Abstract
Dendritic cells (DCs) may arise from multiple lineages and progress through a series of intermediate stages until fully mature, at which time they are capable of optimal antigen presentation and T-cell activation. High cell surface expression of CD83 is presumed to correlate with full maturation of DCs, and a number of agents have been shown to increase CD83 expression on DCs. We hypothesized that interleukin 12 (IL-12) expression would be a more accurate marker of functionally mature DCs capable of activating antigen-specific T cells. We used combinations of signaling through CD40, using CD40 ligand trimer (CD40L), and interferon gamma to demonstrate that CD83 expression is necessary but not sufficient for optimal production of IL-12 by DCs. Phenotypically mature DCs could be induced to produce high levels of IL-12 p70 only when provided 2 simultaneous stimulatory signals. By intracellular cytokine detection, we determined that only a subset of cells that express high levels of CD80 and CD83 generate large amounts of IL-12. DCs matured with both signals are superior to DCs stimulated with the individual agents in activating antigen-specific T cell in vitro. These findings have important implications regarding the identification, characterization, and clinical application of functionally mature DCs.
Introduction
Dendritic cells (DCs) play a central role in humoral and cellular immunity because of their ability to take up and process antigen in peripheral tissues and present the antigen to T cells in secondary lymphoid tissues, such as lymph nodes. Potent stimulation of T cells occurs after DC maturation, which has been commonly associated with high levels of CD80 and CD83 expression.1 A number of cellular signals, including cytokines such as tumor necrosis factor alpha (TNF-α), have been shown to lead to high levels of CD83 expression.1-3 In addition, other agents such as bacterial lipopolysaccharide (LPS) and monocyte-conditioned medium have been shown to lead to high levels of CD80 and CD83 expression.4-6 Finally, interactions with other cells may have a role in maturation and activation of DCs, as some signals for DC maturation, such as CD40 ligation by CD40L, are mediated by T cells.7
Mature DCs are thought to be functionally competent and have been used in clinical studies to induce antigen-specific T cells.8,9Nonetheless, not all mature DCs may be optimized to induce antigen-specific T-cell responses. It has been shown that some mature DCs may also stimulate T helper-1 cells by secreting IL-12.10-12 IL-12 has been shown to enhance the antigen-specific CD8+ T-cell response to a peptide antigen in a murine model.13 Because of the important role IL-12 has in activating T cells, we hypothesized that expression of IL-12 would be a more specific marker of functionally activated dendritic cells. We demonstrated that IL-12 is expressed by only a subset of phenotypically mature DCs in response to combinations of signals commonly used to mature DCs.
Materials and methods
Generation and maturation of dendritic cells
After provision of written informed consent, healthy human donors underwent leukapheresis according to an institutional review board–approved protocol. Peripheral blood mononuclear cells (PBMCs) were isolated by gradient centrifugation over Ficoll-Hypaque Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden). DCs were generated from PBMCs by culturing plastic-adherent cells at 37°C in an incubator with 5% humidified CO2 in AIM V (GibcoBRL, Grand Island, NY) medium, supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 800 U/mL; Schering-Plough, Kennilworth, NJ) and IL-4 500 U/mL (Schering-Plough) for 7 days. Cells were harvested by using enzyme-free dissociation buffer (GibcoBRL) and were used immediately or were cryopreserved in autologous plasma with 10% dimethylsulfoxide for later use. Cells were rapidly thawed in cold AIM V medium and were plated in 24-well tissue culture plates (Costar, Corning, NY) at 0.5 to 1 million cells/mL in AIM V alone or AIM V supplemented with: (1) tumor necrosis factor alpha (TNF-α) (100 ng/mL; Endogen, Woburn, MA); (2) soluble CD40 ligand trimer (1 μg/mL; Immunex, Seattle, WA); (3) Interferon-γ 1b (IFN-γ, Actimmune; 1000 U/mL; InterMune, Palo Alto, CA); (4) lipopolysaccharide (100 ng/mL; Sigma Chemicals, St Louis, MO); or combinations thereof, as described in the “Results” section.
Interleukin-12 enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were performed on 24-hour culture supernatants using the Quantikine IL-12 HS ELISA kit (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Intracellular interleukin-12 analysis
After periods ranging from 9 to 24 hours in the various cytokine-supplemented media, 10 μg/mL brefeldin A (Sigma) was added to each well to inhibit cytokine secretion. DCs were incubated for 4 to 7 hours, then harvested using cell dissociation buffer, and placed on ice until all samples were ready for centrifugation. Cells were washed with 5 mL Dulbecco phosphate-buffered saline (PBS; GibcoBRL) and were fixed with 1% formaldehyde and 1% bovine serum albumin (BSA; Sigma) in PBS for 10 minutes at room temperature (RT). Cells were then permeabilized with 0.5% saponin (Sigma) in PBS for 20 minutes at 37°C, followed by vortexing. Samples were washed and stained with a cocktail of fluorophor-conjugated monoclonal antibodies, according to the manufacturer's instructions. Antibodies included anti-CD80, anti–CD83-FITC (Immunotech, Marseille, France), or anti-PE (Becton Dickinson, San Jose, CA); anti-CD14-PerCP (Becton Dickinson); anti-CD11c-FITC (DAKO, Carpenteria, CA) or anti-APC (Becton Dickinson); and anti-IL-12 (p40/p70)-PE, or anti-APC (Pharmingen, San Diego, CA). Isotype controls were used to define regions so as to include only cells brighter than approximately 99% of those in the isotype control. Samples were incubated at RT for 30 minutes, protected from light. Samples were washed twice and analyzed with a FACSCalibur (Becton Dickinson) flow cytometer using Cellquest software (Becton Dickinson). Data were analyzed by gating on large CD11c+/CD14− cells (dendritic cells); 1 to 5 × 104 gated events were analyzed for each sample.
Cell lines
Induction of antigen-specific primary cytotoxic T-cell responses in vitro
Nonadherent PBMCs were used as responder cells and were resuspended in complete RPMI (RPMI with 10% FCS, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, 5 × 10−5 mol/L β-mercaptoethanol) at 2 × 106 cells/mL. Cells were cocultured with peptide-pulsed DCs at a responder:stimulator ratio of 10:1 in 10 mL of complete RPMI and 10 ng/mL IL-7 (Genzyme, Cambridge, MA). Tumor antigen peptide sequences included the immunodominant epitope of carcinoembryonic antigen (CEA), Cap-1 (YLSGANLNL16); and a Her2/neu immunogenic sequence, HER2(9369) (KIFGSLAFL17). IL-2 was added on day 3 at a concentration of 20 U/mL (Genzyme). Fresh medium was added every 5 days. Viable cells were harvested on day 12, and CD8+ T cells were isolated using CD8 microbeads (Miltenyi Biotech, Sunnyvale, CA) as per the manufacturer's protocol. The captured CD8+ T cells were cultured in 10 mL complete RPMI and 20 U/mL IL-2 at 37°C. Two days later the CD8+ T-cell blasts were harvested and restimulated with peptide-pulsed DCs. CD8+ T cells were maintained at 5 × 105 cells/mL in complete RPMI and 10 ng/mL IL-7 and 20 U/mL IL-2. Cytotoxic T lymphocyte (CTL) assays were performed 5 days after restimulation.
In vitro cytotoxicity assay
The 10 × 106 target cells were labeled with europium for 20 minutes at 4°C. The 1 × 104europium-labeled targets and serial dilutions of effector cells at varying effector:target (E:T) ratios were incubated in 200 μL of complete RPMI 1640. The plates were centrifuged at 500 × g for 3 minutes and incubated at 37°C for 4 hours. Fifty microliters of the supernatant was harvested, and europium release was measured by time-resolved fluorescence. Specific cytotolytic activity was determined using the formula: % specific release = [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. Spontaneous release of the target cells was less than 20% of total release by detergent.
Graphical and statistical analysis
Microsoft Excel (Microsoft Corporation, Redmond, WA) was used for graphical and statistical analysis of data where applicable. Flow cytometric data were analyzed using the paired Studentt test with a Bonferroni correction. P < .05 was considered statistically significant.
Results
CD40L, IFN-γ, and lipopolysaccharide synergistically induce the production of IL-12 by monocyte-derived dendritic cells. Previous work has shown that the combination of CD40L and IFN-γ induces the production of high levels of IL-12 by dendritic cells.18We compared the level of the functional IL-12 p70 heterodimer produced by DCs treated with the combination of CD40L plus IFN-γ to a known maturation agent, TNF-α. We performed an IL-12 p70 ELISA on 24-hour culture supernatants from DCs treated with each of several agents or combinations of agents. In the absence of added cytokines, or with IFN-γ or TNF-α treatment alone, minimal (less than 1 pg/mL) IL-12 p70 heterodimer was detected, and either CD40L or LPS treatment alone resulted in less than 100 pg/mL IL-12 p70 (Figure1A). In contrast, the combination of CD40L and IFN-γ resulted in the secretion of a substantial quantity of IL-12 p70, which was further augmented by the addition of LPS to CD40L and IFN-γ.
CD40L, IFN-γ, and LPS have synergistic effects on IL-12 p40 and p70 secretion by monocyte-derived dendritic cells.
(A) 24-hour culture supernatants from DCs treated with several maturation agents, including the combination of CD40L and IFN-γ, were examined by ELISA specific for IL-12 p70. The graph shows the mean of triplicate values ± SD. Incubation of cells in medium alone, or with IFN-γ or TNF-α alone, resulted in the production of less than 1 pg/mL IL-12 p70. Data are representative of 2 experiments in different donors. (B) After sampling of culture supernatants for ELISA, brefeldin A was added, and the incubation was continued for another 4 hours. Cells were then harvested, fixed, permeabilized, and stained with α-CD11c-FITC, α-CD14-PerCP, and α-IL-12(p40/p70)-PE. 25 000 large, CD11c+/CD14− cells were analyzed by flow cytometry and are represented in the scatter diagrams shown. The indicated percentage represents the frequency of IL-12+cells. R3 denotes the region containing IL-12+ DCs. Data are representative of 5 experiments with similar results.
CD40L, IFN-γ, and LPS have synergistic effects on IL-12 p40 and p70 secretion by monocyte-derived dendritic cells.
(A) 24-hour culture supernatants from DCs treated with several maturation agents, including the combination of CD40L and IFN-γ, were examined by ELISA specific for IL-12 p70. The graph shows the mean of triplicate values ± SD. Incubation of cells in medium alone, or with IFN-γ or TNF-α alone, resulted in the production of less than 1 pg/mL IL-12 p70. Data are representative of 2 experiments in different donors. (B) After sampling of culture supernatants for ELISA, brefeldin A was added, and the incubation was continued for another 4 hours. Cells were then harvested, fixed, permeabilized, and stained with α-CD11c-FITC, α-CD14-PerCP, and α-IL-12(p40/p70)-PE. 25 000 large, CD11c+/CD14− cells were analyzed by flow cytometry and are represented in the scatter diagrams shown. The indicated percentage represents the frequency of IL-12+cells. R3 denotes the region containing IL-12+ DCs. Data are representative of 5 experiments with similar results.
We next examined IL-12 expression by intracellular cytokine detection. Because we were able to detect intracellular IL-12 production using a p40/p70-specific, but not a p70-specific antibody (data not shown), and because p40 is typically produced in excess of p70,19 20we reasoned that IL-12 detected with the p40/p70 antibody may reflect predominantly intracellular p40 levels. Therefore, to address whether intracellular IL-12 (p40) is associated with bulk IL-12 p70 production, we performed intracellular IL-12 analysis on the same DCs examined in the experiment described previously. As shown in Figure 1B, the relative percentages of DCs expressing IL-12, as determined by intracellular cytokine detection, parallels the IL-12 p70 ELISA results: Combined CD40L and IFN-γ treatment resulted in a much higher frequency of IL-12–producing cells than the individual agents, and the addition of LPS increased the percentage of IL-12–producing DCs even further. The experiment was repeated with each condition represented at least 4 times, and in a total of 5 donors; the mean percentage (± SD) of IL-12–producing DCs was 3.53% ± 2.36% after CD40L/IFN-γ treatment (n = 10). Treatment with CD40L/IFN-γ resulted in a significantly greater percentage of IL-12–producing DCs compared with no cytokine (0.13% ± 0.15%, n = 11, P < .0005), CD40L alone (0.34% ± 0.30%, n = 5, P < .05) or IFN-γ alone (0.12% ± 0.13%, n = 5, P < .05).
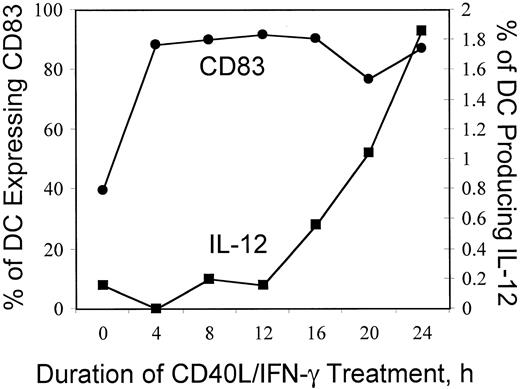
Intracellular IL-12 expression by mature, activated dendritic cells follows CD83 expression and increases over a 24-hour period. We observed that incubation of DCs with the combination of CD40L and IFN-γ induces IL-12 p40 production by a small percentage of cells. Therefore, to test whether IL-12 production is a transient phenomenon, we followed the time course of intracellular IL-12 p40 expression. As shown in Figure 2, CD83 expression begins to rise shortly after treatment is initiated, and within 4 hours, close to 90% of cell are CD83+. In contrast, the frequency of IL-12 expressing DCs increases more slowly and continues to rise for at least 24 hours of treatment with CD40L and IFN-γ. These results indicate that the small percentage of IL-12 p40-producing DCs that we detected does not reflect a transient process that abates rapidly (within hours) of the addition of cytokines.
Time course of intracellular IL-12 expression by mature, activated dendritic cells.
DCs prepared and cryopreserved as described previously were thawed and plated in AIM V medium alone or with the combination of CD40L (1 μg/mL) and IFN-γ (1000 U/mL) for the indicated period, and brefeldin A was added for the last 4 hours. Cells were harvested, fixed, permeabilized, and then stained with α-CD14 PerCP, α-CD11c-APC, α-CD83-FITC, and α-IL-12(p40/p70)-PE and were analyzed with a FACSCalibur multiparameter flow cytometer. Approximately 10 000 CD11c+ large cells were analyzed. The percentage of CD83-expressing DCs (●, left y-axis) and the associated percentage of IL-12–producing DCs (▪, righty-axis) are plotted versus the duration of treatment with CD40L and IFN-γ (x-axis).
Time course of intracellular IL-12 expression by mature, activated dendritic cells.
DCs prepared and cryopreserved as described previously were thawed and plated in AIM V medium alone or with the combination of CD40L (1 μg/mL) and IFN-γ (1000 U/mL) for the indicated period, and brefeldin A was added for the last 4 hours. Cells were harvested, fixed, permeabilized, and then stained with α-CD14 PerCP, α-CD11c-APC, α-CD83-FITC, and α-IL-12(p40/p70)-PE and were analyzed with a FACSCalibur multiparameter flow cytometer. Approximately 10 000 CD11c+ large cells were analyzed. The percentage of CD83-expressing DCs (●, left y-axis) and the associated percentage of IL-12–producing DCs (▪, righty-axis) are plotted versus the duration of treatment with CD40L and IFN-γ (x-axis).
CD40L/IFN-γ induces IL-12 production specifically from CD80+CD83+ dendritic cells. Because flow cytometric detection of cytokine production allows the characterization of individual cytokine-producing cells, we were able to explore the relationship of IL-12 production to the expression of markers of DC maturation. Figure 3 demonstrates the effect of cytokine treatment of DCs on CD80 and CD83 expression (y-axes) and IL-12 expression (x-axis). The IL-12–expressing cells have high levels of CD80 and CD83. After combined treatment with CD40L plus IFN-γ, the mean fluorescence intensity of CD80 for IL-12+ versus IL-12−cells was 24.3 versus 13.1, respectively. This relationship was similar for CD83 (93.2 versus 49.3), as well as several other relevant cell-surface markers, such as CD40, CD58, CD86, and CXCR-4 (data not shown). Hence, IL-12 p40 appears to be produced specifically by DCs with a mature phenotype, based on cell-surface markers. In addition, not all CD83+ cells produce IL-12 p40, indicating that CD83 expression is sensitive, but not specific for identifying the cells capable of IL-12 production.
CD40L/IFN-γ induces IL-12 production specifically from CD80-bright and CD83-bright dendritic cells.
Monocyte-derived DCs were thawed and plated in 1 mL AIM V medium with no added cytokine, or with 1 μg/mL CD40 ligand (CD40L) alone, 1000 U/mL IFN-γ alone, or both 1 μg/mL CD40L and 1000 U/mL IFN-γ. Cells were incubated for 12 hours, 10 μg/mL brefeldin A was added, and incubation was continued for another 7 hours before cell harvest, fixation, and permeabilization. Cells were stained with α-CD11c-FITC, α-CD80 or α-83-PE, α-CD14-PerCP, and α-IL-12-APC. The fluorescence intensity of DCs stained with isotype control antibodies (used to construct regions) is shown in the upper panel. For the remaining scatter diagrams, 25 000 large, CD11c+/CD14− cells analyzed by flow cytometry are shown, and the indicated percentage represents the frequency of CD83+IL-12+ DCs. R3 and R4 denote the regions containing IL-12− and IL-12+ DCs; they are emboldened in the first graph of each set for the sake of clarity. The CD80 and CD83 data are representative of 4 and 11 experiments with similar results, respectively.
CD40L/IFN-γ induces IL-12 production specifically from CD80-bright and CD83-bright dendritic cells.
Monocyte-derived DCs were thawed and plated in 1 mL AIM V medium with no added cytokine, or with 1 μg/mL CD40 ligand (CD40L) alone, 1000 U/mL IFN-γ alone, or both 1 μg/mL CD40L and 1000 U/mL IFN-γ. Cells were incubated for 12 hours, 10 μg/mL brefeldin A was added, and incubation was continued for another 7 hours before cell harvest, fixation, and permeabilization. Cells were stained with α-CD11c-FITC, α-CD80 or α-83-PE, α-CD14-PerCP, and α-IL-12-APC. The fluorescence intensity of DCs stained with isotype control antibodies (used to construct regions) is shown in the upper panel. For the remaining scatter diagrams, 25 000 large, CD11c+/CD14− cells analyzed by flow cytometry are shown, and the indicated percentage represents the frequency of CD83+IL-12+ DCs. R3 and R4 denote the regions containing IL-12− and IL-12+ DCs; they are emboldened in the first graph of each set for the sake of clarity. The CD80 and CD83 data are representative of 4 and 11 experiments with similar results, respectively.
CD40L/IFN-γ–induced production of IL-12 by dendritic cells does not require the presence of T cells. The ability to generate IL-12–producing DCs by the methods used above might be highly variable and fraught by poor reproducibility among laboratories if the maturation process were dependent on accessory cells, such as T lymphocytes. It has been shown previously by using blocking antibodies that the ability of T-helper cells to induce IL-12 production stems from their production of IFN-γ.18 We hypothesized therefore that sorting dendritic cells from contaminating accessory cells, such as lymphocytes, within a DC preparation would not abrogate the ability of DCs to produce IL-12 in response to combined CD40L and IFN-γ treatment. To address this question, we sorted CD11c+/CD14− (DCs) from a monocyte-derived DC preparation. Unsorted or sorted (large, CD11c+/CD14−) cells were then plated in AIM V medium with or without CD40L plus IFN-γ. Intracellular cytokine analysis showed that the sorted DCs remain responsive to CD40L and IFN-γ and produce high levels of IL-12 (4.55% versus 3.07% of large, CD11c+/CD14− cells; Figure4).
CD40L/IFN-γ induced production of IL-12 by dendritic cells occurs independently of T cells.
A preparation of DCs was stained with fluorophor-conjugated monoclonal antibodies against CD11c and CD14; unsorted or CD11c+/CD14− sorted cells were then plated in AIM V medium alone or with the combination of CD40L (1 μg/mL) and IFN-γ (1000 U/mL). After 24 hours, brefeldin A was added, and after 4 more hours cells were harvested, fixed, permeabilized, and stained with α-CD11c-FITC, α-CD83-PE, α-CD14-PerCP, and α-IL-12-APC. The fluorescence intensity of DCs stained with isotype control antibodies (used to construct regions) is shown in the upper panel. The number in the lower right corner of each panel indicates the percentage of IL-12+ DCs. (R3 is the region label generated during data analysis.) Data are representative of 2 experiments with similar results.
CD40L/IFN-γ induced production of IL-12 by dendritic cells occurs independently of T cells.
A preparation of DCs was stained with fluorophor-conjugated monoclonal antibodies against CD11c and CD14; unsorted or CD11c+/CD14− sorted cells were then plated in AIM V medium alone or with the combination of CD40L (1 μg/mL) and IFN-γ (1000 U/mL). After 24 hours, brefeldin A was added, and after 4 more hours cells were harvested, fixed, permeabilized, and stained with α-CD11c-FITC, α-CD83-PE, α-CD14-PerCP, and α-IL-12-APC. The fluorescence intensity of DCs stained with isotype control antibodies (used to construct regions) is shown in the upper panel. The number in the lower right corner of each panel indicates the percentage of IL-12+ DCs. (R3 is the region label generated during data analysis.) Data are representative of 2 experiments with similar results.
Dendritic cells treated with the combination of CD40L and IFN-γ are more potent antigen-presenting cells in vitro than those treated with either cytokine alone. We next wanted to establish whether human DCs stimulated to express both CD83 and IL-12 were more potent antigen-presenting cells (APCs) than those expressing only CD83. We explored this question using the HLA-A2–restricted tumor antigen peptide (Cap-1) that we are currently using in cancer vaccine trials. DCs were treated for 24 hours without added cytokines or with CD40L alone, IFN-γ alone, CD40L plus IFN-γ, or GM-CSF plus IL-4. Each preparation of DCs was then pulsed with CEA peptide and was used to stimulate PBMCs to generate cytotoxic T cells. After 2 cycles of in vitro stimulation, cytotoxic T-cell activity was assessed by microcytotoxicity using T2 cells pulsed with the indicated peptide as target cells. As shown in Figure 5, DCs incubated with CD40L plus IFN-γ were more potent inducers of cytolytic activity than DCs incubated without added cytokines or DCs treated with either CD40L or IFN-γ alone. These data are consistent with the interpretation that IL-12 production, rather than CD83 expression alone, is a specific marker of mature DCs with optimal antigen-presenting activity.
Stimulatory activity of DCs pulsed with CEA peptide after treatment with various cytokines.
DCs, either untreated or treated with various cytokine combinations, were pulsed with CEA peptide (Cap-1) or Her2/neu peptide (each at 25 μg/mL) for 2 hours at 37°C in the presence 3 μg of β2-microglobulin. DCs were washed and were used as stimulators for autologous nonadherent PBMCs. Cells were cultured in the presence of IL-7 (10 ng/mL) and IL-2 (20 U/mL) for 12 days, followed by isolation of CD8+ T cells. The CD8+ T cells were restimulated with the corresponding DCs in the presence of IL-7 and IL-2. The CTL assay was performed 5 days after restimulation. T2 cells pulsed with CEA peptide or Her2/neu peptide were used as targets. Data are expressed as the mean percentage specific lysis of triplicate samples ± SD.
Stimulatory activity of DCs pulsed with CEA peptide after treatment with various cytokines.
DCs, either untreated or treated with various cytokine combinations, were pulsed with CEA peptide (Cap-1) or Her2/neu peptide (each at 25 μg/mL) for 2 hours at 37°C in the presence 3 μg of β2-microglobulin. DCs were washed and were used as stimulators for autologous nonadherent PBMCs. Cells were cultured in the presence of IL-7 (10 ng/mL) and IL-2 (20 U/mL) for 12 days, followed by isolation of CD8+ T cells. The CD8+ T cells were restimulated with the corresponding DCs in the presence of IL-7 and IL-2. The CTL assay was performed 5 days after restimulation. T2 cells pulsed with CEA peptide or Her2/neu peptide were used as targets. Data are expressed as the mean percentage specific lysis of triplicate samples ± SD.
Discussion
The data presented in this work show that intracellular IL-12 p40 expression is associated with phenotypically and functionally mature DCs. We also showed that not all CD83+ DCs produce IL-12 or are functionally mature. The use of intracellular cytokine detection demonstrated that the combination of CD40L and IFN-γ induces a greater quantity of IL-12 than does either cytokine alone. Interestingly, agents commonly considered maturation factors (such as TNF-α) do not necessarily induce IL-12 secretion even though they are known to augment CD80 and CD83 expression.
We observed that only a minority of CD83+ DCs produced significant amounts of IL-12 after CD40L and IFN-γ treatment. As shown in Figure 2, CD83 expression increases earlier than that of IL-12. In addition, a significant, though variable degree of CD83 expression occurs after plastic adherence of DCs in AIM V alone overnight. Therefore, CD83 is necessary, but not sufficient for IL-12 production by DCs, and intracellular IL-12 p40 appears to be a more reliable marker of functionally mature DCs than CD83.
CD40L alone induces a variably small amount of IL-12 p70 production by ex vivo monocyte-derived DCs, as well as a very low percentage of IL-12 p40 positive DCs; this contrasts with the substantially greater quantity of IL-12 p70 and p40 production induced by treatment with the combination of CD40 ligand plus IFN-γ (Figure1). Although under certain conditions CD40L alone can induce IL-12 secretion, the low level of IL-12 secretion in response to soluble CD40L trimer alone is well supported in the literature. Studies by Cella et al21 showed that CD40L-transfected J558L cells induce human DCs to produce IL-12 p40 and p70, but the contributions of other cell-cell interactions are unknown. Vieira et al22have shown that the combination of soluble CD40L trimer and LPS does not increase IL-12 p70 production above that induced by LPS alone. Finally, Snijders et al18 detected a large quantity of IL-12 p40 secretion in response to CD40L treatment alone, but IL-12 p70 secretion was undetectable. Factors that may explain the variable results among laboratories and among experiments include differences among donors and among DC preparations, the particular cytokines and media used, and whether p40 or p70 is measured. Despite this variability, however, a consistent finding is that the combination of IFN-γ and CD40L enhances IL-12 production compared with CD40L alone.18 23
An important issue is whether further optimization or the addition of other signals would increase IL-12 production above that observed after treatment with CD40L and IFN-γ. We showed that an additional signal, mediated by LPS in this study, could further increase the frequency of IL-12–producing DCs. The LPS-induced augmentation of IL-12 production by DCs treated with CD40L and IFN-γ was initially surprising, however, because the activity of LPS in monocytes is primarily mediated through CD14,24,25whereas the DCs in our study were CD14−. This finding may be explained by CD14-independent effects of LPS.26-29 In addition, other factors may further increase the frequency of IL-12–producing DCs, such as optimal cell density, the composition of the substratum (and degree of cell attachment), and the presence or concentration of protein present in the medium (P.J.M., unpublished data, June 2000). Therefore, further optimization of maturation conditions is possible, and we are currently exploring ways to maximize the percentage of functionally mature cells in a DC preparation for use in DC-based immunotherapy trials.
Although we used a p40/p70-specific mononclonal antibody in the intracellular cytokine experiments, we believe that we primarily detected IL-12 p40 because similar experiments using a p70-specific antibody failed to detect p70 expression (P.J.M., unpublished data, December 1999). The active IL-12 heterodimer, p70, consists of 2 subunits, p40 and p35. Studies have shown that large quantities of p40 may be generated in the absence of substantial amounts of p70.30,31 It is unclear how closely the production of p40 correlates with the production of p70 in human DCs in vivo, but the relationship is dictated at least in part by the cytokine milieu in vitro.21 IL-12 p70-specific ELISA on culture supernatants performed in this work showed that the expression of p40 was associated with that of p70. IL-12 p40-producing cells also express maturation markers consistent with functionally mature DCs. Furthermore, increased intracellular IL-12 p40 expression was also associated with the ability to induce potent cytolytic T-cell activity in vitro. The precise mechanism of this enhanced APC function is unclear, but may be mediated directly by IL-1232; by another cytokine, such as IL-1523 or IL-1833; by the up-regulation of certain cell-surface molecules; or by a combination of factors. Identifying all the important factors that mediate potent APC function is an area of intense interest in several laboratories, including our own, and exploring these signals should be facilitated by a reliable marker of functionally mature DCs, such as intracellular IL-12 p40 expression.
The implications of this work also may impact DC-based immunotherapy trials. IL-12 production by intracellular cytokine staining may be a more reliable criterion on which to base assessments of DC function in the context of these trials. The hypothesis that functionally mature human DCs will serve as more potent inducers of antigen-specific cytolytic activity in vivo can then be tested. Of note, although IL-12 production was produced only by a small fraction of the DCs in this study, there was a dramatic effect on the ability to induce cytolytic T-cell activity in vitro. Furthermore, because DCs exposed to the appropriate signals (such as the combination of CD40L and IFN-γ) express CD83 before producing IL-12, many CD83+IL-12− DCs may eventually produce IL-12 after administration to the patient and homing to the appropriate site in vivo. The ability to monitor the relationship of DC function to the expression of IL-12 of individual cells should facilitate progress toward identifying the optimal maturation state and critical gene products associated with DCs that are clinically effective in human immunotherapy trials.
Acknowledgments
We acknowledge the technical assistance of Mike Cook, Ian Cumming, Eva Fisher, Michelle St. Peter, and Rhonda Williams.
Supported by NIH NRSA CA77894-02 and the Ethicon-Society of University Surgeons fellowship award (P.J.M.); M.A.M. is a recipient of an American Society of Clinical Oncology Career Development Award and is supported by NIH grant M01RR00030. A.C.H, T.M.C, S.K.N., and H.K.L. are supported by NIH P01 CA 78673 01A1.
E.K.T. is employed by and has significant financial interest in Immunex Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Kim Lyerly, Departments of General and Thoracic Surgery, Pathology, and Immunology, Center for Genetic and Cellular Therapies, Duke University Medical Center, Durham, NC 27710; e-mail: k.lyerly@cgct.duke.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal