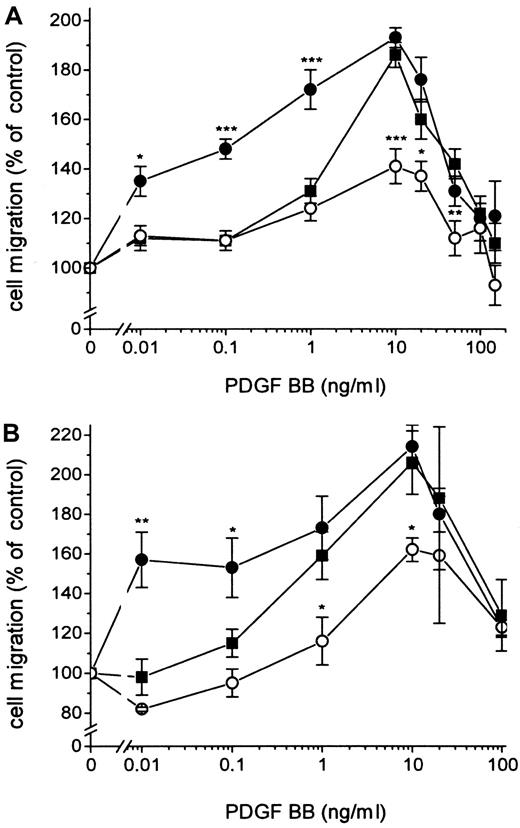
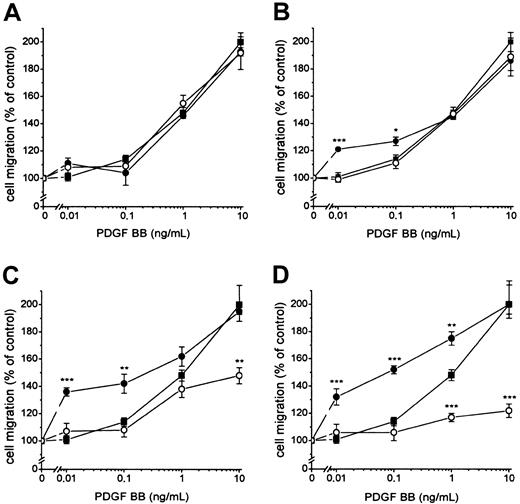
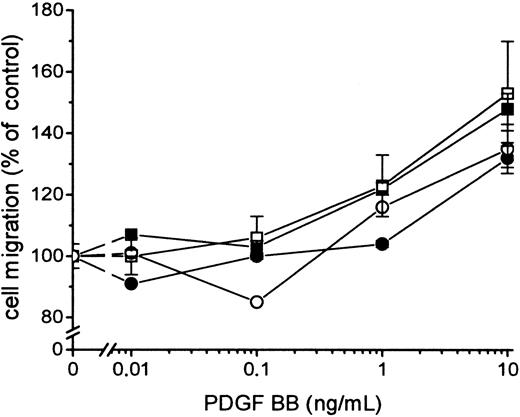
Fibroblasts expressing active TF (Figure1) were incubated with 100 nmol/L FVIIa and seeded in the upper part of the modified Boyden chamber; and media containing 10% FBS and PDGF-BB at different concentrations were added below the 150 μm micropore filter. The migration of the cells under conditions in which medium containing 10% FBS without PDGF-BB was added below the filter was used as a measure of random migration and was calculated as 100% migration. A significant migration response was recorded at a concentration of 0.01 ng/mL PDGF-BB in cells stimulated by FVIIa compared to 1 ng/mL PDGF-BB for cells not ligated with FVIIa (ie, a 100-fold difference in concentration) (Figure2A). At 0.01 to 0.1 ng/mL PDGF-BB, the migration response to FVIIa increased dose dependently, starting at 25 nmol/L with a maximal effect at 50 to 100 nmol/L FVIIa (Figure3A-D). No enhancement of random migration was observed after activation with FVIIa. To test whether the proteolytically active FVIIa was mandatory for the hyperchemotactic response to PDGF-BB, fibroblasts were also incubated with 100 nmol/L FFR-FVIIa and assayed in the Boyden chamber in the same way (Figure2A). No increased chemotaxis was observed with FFR-FVIIa at low concentrations of PDGF-BB, 0.01 to 1 ng/mL. In contrast, a pronounced suppression of chemotaxis induced by 10 to 50 ng/mL PDGF-BB was achieved by 100 nmol/L FFR-FVIIa (Figure 2A, 3A-D). To clarify whether these results are of a more general nature, migration experiments with 2 human vascular smooth muscle cell lines were performed under the same conditions. These cells have spontaneous expression of TF and PDGF β-receptors (analyzed by flow cytometry; data not shown). Incubation with 100 nmol/L FVIIa and FFR-FVIIa resulted in the same migration pattern (Figure 2B).
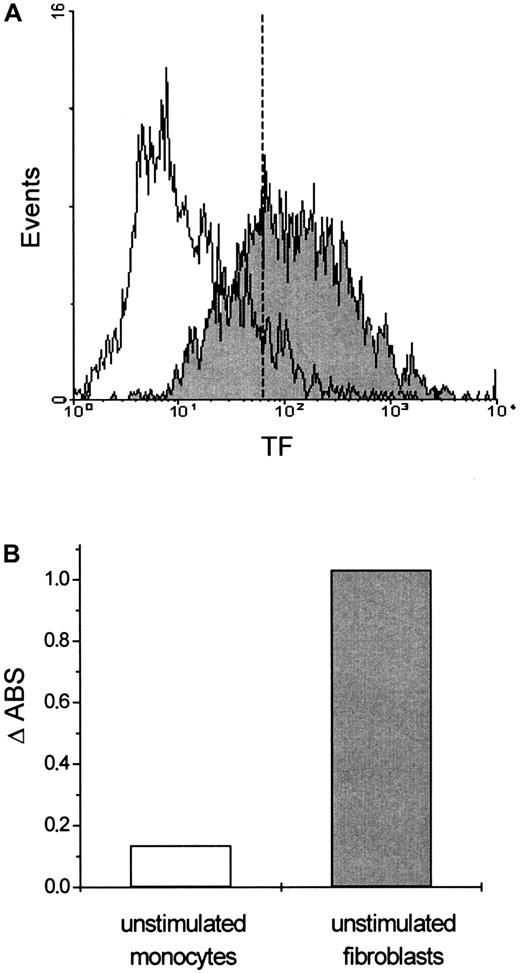
Flow cytometric analysis of TF expression in fibroblasts.
(A) Cells were stained with either a murine monoclonal FITC-conjugated mouse anti-IgG antibody (unfilled area) that was used as a negative control or a monoclonal FITC-conjugated anti-tissue factor (TF) antibody (filled area). Serum-starved cells expressed almost the same levels of TF (data not shown). (B) Procoagulant activity of fibroblasts. Fibroblasts with TF expression generated a 10-fold increase in PCA compared to monocytes without TF expression.
Flow cytometric analysis of TF expression in fibroblasts.
(A) Cells were stained with either a murine monoclonal FITC-conjugated mouse anti-IgG antibody (unfilled area) that was used as a negative control or a monoclonal FITC-conjugated anti-tissue factor (TF) antibody (filled area). Serum-starved cells expressed almost the same levels of TF (data not shown). (B) Procoagulant activity of fibroblasts. Fibroblasts with TF expression generated a 10-fold increase in PCA compared to monocytes without TF expression.
Effects of FVIIa and FFR-FVIIa on PDGF-BB–induced chemotaxis.
Effects of FVIIa and FFR-FVIIa on PDGF-BB–induced chemotaxis in human fibroblasts (A) and human aortic SMC (B). ▪ shows the chemotactic response of cells to different concentrations of PDGF-BB. Cells incubated with 100 nmol/L FVIIa (●) or 100 nmol/L FFR-FVIIa (○) migrated to different concentrations of PDGF-BB. Results are means± SEM of 3 separate experiments (A) or mean± SD of 2 separate experiments (B). *P < .05 was considered statistically significant (Student t test); **P < .01; ***P < .001. The same migration response to PDGF-BB was obtained with coronary artery SMC (data not shown).
Effects of FVIIa and FFR-FVIIa on PDGF-BB–induced chemotaxis.
Effects of FVIIa and FFR-FVIIa on PDGF-BB–induced chemotaxis in human fibroblasts (A) and human aortic SMC (B). ▪ shows the chemotactic response of cells to different concentrations of PDGF-BB. Cells incubated with 100 nmol/L FVIIa (●) or 100 nmol/L FFR-FVIIa (○) migrated to different concentrations of PDGF-BB. Results are means± SEM of 3 separate experiments (A) or mean± SD of 2 separate experiments (B). *P < .05 was considered statistically significant (Student t test); **P < .01; ***P < .001. The same migration response to PDGF-BB was obtained with coronary artery SMC (data not shown).
Influence of different concentrations of FVIIa or FFR-FVIIa on PDGF-BB–induced chemotaxis in fibroblasts.
▪ shows migration of fibroblasts to different concentrations of PDGF-BB alone. Cells were incubated with 12.5 (A), 25 (B), 50 (C) and 100 (D) nmol/L FVIIa (●) or FFR-FVIIa (○) and assayed in the Boyden chamber to different concentrations of PDGF-BB. Results are mean ± SEM of 3 different experiments. *P < .05; **P < .01; ***P < .001 (Studentt tests).
Influence of different concentrations of FVIIa or FFR-FVIIa on PDGF-BB–induced chemotaxis in fibroblasts.
▪ shows migration of fibroblasts to different concentrations of PDGF-BB alone. Cells were incubated with 12.5 (A), 25 (B), 50 (C) and 100 (D) nmol/L FVIIa (●) or FFR-FVIIa (○) and assayed in the Boyden chamber to different concentrations of PDGF-BB. Results are mean ± SEM of 3 different experiments. *P < .05; **P < .01; ***P < .001 (Studentt tests).
When fibroblasts were preincubated with a mixture of 3 different TF antibodies and then with FVIIa or FFR-FVIIa, the migration response to PDGF-BB was identical to the response of fibroblasts without the presence of ligand bonded to TF. An irrelevant monoclonal IgG antibody prevented neither hyperchemotaxis induced by FVIIa nor inhibition of the migration response induced by FFR-FVIIa (data not shown). The presence of the IgG antibodies or the 3 TF antibodies did not change the random migration of the fibroblasts (data not shown). To analyze whether the enhanced chemotaxis was a result of increased numbers of PDGF β-receptors induced by FVIIa/TF, binding of125I-PDGF-BB to fibroblasts, with or without stimulation with 100 nmol/L FVIIa, was measured. FVIIa/TF did not induce enhanced exposure of PDGF β-receptors (data not shown).
The hyperchemotactic response is not mediated by FXa or by thrombin
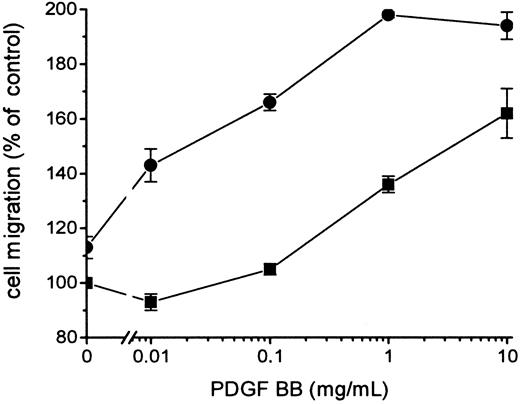
Because FVIIa-induced signal transduction leading to the hyperchemotactic response to PDGF-BB was dependent on the catalytic activity of FVIIa, it was important to determine whether signaling occurred directly or through FXa or thrombin generated by the FVIIa/TF complex. The enhanced migration response transduced by FVIIa/TF was not blocked by 0.2 to 10 μmol/L Tick anticoagulant peptide (TAP), which specifically blocks the active site of FXa and prevents a further activation of the coagulation cascade leading to thrombin formation (Figure 4). Addition of 5 U/mL Hirudin, a specific thrombin inhibitor, had no effect on FVIIa/TF-induced hyperchemotaxis (data not shown). Neither TAP (Figure 4) nor Hirudin (data not shown) influenced the migration of fibroblasts in response to PDGF-BB without the presence of the ligand FVIIa. Stimulation of the fibroblasts with 1 to 100 nmol/L FXa or 0.1 to 100 nmol/L thrombin in the absence or the presence of TAP or Hirudin, respectively, resulted in identical chemotaxis toward PDGF-BB, as recorded with fibroblasts without ligands (Figure 5). Thus, it is unlikely that the effect of FVIIa on chemotaxis is mediated by the activation of FXa or thrombin.
Influence of FXa on the chemotactic response to PDGF-BB induced by FVIIa.
Fibroblasts were preincubated with 200 nmol/L TAP (▪) and then with 100 nmol/L FVIIa (●). TAP was present at all times during the experiments. Chemotaxis was induced by different concentrations of PDGF-BB. Results are mean ± SD of 2 separate experiments. Increased concentrations of TAP, 0.2 to 10 μmol/L, gave the same results (data not shown).
Influence of FXa on the chemotactic response to PDGF-BB induced by FVIIa.
Fibroblasts were preincubated with 200 nmol/L TAP (▪) and then with 100 nmol/L FVIIa (●). TAP was present at all times during the experiments. Chemotaxis was induced by different concentrations of PDGF-BB. Results are mean ± SD of 2 separate experiments. Increased concentrations of TAP, 0.2 to 10 μmol/L, gave the same results (data not shown).
Effects of FXa and thrombin on PDGF-BB–induced chemotaxis in human fibroblasts.
Cells were preincubated with 100 nmol/L FXa in the absence (▪) or presence (■) of 200 nmol/L TAP and with 100 nmol/L thrombin in the absence (●) or presence (○) of 15 U/mL Hirudin. TAP and Hirudin were present at all times during the experiments. Chemotaxis was induced by different concentrations of PDGF-BB. Results are mean ± SEM of 3 separate experiments.
Effects of FXa and thrombin on PDGF-BB–induced chemotaxis in human fibroblasts.
Cells were preincubated with 100 nmol/L FXa in the absence (▪) or presence (■) of 200 nmol/L TAP and with 100 nmol/L thrombin in the absence (●) or presence (○) of 15 U/mL Hirudin. TAP and Hirudin were present at all times during the experiments. Chemotaxis was induced by different concentrations of PDGF-BB. Results are mean ± SEM of 3 separate experiments.
FVIIa/TF-induced activation of PLC
To investigate whether the FVIIa/TF-induced chemotactic response involved the activation of phosphatidylinositol-specific phospholipase C (PLC), we analyzed the direct effects of FVIIa/TF on PLC activity in fibroblasts. Activation of PLC leads to the production of 2 second messengers, inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. Fibroblasts were incubated withmyo-[3H]-inositol overnight and then with 100 nmol/L FVIIa or FFR-FVIIa for 60 minutes, followed by incubation with or without PDGF-BB at indicated concentrations. Treatment with 100 nmol/L FVIIa alone for 60 minutes induced IP3 release in fibroblasts at the same level as 10 ng/mL and 100 ng/mL PDGF-BB alone (Figure 6). Moreover, the combination of 100 nmol/L FVIIa and 10 ng/mL or 100 ng/mL PDGF-BB doubled the IP3 release. The active site-inhibited FVIIa did not induce the release of IP3. These results clearly show that PLC is activated on binding of FVIIa to TF.
Release of IP3 from fibroblasts stimulated with FVIIa, FFR-FVIIa alone, or FFR-FVIIa in combination with PDGF-BB.
Cells were labeled overnight withmyo-[3H]-inositol, incubated with or without 100 nmol/L FVIIa or FFR-FVIIa in the absence or presence of 10 ng/mL or 100 ng/mL PDGF-BB. Cells were then analyzed for release of IP3. Open bars show cells without FVIIa or FFR-FVIIa (control), hatched bars show cells with FFR-FVIIa, and filled bars show cells incubated with FVIIa.
Release of IP3 from fibroblasts stimulated with FVIIa, FFR-FVIIa alone, or FFR-FVIIa in combination with PDGF-BB.
Cells were labeled overnight withmyo-[3H]-inositol, incubated with or without 100 nmol/L FVIIa or FFR-FVIIa in the absence or presence of 10 ng/mL or 100 ng/mL PDGF-BB. Cells were then analyzed for release of IP3. Open bars show cells without FVIIa or FFR-FVIIa (control), hatched bars show cells with FFR-FVIIa, and filled bars show cells incubated with FVIIa.
Phosphorylation of PLC-γ1 is not enhanced by FVIIa/TF signaling in fibroblasts
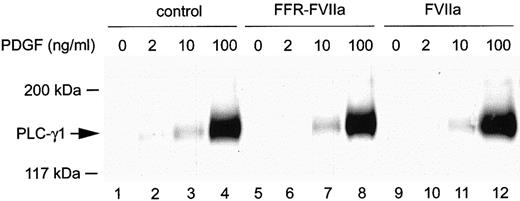
To determine whether the PLC-γ1 isoform, which is activated by certain tyrosine kinase receptors, was responsible for the increased PLC activity induced by FVIIa/TF, tyrosine phosphorylation of PLC-γ1 was studied. Fibroblasts were incubated in the absence or presence of 100 nmol/L FVIIa or FFR-FVIIa for 1 hour, followed by stimulation with 0, 2, 10, or 100 ng/mL PDGF-BB. After 5 minutes of incubation, the cells were lysed and PLC-γ1 was immunoprecipitated, separated by SDS-PAGE, and immunoblotted with anti-phosphotyrosine antibodies. Whereas a significant increase in tyrosine phosphorylation of PLC-γ1 was recorded with increasing concentrations of PDGF-BB, the addition of FVIIa alone to the fibroblasts did not induce any tyrosine phosphorylation of PLC-γ1 (Figure 7). Moreover, the combination of FVIIa and PDGF-BB at different concentrations did not induce any further phosphorylation compared to stimulation with PDGF-BB alone (Figure 7). FFR-FVIIa had no effect on PLC-γ1 tyrosine phosphorylation (Figure 7). Thus, PLC isoforms other than PLC-γ1 are responsible for the increased PLC activity after FVIIa stimulation.
Tyrosine phosphorylation of PLC-γ1 in response to PDGF-BB alone (control), FVIIa, or FFR-FVIIa in combination with PDGF-BB.
Cells were incubated with 100 nmol/L FVIIa or FFR-FVIIa for 1 hour and then with or without PDGF-BB at indicated concentrations. Cell were lysed, and tyrosine phosphorylation of PLC-γ1 was detected as described in “Materials and methods.”
Tyrosine phosphorylation of PLC-γ1 in response to PDGF-BB alone (control), FVIIa, or FFR-FVIIa in combination with PDGF-BB.
Cells were incubated with 100 nmol/L FVIIa or FFR-FVIIa for 1 hour and then with or without PDGF-BB at indicated concentrations. Cell were lysed, and tyrosine phosphorylation of PLC-γ1 was detected as described in “Materials and methods.”
Discussion
Tissue factor is constitutively expressed on the plasma membrane of many extravascular cells, such as stromal fibroblasts in vascular adventitia and in fibrous capsules of liver, spleen, and kidney.1 Thus, the expression of TF is found at sites physically separated from the circulating blood providing a hemostatic envelope. With injury this barrier is thought to protect the organism against bleeding. TF can, however, be induced in monocytes/macrophages, vascular smooth muscle cells, endothelial cells, and tumor cells by various agents, including cytokines and growth factors.1 Induction at the transcriptional level occurs rapidly after stimulation, identifying TF as a growth-related immediate-early gene.26 Data from studies in which the TF gene was inactivated in mice demonstrated that the deficiency of TF results in embryonic lethality because of the defective development of blood vessels.27-29 Insufficient accumulation and differentiation of peri-endothelial mesenchymal cells to pericytes/primitive smooth muscle cells occurred in the TF-deficient embryos.29
A role for TF in tumor angiogenesis has also been proposed.30,31 However, contradictory results—with no essential role for TF in embryonic vascular development but the requirement for uterine hemostasis—have also been presented.32,33 Very recent studies10,11,34clearly demonstrate the up-regulation of expression of certain genes by the FVIIa/TF complex. Responders include genes coding for growth factors, such as connective tissue growth factor, and proteins involved in cellular reorganization and migration.11 34 The results indicate an active role of FVIIa/TF in wound healing. Thus, accumulating data clearly suggest that TF is involved not only in coagulation but also in several cellular responses, such as cell migration.
In this study we have investigated the role of TF as a signaling receptor. We show that human fibroblasts with a constitutive expression of TF on ligand binding of FVIIa migrate toward extremely low concentrations of PDGF-BB. FVIIa/TF alone did not induce enhanced spontaneous migration (ie, random migration). Thus, a combination of intracellular signal transduction by FVIIa/TF and the growth factor PDGF-BB was necessary to achieve the motility response. Not only was binding to TF mandatory, but so was the catalytic activity of FVIIa/TF because active site-inhibited FVIIa did not elicit an enhanced migration response. Furthermore, inhibitory monoclonal antibodies prevented enhancement of the chemotactic response by FVIIa. We also excluded that indirect signaling occurred as a result of FXa or thrombin because TAP and hirudin had no effect on FVIIa/TF-induced chemotaxis. Moreover, FXa and thrombin themselves did not enhance the migration response to PDGF-BB. We instead found that increasing concentrations of FFR-FVIIa actively inhibited PDGF-BB–induced chemotaxis. Fibroblasts incubated with FFR-FVIIa showed completely normal random migration. The inhibitory effect of FFR-FVIIa on PDGF-BB–induced chemotaxis was not observed in the presence of the combination of anti-TF antibodies, thereby ruling out the possibility that FFR-FVIIa was toxic. The results suggest, rather, that in cells expressing PDGF β-receptors and TF, the FVIIa/TF complex is of importance for the chemotactic response to PDGF-BB. Our observations on the effect of FFR-FVIIa on migration may have a bearing on the findings recently reported by Mueller and Ruf35 with respect to cancer cell metastasis; they found that increasing doses FFR-FVIIa to TF-expressing cells resulted in a nearly complete inhibition of metastasis in SCID mice.
A chemoattractant property of FVIIa/TF complexes in solution has previously been demonstrated.36 37 In these experiments, aortic smooth muscle cells migrate toward a concentration gradient of 0.3 nmol/L FVIIa/TF complexes and thereby exclude signaling events transduced by the TF–intracellular domain.
Our finding that FVIIa increases IP3 production, and the previously reported data on FVIIa/TF-induced Ca++oscillations, especially in MDCK cells, strongly support the notion that PLC is activated by FVIIa/TF signaling in a number of cells.7,8 We previously found a similar hyperchemotactic response to PDGF-BB in PDGF β-receptor Y934F mutant cells that showed increased phosphorylation and activation of PLC-γ1.38 In these cells, the enhanced phosphorylation of PLC-γ1 correlated with a 3-fold higher IP3 production rate than wild-type PDGF β-expressing cells.39 The combination of FVIIa/TF and PDGF-BB induced an approximately 2-fold increase in IP3production in human fibroblasts. FVIIa/TF-induced IP3production, however, did not correlate with phosphorylation of PLC-γ1. Tyrosine phosphorylation of PLC-γ2 induced by FVIIa/TF cannot be excluded, but it seems unlikely because the expression of PLC-γ2 is low in human fibroblasts. Moreover, the intracellular part of TF is not endowed with intrinsic protein tyrosine kinase activity. These results suggest that FVIIa/TF induces the activation of β or δ PLC isozymes. We cannot fully exclude that the PLC activation is mediated by FXa or by thrombin. In the assay for IP3release, the cell culture medium was supplemented with 0.1% FBS containing only approximately 0.1 nmol/L FXa. We found that a concentration of more than 20 nmol/L FXa is necessary to induce IP3 production (data not shown). The mechanism by which β or δ PLC isozymes are activated remains to be elucidated. Because the protease activity is mandatory for FVIIa/TF signaling, it has been hypothesized that activation involves cooperation with the protease activated receptor 2 (PAR2).40,41 So far, however, conflicting results have been shown. Petersen et al40present results that show intracellular activity induced by FVIIa does not involve any of the known PARs. In contrast, a recent study using coexpression of TF and PAR2 suggests that PAR2 may be activated directly by FVIIa/TF and indirectly by FVIIa/TF-generated FXa.41
Lately, the connection between TF and the cytoskeleton was identified.14,42 A molecular interaction between the cytoplasmic domain of TF and the actin filament-binding protein ABP-280 was shown.14 Furthermore, TF was found to be in close contact with actin and actin filament-binding proteins, such as α-actinin and ABP-280 in lamellipodia and ruffled membrane areas in spreading epithelial cells.42 ABP-280, a member of the filamen subfamily, is required for the normal function of lamellipodia; thus, it is highly important for cell motility.43PI3-kinase and PLC isozymes are implicated in chemotactic responses, such as mobilization of actin-binding proteins.43-46 In previous studies38 39 we observed that the PI3-kinase pathway in PDGF-β receptor-induced chemotaxis seems less important in cells with overexpression and enhanced activity of PLC-γ. This was also the case for cells with FVIIa bonded to TF. Taken together, our data strongly support the idea that cell migration is an important morphogenic function induced by FVIIa/TF signaling. A cellular migration response is probably mediated in cooperation with different chemotactic factors.
Chemotaxis plays a pivotal role in wound healing, angiogenesis, and metastasis. Chemotaxis is also an important component in the development of atherosclerotic plaques. In these processes various cells express TF, PDGF, and PDGF receptors. Restenosis is a major complication after interventional procedures to open obstructed arteries. PDGF has been implicated in vessel wall response (neointima formation) to mechanical injury by mediating the migration and proliferation of smooth muscle cells and fibroblasts. We have shown now, for the first time, that FVIIa binding to TF-expressing cells includes an increased chemotactic response to PDGF independent of coagulation.
This finding can explain the efficacy of blocking FVIIa/TF activity in reducing neointima formation in animal models of restenosis.47 In addition to limiting thrombin generation, thrombus formation, and the cellular events caused by thrombin signaling, the inhibition of FVIIa/TF would locally inhibit the migration of TF-expressing cells at the site of injury. Our observations suggest that the inhibition of FVIIa may become clinically important for regulating these events.
Acknowledgment
We thank Dr Lars C. Petersen (Novo Nordisk A/S) for fruitful discussions.
Supported by the Swedish Cancer Society and the Swedish Medical Research Council.
A.S. and L.R. are Senior Researchers supported by the Swedish Medical Research Council.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Agneta Siegbahn, Department of Medical Sciences, Laboratory for Coagulation Research, Clinical Chemistry, University Hospital, SE-751 85 Uppsala, Sweden; e-mail:agneta.siegbahn@klinkem.uas.lul.se.

![Fig. 6. Release of IP3 from fibroblasts stimulated with FVIIa, FFR-FVIIa alone, or FFR-FVIIa in combination with PDGF-BB. / Cells were labeled overnight withmyo-[3H]-inositol, incubated with or without 100 nmol/L FVIIa or FFR-FVIIa in the absence or presence of 10 ng/mL or 100 ng/mL PDGF-BB. Cells were then analyzed for release of IP3. Open bars show cells without FVIIa or FFR-FVIIa (control), hatched bars show cells with FFR-FVIIa, and filled bars show cells incubated with FVIIa.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/10/10.1182_blood.v96.10.3452/5/m_h82200358006.jpeg?Expires=1769082131&Signature=bufonzchVp9GQnn51bohdI13V9ENofrZSIHPui2yHH2tjVrHO9~-KdSTqwsKusGcYB2KwdYrqMPDm9zxWz7JDdgEQlC~b28qMCCaBD4ZT98VOsdpRWfiRcq8cYLWtZqO4N-245KSLoPN5UXqp53CSdS6lY0wtzi8pImSYUANvb2y5hos2QPkV7SrmogaF727efggHEyzAPrzO7HBJKZrOYuMzGzJyqCrjJQygTyBrvAg6jWvnPYuf-pb1hui4jc~~yXkAcO3BPFPVLBXbnL7PAL4XjEsoUNBC75~xsI8YhLBkL7WA~jlEG9dBDPCxa5SZihU34Q~DkkL234cDWtRbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal