Abstract
Interleukin 6 (IL-6), the major growth factor for myeloma cells, signals through the activation of signal transducers and activators of transcription (STAT) proteins. An important step in the malignant progression of murine plasmacytomas is the transition from dependence on IL-6 to a state of IL-6 independence. To elucidate the mechanism whereby IL-6 independence occurs, intracellular signaling events elicited by IL-6 in both IL-6–dependent and –independent plasmacytomas and hybridomas were compared. It was found that STAT3, a key molecule involved in IL-6 signaling, was constitutively activated and phosphorylated in IL-6–independent cell lines compared to the IL-6–dependent cells. Further comparison of upstream signaling pathways revealed that JAK-1 was constitutively present in anti-phosphotyrosine immunoprecipitates of IL-6–independent cells; gp130 was constitutively phosphorylated in a subset of IL-6–independent plasmacytomas, whereas other IL-6–independent lines showed no detectable gp130 phosphorylation in the absence of exogenous IL-6. Secretion of a factor capable of supporting the growth of IL-6–dependent cells was observed in one of the IL-6–independent plasmacytomas, but not in others, making an autocrine mechanism an unlikely explanation for IL-6 independence. These findings provide evidence that the constitutive activation of STAT3, either in the absence of detectable receptor-proximal events or associated with the concomitant activation of gp130, can contribute to the process of IL-6 independence.
Introduction
Interleukin 6 (IL-6) is a cytokine with pleiotropic effects.1-4 In addition to its activity in normal cellular response, IL-6 is associated with a number of pathologic processes, including polyclonal B-cell abnormalities and plasma cell neoplasia. The role of IL-6 in plasma cell neoplasia or myeloma was first described by using the mouse plasmacytoma model of myeloma.5 A striking aspect of plasmacytoma biology, priming dependence, provides an opportunity to gain insights into one of the steps of the multi-event process of carcinogenesis. In murine plasmacytomas the newly induced tumors failed to grow intraperitoneally or elsewhere when transplanted to normal (unprimed) syngeneic Balb/c AnN mice. If priming, in the form of the induction of a peritoneal oil granuloma, is first created within the host animal, the primary transplanted tumor cells readily grow and kill the host.6 7 After repeated serial transplantation in primed mice, it is possible to isolate tumors that are able to grow autonomously in unprimed mice. Therefore, for in vivo growth, early/primary plasmacytoma tumors require the microenvironment of the oil granuloma, whereas later transplant generations of the same tumor eventually progress to fully malignant phenotype able of autonomous growth in animals. Established IL-6–dependent plasmacytoma cell lines also require the microenvironment of an oil granuloma for growth when transplanted in vivo. This requirement, however, is not shared by established IL-6–independent plasmacytoma cell lines, which grow autonomously in vivo in the absence of the oil granuloma.
B-cell hybridomas, generated by the fusion of splenic B cells with a plasmacytoma cell line (eg, SP2/0), also depend on IL-6 for their initial growth. This cytokine, however, can generally be omitted from the culture medium on further expansion. The ease with which B-cell hybridomas can be grown in vitro in the absence of IL-6 suggests that the loss of IL-6 dependence occurs at high frequency in these cells. As do plasmacytoma lines, IL-6–independent hybridomas grow as tumors in vivo, whereas IL-6–dependent hybridomas do not give rise to tumor development when injected into mice unless IL-6 is provided.8 Therefore, B-cell hybridomas constitute an additional model for studying the role of IL-6 signal transduction in IL-6–mediated proliferation of plasma cells.
A mechanism by which tumor cells can escape growth factor dependence is the autocrine production of the growth factor; an autocrine loop may exist in some tumors.9 10 Other mechanisms, however, may account for growth factor independence. One such alternative is the constitutive activation of signaling intermediates.
On binding ligand, the gp130 component of the IL-6 receptor becomes tyrosine phosphorylated by Janus family kinases (JAK), which results in the binding, phosphorylation, and activation of signal transducers and activators of transcription (STAT) proteins.11,12Activated STAT proteins dimerize and translocate to the nucleus, where they bind to specific DNA response elements and induce the expression of STAT-regulated genes.13 In the case of IL-6 signaling, one STAT family member, STAT3 (also described as acute-phase response factor [APRF]), plays a critical role in activating the transcription of IL-6–responsive genes.14,15 Recent studies have shown that the constitutive activation of STAT proteins has been linked to cellular transformation with various oncoproteins.16-18Constitutive activation of STAT proteins occurs in cells transformed by oncogenic tyrosine kinases, such as Src or Lck.16,19 In IL-2–dependent cells, the constitutive activation of the JAK/STAT pathway has been shown to result in growth factor independence.20 These observations raised the possibility that the constitutive activation of any of these signaling events may play a role in the process of growth factor independence of plasmacytoma cells. Here we show that in IL-6–dependent murine plasmacytoma and hybridoma cell lines, gp130 phosphorylation was present in cells maintained in IL-6, whereas it was absent in cells withdrawn from IL-6. In IL-6–independent plasmacytoma–hybridoma lines, one subset showed gp130 constitutive phosphorylation, but in the other gp130 phosphorylation was not detected. STAT3, however, was constitutively phosphorylated in both subsets of autonomously growing plasmacytoma–hybridoma lines. Our findings provide evidence that constitutive phosphorylation and activation of STAT3 in mouse myeloma cells can contribute to IL-6 independence.
Materials and methods
Cell lines and cell culture
The IL-6–independent plasmacytomas MOPC31C and MOPC315 and the IL-6–independent hybridomas SP2/0, 7TD1ind, and B9ind were used in this study. 7TD1ind and B9ind were derived from the IL-6–dependent hybridomas, 7TD1 and B9, respectively. Other IL-6–dependent cell lines used were the plasmacytomas TEPC 1165 and TEPC 2027. All cells were grown in RPMI 1640 (BioWhittaker, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (BioWhittaker), 50 μmol/L 2-mercaptoethanol, and 10 μg/mL gentamicin. T1165 and T2027 cells were maintained with the addition to their culture medium of 100 plasmacytoma growth factor units of recombinant murine IL-6 purified by affinity chromatography21 and were titrated as previously described.5 IL-6 (4 U/mL) was added to the culture medium of B9 and 7TD1 cells.
Immunoprecipitation and Western blot analysis
IL-6–dependent cells were grown continuously in the presence of IL-6 or were withdrawn from IL-6 for 10 hours (T1165), 30 hours (T2027), or 48 hours (B9 and 7TD1) and were restimulated with 500 U/mL (T1165 and T2027) or 100 U/mL (B9 and 7TD1) of IL-6 for 20 minutes at 37°C. IL-6–independent cells were grown in the absence of exogenous growth factors. These cells were stimulated with IL-6 (100 U/mL) for 20 minutes or 24 hours at 37°C. Cells were washed twice with ice-cold phosphate-buffered saline containing 1 mmol/L sodium orthovanadate and lysed for 30 minutes on ice in 1 mL lysis buffer (50 mmol/L Tris-HCl, pH 7.5; 1% Triton X-100; 150 mmol/L NaCl; 1 mmol/L EDTA; 2 mmol/L sodium orthovanadate; 1 mmol/L phenylmethylsulfonyl fluoride; 1 mmol/L AEBSF; 5 μg/mL leupeptin; and 5 μg/mL aprotinin). Postnuclear extracts were obtained by centrifugation at 14 000 rpm for 30 minutes, and lysates were immunoprecipitated with anti gp130 antibody overnight at 4°C. Immunocomplexes were then precipitated with protein A/G beads (Pierce, Rockford, IL), washed 3 times with lysis buffer, and boiled in 2× SDS sample buffer for 5 minutes. Samples were analyzed by SDS-PAGE and immunoblotting with specific antibodies using an enhanced chemiluminescence detection system (Renaissance; New England Nuclear, Boston, MA). For STAT3 phosphorylation, crude cell lysates were run on SDS-PAGE and blotted with tyrosine-phosphorylated STAT3 antibodies (NEB, Beverly, MA).
For phosphotyrosine immunoprecipitation, cells were lysed in 50 mmol/L Tris-HCl, pH 7.8, 1% NP-40, 150 mmol/L NaCl, 1 mmol/L sodium molybdate, 1 mmol/L MgCl2, 10 mmol/L sodium β-glycerophosphate, 10% glycerol, 2 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L AEBSF, 5 μg/mL leupeptin, and 5 μg/mL aprotinin. Immunoprecipitation was performed by using polyclonal antiphosphotyrosine antibodies (UBI, Lake Placid, NY) at 4°C for 3 hours immobilized on protein A beads (Pierce). JAK-1 was detected using anti-JAK-1 monoclonal antibodies (Transduction Laboratories, Lexington, KY) using an enhanced chemiluminescence detection system (New England Nuclear).
Reverse transcription–polymerase chain reaction analysis
To detect mRNA for IL-6, CNTF, LIF, OSM, IL-11, and GAPDH, total RNA was isolated by using an RNA isolation kit (Qiagen, Santa Clara, CA). First-strand cDNA synthesis was performed with the Superscript Preamplification system (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instructions. Polymerase chain reaction (PCR) was performed in the presence of 1.5 mmol/L MgCl2, 200 μmol/L dNTP (GIBCO BRL), 0.2 μmol/L each of forward and reverse primers (synthesized by Cruachem, VA), and 2.5 U of Taq polymerase (Perkin Elmer, Foster City, CA). The sense and anti-sense oligonucleotide sequences and the expected size of the products for the above cytokines are as follows: IL-6, 5′-GTA CTC CAG AAG ACC AGA GG-3′ and 5′-TGC TGG TGA CAA CCA CGG C-3′ (306 bp); CNTF, 5′-TGG AGG TTC TCT TGG AGT CGC TCT G-3′ and 5′-GGC TAG CAA GGA AGA TTC GTT CAG A-3′ (169 bp); LIF, 5′-GCC ATT GAG CTG TGC CAG TTG-3′ and 5′-GAA AAC GGC CTG CAT CTA AGG-3′ (200 bp); OSM, 5′-CAA GGG GTG CTC TCG AGG CTA-3′ and 5′-CAG ACT GGC CGA CTT AGA-3′ (454 bp); IL-11, 5′-GAA GCC TTG TCA CCA CAC CAG GAA GCT GCA AA-3′ and 5′-GAC ATG AAC TGT GTT TGT CGC CTG GT-3′ (295 bp); and GAPDH, 5′-CTC AGT GTA GCC CAG GAT GC-3′ and 5′-ACC ACC ATG GAG AAG GCT GG-3′ (560 bp). Each PCR sample was cycled at 94°C for 1 minute, 65°C for 2 minutes, and 72°C for 3 minutes for 30 cycles, followed by a 10-minute final extension at 72°C. Each PCR reaction was carried out with appropriate negative and positive controls. The products were electrophoresed on 1.5% agarose gels containing ethidium bromide and were photographed.
Electrophoretic mobility shift assay
Nuclear extracts were prepared from IL-6–dependent cells withdrawn from IL-6 and subsequently restimulated for 20 minutes with IL-6, whereas the nuclear extracts for IL-6–independent cells were prepared from cells growing without IL-6 and stimulated with IL-6. Probes for gel shift assays were prepared from oligonucleotides containing junB enhancer sequences, which bind activated STAT3.22 Annealed oligonucleotides were 5′-end-labeled with [32P]-γadenosine triphosphate and T4 polynucleotide kinase and used in gel shift reactions performed with the Gel Shift System (Promega, Madison, WI) before electrophoresis on a 4% nondenaturing polyacrylamide gel. Supershift assays were performed by incubating the nuclear extracts with anti-STAT3 antibodies (a gift from A. C. Larner, Cleveland Clinic, Cleveland, OH).
Co-culture assay
The IL-6–dependent cell lines, B9 and T1165 (1 × 104/well), were cultured with medium alone or with 2 × 104 irradiated (8.0 Gy) MPC11, MOPC31C, MOPC315, or SP2/0 as feeder cells in a volume of 200 μL. Twenty-four hours (T1165 cells) or 72 hours (B9 cells) later, the cells were pulsed with [3H]-thymidine for 4 hours, and the radioactivity was incorporated into DNA determined by β-scintillation counting. MPC11 cells, which produce IL-6,23 were included as a positive control.
Results
Inducible and constitutive STAT3 tyrosine phosphorylation and activation in IL-6–dependent and –independent hybridomas and plasmacytomas
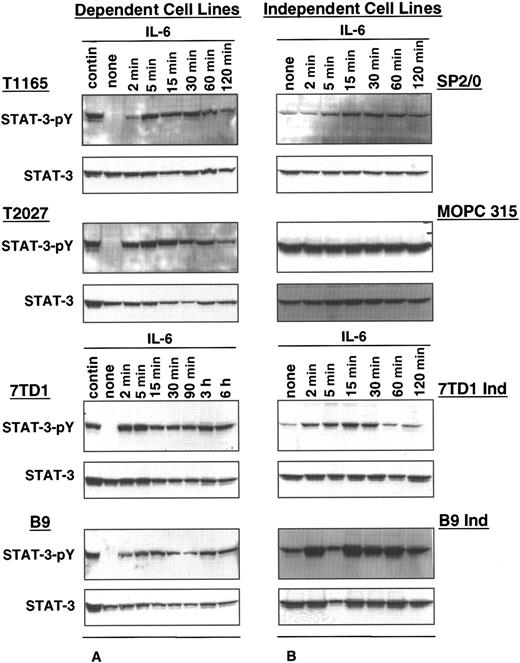
The status of STAT3 activation in IL-6–dependent and –independent cell lines was determined by comparing the phosphorylation levels of STAT3 in the absence or presence of exogenous IL-6. In IL-6–dependent cells, constitutive levels of STAT3 phosphorylation were observed in cells continuously maintained in IL-6, whereas phosphorylated STAT3 was undetectable in cells withdrawn from IL-6 for 10 to 48 hours (Figure 1A). When these cells were restimulated with IL-6, high levels of STAT3 phosphorylation were observed. STAT3 phosphorylation was detected within 2 minutes of restimulation, the earliest time point evaluated, and was increased gradually for up to 15 minutes. These levels were then maintained for approximately 2 hours after stimulation.
STAT3 phosphorylation in IL-6–dependent and –independent cell lines.
IL-6–dependent cells were cultured continuously in IL-6–containing medium or starved from IL-6 for 10 hours (T1165), 30 hours (T2027), or 48 hours (7TD1 and B9) and restimulated with IL-6 (500 U/mL for T1165 and T2027,100 U/mL for 7TD1 and B9) for the indicated times (A). IL-6–independent cells (SP2/0, MOPC315, 7TD1Ind, and B9Ind) were cultured continuously in medium without IL-6 or were stimulated with IL-6 (100 U/mL) for the indicated times (B). Crude cell lysates were resolved by 10% SDS-PAGE, and phosphorylation of STAT3 was detected by immunoblotting with an anti-phosphorylated STAT3 antibody (STAT3 pY). Blots were stripped and reprobed with anti-STAT3 antibody.
STAT3 phosphorylation in IL-6–dependent and –independent cell lines.
IL-6–dependent cells were cultured continuously in IL-6–containing medium or starved from IL-6 for 10 hours (T1165), 30 hours (T2027), or 48 hours (7TD1 and B9) and restimulated with IL-6 (500 U/mL for T1165 and T2027,100 U/mL for 7TD1 and B9) for the indicated times (A). IL-6–independent cells (SP2/0, MOPC315, 7TD1Ind, and B9Ind) were cultured continuously in medium without IL-6 or were stimulated with IL-6 (100 U/mL) for the indicated times (B). Crude cell lysates were resolved by 10% SDS-PAGE, and phosphorylation of STAT3 was detected by immunoblotting with an anti-phosphorylated STAT3 antibody (STAT3 pY). Blots were stripped and reprobed with anti-STAT3 antibody.
In contrast, all IL-6–independent cell lines displayed constitutively phosphorylated STAT3 in the absence of exogenous growth factor (Figure1B). Stimulation with IL-6 resulted in a further increase in STAT3 phosphorylation, suggesting that constitutive phosphorylation of STAT3 may be responsible for the IL-6 independence in these cell lines.
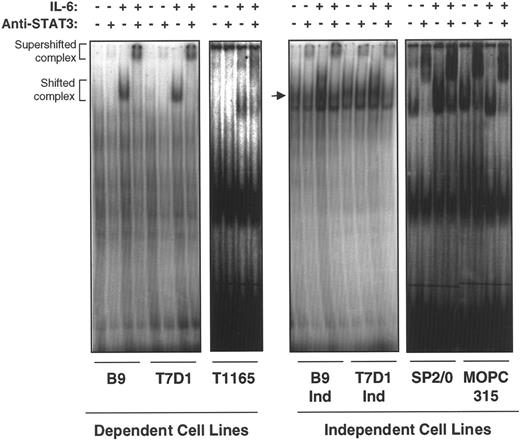
On phosphorylation and dimerization, STAT3 translocates to the nucleus where it binds to specific DNA response elements and induces the expression of STAT-regulated IL-6–responsive genes. To confirm the transcriptional activation status of STAT3 in IL-6–independent cells, nuclear extracts from IL-6–dependent and –independent cell lines were subjected to electrophoresis mobility shift assay (EMSA) by using a junB enhancer element containing the STAT3 binding site as a probe. All IL-6–independent cell lines tested (B9IND, 7TD1IND, SP2/0, and MOPC315) showed constitutive binding of STAT3 to a junBenhancer element in the absence of exogenous IL-6, though the addition of IL-6 could further enhance binding (Figure2). These complexes were super-shifted with anti-STAT3 antibodies, further confirming that they contained STAT3. In IL-6–dependent cell lines (T1165, B9, and 7TD1), shifted and anti-STAT3 super-shifted complexes were observed exclusively in the presence of IL-6. These results suggest that constitutive activation of STAT3 may be responsible for the proliferation of IL-6–independent plasmacytomas and hybridomas in the absence of exogenous growth factors.
IL-6–independent cells express constitutive STAT3 binding to a STAT3/APRF element.
Nuclear extracts were prepared from IL-6–dependent cells cultured in the absence of IL-6 for 10 hours (T1165) or 48 hours (7TD1 and B9) and restimulated with medium alone or IL-6 (100 U/mL) for 20 minutes. IL-6–independent cells were cultured continuously in medium without IL-6 or stimulated with IL-6 (100 U/mL) for 20 minutes. EMSA was performed with the extracts, and a probe was derived from the overlapping APRF/NF-κB site in the junB enhancer region.22 The probe, GGG CAG ATT CCGGGAATCGAG TCC CCC C, contains an APRF element (bold) that binds STAT3 and a mutation in the overlapping NF-κB site (underlined) to avoid interference with binding to the STAT3 site. The arrow in the second panel points to STAT3-specific band.
IL-6–independent cells express constitutive STAT3 binding to a STAT3/APRF element.
Nuclear extracts were prepared from IL-6–dependent cells cultured in the absence of IL-6 for 10 hours (T1165) or 48 hours (7TD1 and B9) and restimulated with medium alone or IL-6 (100 U/mL) for 20 minutes. IL-6–independent cells were cultured continuously in medium without IL-6 or stimulated with IL-6 (100 U/mL) for 20 minutes. EMSA was performed with the extracts, and a probe was derived from the overlapping APRF/NF-κB site in the junB enhancer region.22 The probe, GGG CAG ATT CCGGGAATCGAG TCC CCC C, contains an APRF element (bold) that binds STAT3 and a mutation in the overlapping NF-κB site (underlined) to avoid interference with binding to the STAT3 site. The arrow in the second panel points to STAT3-specific band.
JAK-1 activation status in IL-6–dependent and –independent hybridomas
Immunoprecipitation with anti-phosphotyrosine antibodies followed by immunoblotting with anti-JAK-1 antibodies was used to detect the level of JAK-1 in the tyrosine-phosphorylated protein fraction as an indicator of JAK-1 activation. In IL-6–dependent cells, the presence of JAK-1 in anti-phosphotyrosine immunoprecipitates was only detected on restimulation with IL-6, whereas the levels of JAK-1 decreased substantially when these cells were withdrawn from IL-6 (Figure3). In contrast, a constitutive presence of JAK-1 in anti-phosphotyrosine immunoprecipitates was observed in IL-6–independent cell lines.
Recovery of JAK-1 in anti-phosphotyrosine immunoprecipitates from IL-6–dependent and –independent cell lines.
IL-6–dependent cells (7TD1 and B9) were cultured continuously in IL-6–containing medium or starved from IL-6 for 48 hours and restimulated with 100 U/mL IL-6 for 20 minutes. IL-6–independent cells (SP2/0, 7TD1Ind, and B9Ind) were cultured continuously in medium without IL-6 or stimulated with 100 U/mL IL-6 for 15 minutes. Cell lysates were prepared and subjected to immunoprecipitation with anti-phosphotyrosine antibodies, resolved by 8% SDS-PAGE, and immunoblotted with anti-JAK-1 antibodies.
Recovery of JAK-1 in anti-phosphotyrosine immunoprecipitates from IL-6–dependent and –independent cell lines.
IL-6–dependent cells (7TD1 and B9) were cultured continuously in IL-6–containing medium or starved from IL-6 for 48 hours and restimulated with 100 U/mL IL-6 for 20 minutes. IL-6–independent cells (SP2/0, 7TD1Ind, and B9Ind) were cultured continuously in medium without IL-6 or stimulated with 100 U/mL IL-6 for 15 minutes. Cell lysates were prepared and subjected to immunoprecipitation with anti-phosphotyrosine antibodies, resolved by 8% SDS-PAGE, and immunoblotted with anti-JAK-1 antibodies.
Tyrosine phosphorylation status of gp130 in IL-6–dependent and –independent hybridomas and plasmacytomas
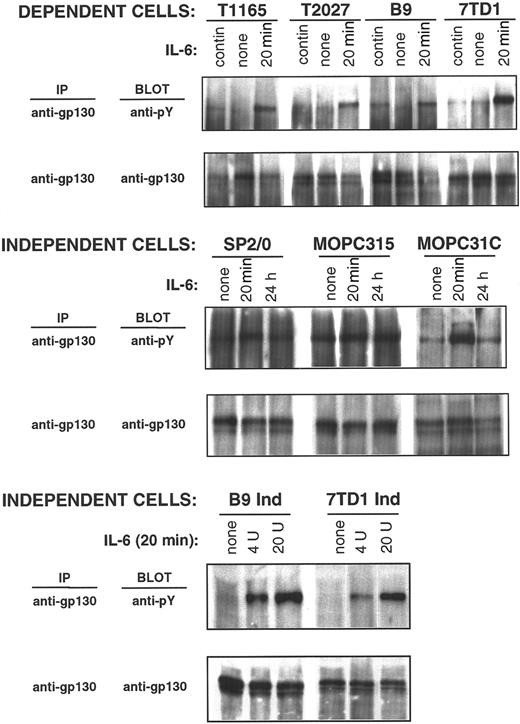
IL-6–induced receptor oligomerization and ensuing JAK-1 activation results in the phosphorylation of tyrosine residues on the intracellular tail of the receptor-associated protein, gp130, thus forming a docking site for the STAT3 Src-homology 2 domain. It was therefore of interest to evaluate the tyrosine phosphorylation status of gp130 in both IL-6–dependent and –independent cell lines to gain information on receptor proximal events that may lead to STAT3 activation. Low-level constitutive tyrosine phosphorylation of gp130 was observed in IL-6–dependent cells (T1165, T2027, B9, and 7TD1 cells) when they were maintained in IL-6 (Figure4). Phosphorylation was absent or decreased when cells were withdrawn from IL-6, whereas high gp130 phosphorylation levels were observed when these cells were restimulated for 20 minutes with IL-6. Therefore, gp130 phosphorylation closely correlates with STAT3 phosphorylation and activation, indicating the presence of continuous stimulation of gp130-mediated signaling in IL-6–dependent cells maintained in the presence of IL-6.
gp130 phosphorylation in IL-6–dependent and –independent cell lines.
IL-6–dependent cells were cultured continuously in IL-6–containing medium or starved from IL-6 for 10 hours (T1165), 30 hours (T2027), or 48 hours (7TD1 and B9) and restimulated with IL-6 (500 U/mL) for 20 minutes. IL-6–independent cells were cultured continuously in medium without IL-6 and stimulated with IL-6 (100 U/mL) for the indicated times (SP2/0, MOPC315, and MOPC31C) or stimulated for 20 minutes with the indicated doses of IL-6 (B9Ind and 7TD1Ind). Anti-gp130 immunoprecipitates were resolved by 10% SDS-PAGE, and phosphorylation of gp130 was detected by immunoblotting with an anti-phosphotyrosine antibody. Blots were stripped and reprobed with anti-gp130 antibody.
gp130 phosphorylation in IL-6–dependent and –independent cell lines.
IL-6–dependent cells were cultured continuously in IL-6–containing medium or starved from IL-6 for 10 hours (T1165), 30 hours (T2027), or 48 hours (7TD1 and B9) and restimulated with IL-6 (500 U/mL) for 20 minutes. IL-6–independent cells were cultured continuously in medium without IL-6 and stimulated with IL-6 (100 U/mL) for the indicated times (SP2/0, MOPC315, and MOPC31C) or stimulated for 20 minutes with the indicated doses of IL-6 (B9Ind and 7TD1Ind). Anti-gp130 immunoprecipitates were resolved by 10% SDS-PAGE, and phosphorylation of gp130 was detected by immunoblotting with an anti-phosphotyrosine antibody. Blots were stripped and reprobed with anti-gp130 antibody.
The tyrosine phosphorylation status of gp130 in autonomously growing cells (SP2/0, MOPC31C, MOPC315, 7TD1ind, and B9ind) was then examined. In 3 cell lines (SP2/0, MOPC 31C, and MOPC315), gp130 was constitutively phosphorylated (Figure 4). Stimulation of these cells with IL-6 for 20 minutes resulted in a further increase in gp130 phosphorylation, which either returned to basal levels within 24 hours (SP2/0 and MOPC31C) or remained constant (MOPC315). In the remaining 2 cell lines (7TD1Ind and B9Ind), no constitutive gp130 phosphorylation was detected in the absence of exogenous IL-6. Phosphorylation of gp130 was observed in 7TD1Ind and B9Ind cells on IL-6 stimulation, confirming that the receptor-proximal events of the IL-6 signaling pathway were intact in these cells.
The common receptor component for multiple cytokines, including IL-6, IL-11, CNTF, LIF, and OSM, is gp130.11 It was recently reported that IL-11, CNTF, LIF, and OSM could support the growth of human myelomas and plasmacytomas in a paracrine manner.24 25 To account for the constitutive gp130 phosphorylation in the IL-6–independent cells, all lines were screened by reverse transcription (RT)-PCR for the de novo production of the cytokines known to interact with gp130. As shown in Figure5, mRNA for IL-6, LIF, and OSM were undetectable in any of the IL-6–dependent and –independent cell lines, whereas IL-11 mRNA was present in 2 IL-6–dependent (T1165 and 7TD1) and 2 IL-6–independent (MOPC31C and 7TD1ind) cell lines. Detectable mRNA levels for CNTF were present in 1 IL-6–dependent (T1165) and several IL-6–independent (SP2/0, MOPC31C, and MOPC315) cell lines. The latter finding, though suggestive of a CNTF autocrine mechanism for SP2/0, MOPC315, and MOPC31C, is inconsistent with the lack of constitutive gp130 phosphorylation in the IL-6–dependent line, T1165. Similarly, the presence of IL-11 mRNA levels in MOPC31C and 7TD1ind is inconsistent with the lack of detectable gp130 phosphorylation in the IL-6–dependent lines, T1165 and 7TD1. Although an autocrine loop by a yet unidentified factor cannot be ruled out at this time, these data argue against a role for cytokines known to use gp130 as the mechanism underlining the constitutive phosphorylation of gp130 in IL-6–independent cells.
RT-PCR analysis for mRNA expression of gp130-sharing cytokines in IL-6–dependent and –independent cells.
Messenger RNA was isolated from the indicated cell lines, reverse transcribed, and added to PCR reactions containing primers specific for IL-6, IL-11, OSM, CNTF, LIF, or GAPDH.
RT-PCR analysis for mRNA expression of gp130-sharing cytokines in IL-6–dependent and –independent cells.
Messenger RNA was isolated from the indicated cell lines, reverse transcribed, and added to PCR reactions containing primers specific for IL-6, IL-11, OSM, CNTF, LIF, or GAPDH.
Growth support of IL-6–dependent hybridomas and plasmacytomas by IL-6–independent cell lines
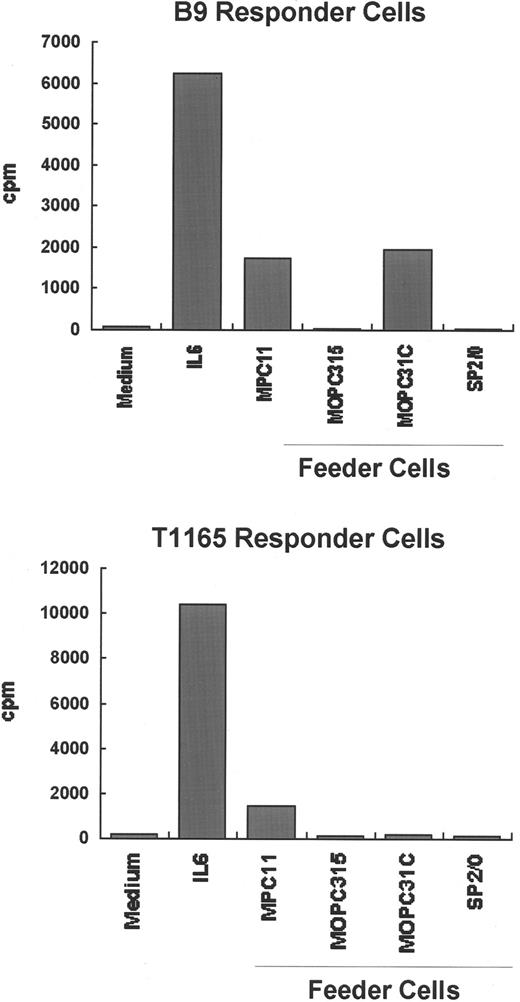
To further explore whether the IL-6–independent cell lines produce any growth factors capable of supporting plasmacytoma cell growth, 3 IL-6–independent cell lines (SP2/0, MOPC315, and MOPC31C) were irradiated and incubated together with either of 2 IL-6–dependent cell lines, T1165 and B9, in the absence of exogenous growth factors. As shown in Figure 6, T1165 and B9 cells proliferate only in the presence of exogenous IL-6 or when irradiated MPC11 cells were used as feeder cells. MPC11 cells have been shown to constitutively produce IL-623 and were used as a positive control in these experiments. Co-culture of MOPC315 or SP2/0 with either T1165 or B9 cells showed that these cell lines do not support the proliferation of either indicator cell, whereas MOPC31C cells support the proliferation of B9 but not T1165 cells. This response by MOPC31C may be owing to the presence of cytokines other than IL-6 that signal through gp130. MOPC31C is the only cell line capable of producing both IL-11 and CNTF, as shown by RT-PCR (Figure 5). It is possible that the combined presence of both cytokines is responsible for the proliferative effect on B9 cells, whereas CNTF alone is unlikely to support proliferation because mRNA for CNTF was also found in other cell lines (SP2/0 and MOPC315) incapable of supporting the growth of IL-6–dependent cells.
Ability of IL-6–independent cell lines to support the growth of IL-6–dependent cell lines.
The IL-6–dependent cell lines B9 and T1165 were independently cultured (1 × 104 cells/200 μL) with medium alone or medium containing 2 × 104 irradiated (8.0 Gy) IL-6–independent plasmacytoma/hybridoma cells (MPC11, MOPC31C, MOPC315, or SP2/0) as feeders. Twenty-four (T1165 cells) or 72 hours (B9 cells) later, the cells were pulsed with 3H-thymidine for an additional 4 hours, and the radioactivity incorporated into DNA was determined. MPC11 cells, which are known to produce small amounts of IL-6, were included as a positive control.
Ability of IL-6–independent cell lines to support the growth of IL-6–dependent cell lines.
The IL-6–dependent cell lines B9 and T1165 were independently cultured (1 × 104 cells/200 μL) with medium alone or medium containing 2 × 104 irradiated (8.0 Gy) IL-6–independent plasmacytoma/hybridoma cells (MPC11, MOPC31C, MOPC315, or SP2/0) as feeders. Twenty-four (T1165 cells) or 72 hours (B9 cells) later, the cells were pulsed with 3H-thymidine for an additional 4 hours, and the radioactivity incorporated into DNA was determined. MPC11 cells, which are known to produce small amounts of IL-6, were included as a positive control.
Discussion
This report is the first observation of a constitutive activation of STAT3 in IL-6–independent plasmacytomas and hybridomas. In IL-6–dependent lines, the activation of the IL-6 signal transduction pathway is completely contingent on the addition of exogenous growth factor. Cells grown in the absence of IL-6 do not demonstrate detectable gp130 and STAT3 phosphorylation, but such activation occurs rapidly with the addition of IL-6. These cells, when continuously grown in the presence of IL-6, demonstrate detectable levels of phosphorylation of these molecules, albeit decreased when compared to the level induced by acute stimulation. In contrast, constitutive activation of JAK-1/STAT3 was observed in the IL-6–independent cell lines tested. Two different mechanisms of IL-6 independence were observed in these cell lines that can be discriminated based on the activation status of gp130. In one set of IL-6–independent lines (7TD1Ind and B9Ind), JAK-1/STAT3 are constitutively activated in the absence of detectable gp130 phosphorylation, whereas in the other set of IL-6–independent cells (SP2/0, MOPC31C, and MOPC315), JAK-1/STAT3 activation was associated with the constitutive phosphorylation of gp130.
The mechanism underlying the constitutive gp130 phosphorylation observed in certain IL-6–independent lines remains unclear. Although it has no intrinsic tyrosine kinase activity, gp130 is phosphorylated on tyrosine residues by JAK kinases after stimulation by the IL-6 family of cytokines.1 An autocrine cytokine production mechanism has great potential for regulating biologic responses because the factor is released in proximity to the receptor on the surface of the cells that produced it. Autocrine regulation of tumor growth has been observed in many malignancies, including multiple myeloma.24 25 Our data, however, argue against an autocrine loop as the principal mechanism for IL-6 independence in these plasmacytoma cell lines. RT-PCR detection of mRNA for several members of the IL-6 family of cytokines and the co-culture data, when taken together, strongly suggest that at least 2 IL-6–independent cell lines, MOPC315 and SP2/0, do not have an autocrine loop responsible for their growth factor independence. No mRNA for IL-6, IL-11, LIF, or OSM was detected in these lines nor did they support the proliferation of IL-6–dependent cells.
Despite the presence of CNTF mRNA, co-cultivation experiments showed that MOPC315 and SP2/0 cells failed to support the growth of IL-6–dependent plasmacytomas (T1165 and B9), indicating that these cells are factor-independent by mechanisms other than the autocrine production of this growth factor. It should be noted that the absence of a canonical signal sequence in CNTF might limit the ability of this protein to be exported.26 A direct intracellular interaction of CNTF with gp130 cannot be completely excluded. Furthermore, an intracrine stimulatory mechanism, in which ligand-receptor binding and signal induction takes place inside the cell, cannot be entirely ruled out for all cytokines. Secretory granules, containing both the growth factors and their receptors,27-29 may be a place for the interaction to occur. The presence of a similar spectrum and similar levels of cytokine mRNA in IL-6–dependent cells, in which no constitutive gp130 phosphorylation was detected, strongly argues against this possibility. For instance, CNTF mRNA was detected in the IL-6–dependent line, T1165. IL-11 mRNA was detected in the IL-6–independent cell line, 7TD1Ind, though no gp130 phosphorylation was observed. Furthermore, IL-11 mRNA was also observed in the parental IL-6–dependent line, 7TD1, and in another IL-6–dependent line, T1165, suggesting that IL-11 per se is unlikely to play a role as an autocrine factor. An autocrine mechanism for MOPC31C, through the combined secretion of IL-11 and CNTF, cannot be entirely ruled out because this cell line supports the growth of the IL-6–dependent line, B9. The continuous activation of gp130 may be attributable to an autocrine loop in this cell line.
IL-6–independent lines might display a higher sensitivity to growth factor by expressing a higher number of cytokine receptor(s). However, the level of gp130, the common signal-transducing unit of the IL-6 family of cytokines, was similar on a per cell basis in both IL-6–dependent and –independent cell lines (data not shown). This suggests that an increased level of signaling potential or receptor expression cannot be responsible for the observed effects.
The role of another signaling pathway, the Ras-dependent activation of mitogen-activated protein kinases (MAPK), in controlling myeloma cell growth is controversial. Activation of Ras/MAPK has been implicated in IL-6–mediated myeloma cell growth, though constitutive activation of the Ras/MAPK pathway was not observed in IL-6–nonresponsive myelomas.30 In contrast, Zauberman et al31detected no MAPK activation in response to IL-6 in IL-6–dependent hybridoma B9. We did not detect increased MAPK phosphorylation in response to IL-6 in IL-6–dependent cells or constitutive MAPK phosphorylation in IL-6–independent cells (data not shown). These data are consistent with the observation of Zauberman et al31and do not support a role for the Ras/MAPK pathway in IL-6 signaling or the process of IL-6–independent growth of plasmacytomas or hybridomas.
Two of the IL-6–dependent cell lines used in these studies, B9 and 7TD1, are hybridomas derived from fusing SP2/0 with Balb/c spleen cells. Thus, IL-6 dependence can be restored from an IL-6–independent plasmacytoma by the introduction of wild-type DNA. Furthermore, IL-6–independent clones can be derived from these IL-6–dependent cell lines by slowly withdrawing them from IL-6, as in the case of B9Ind and 7TD1Ind cells. In these IL-6–independent cells, gp130 phosphorylation was undetectable, albeit still inducible, by IL-6. STAT3 was nonetheless constitutively activated, strongly supporting the hypothesis that these hybridomas become independent of IL-6 through a mechanism that does not involve the autocrine production of cytokines.
Phosphotyrosine residues on gp130 recruit various Src-homology 2 (SH2) domain-containing signal-transducing molecules, including STAT3,14,32,33 promoting their phosphorylation. Tyrosine-phosphorylated STAT3 molecules dimerize through SH2 domain-mediated intermolecular interactions. It has recently been shown34 that constitutive STAT3 dimerization, by means of artificial intermolecular disulfide linkages, renders STAT3 tumorigenic. We have found that the constitutive activation of STAT3 in growth- factor–independent plasmacytomas and hybridomas was associated with constitutive STAT3 tyrosine phosphorylation, presumably leading to constitutive dimerization. Constitutive activation of JAK kinases has been shown to result in the constitutive activation of STAT molecules, thus contributing to growth factor independence of several leukemic cell lines.20,35 Consistent with the above observation, we have found that JAK-1 was constitutively present in anti-phosphotyrosine immunoprecipitates from IL-6–independent cell lines, suggesting that a constitutively active JAK-1 contributed to STAT3 activation in these cells. Aberrant JAK activation in human cancers has been attributed to different mechanisms. In acute lymphocytic leukemia, constitutive activation was associated with a chromosomal translocation involving JAK-2.36 Furthermore, oncogenic transformation by Src or Abl may involve JAK, leading to the activation of STAT molecules.16,18 19 Additional studies will be needed to elucidate the mechanism of constitutive JAK activation in IL-6–independent hybridomas.
STAT3 signaling has been implicated in the regulation of differentiation, cell proliferation, and apoptosis.37-40Constitutive activation of STAT3 has been linked to cellular transformation by various oncoproteins.41-44 STAT3 is required for gp130-mediated activation of c-myc,45 a critical regulator of cell growth involved in cell cycle progression from the G1 to the S phase.46 Constitutive activation of STAT3 signaling occurs in the IL-6–producing human myeloma cell line, U266, conferring resistance to apoptosis through Bcl-xLinduction.47 Furthermore, STAT3 activation occurs frequently in human tumors of diverse origin.17,41,42,44,48-50 STAT3, therefore, plays a critical role in regulating fundamental processes associated with malignant transformation and cell survival.51
Acknowledgments
We thank Drs Ezio Bonvini, Gerald Feldman, Gibbes Johnson, and Michael Potter for critical discussion and review of the manuscript. This work is dedicated to the memory of Dr Richard P. Nordan.
R.R. and G.J.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rashmi Rawat, Laboratory of Immunobiology, Division of Monoclonal Antibodies, Center for Biologics Evaluation and Research, Building 29B, Room 4G07, 29 Lincoln Drive, Bethesda, MD 20892; e-mail: rawat@cber.fda.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal