Abstract
Sickle red blood cells (RBCs) become depleted of potassium, leading to dehydration and abnormally elevated cellular density. The increased sickling that results is important for both hemolysis and vasocclusion. In this study, sickle cells were subjected to high-speed centrifugation, and the bottom 15% were isolated. This procedure removed light cells and to a variable degree enriched cells that were denser than normal to produce a high-density–enriched (HDE) population of sickle cells. Autologous HDE cells from 3 subjects were labeled with biotin and re-infused. The following determinations were performed: (1) the survival and density changes of HDE cells; (2) the amount of fetal hemoglobin (HbF) in labeled cells after magnetic isolation; (3) the percentage of labeled F cells; (4) the percentage of labeled cells displaying external phosphatidylserine (PS). For patients with 3.5%, 4.5%, and 24% HbF in the HDE RBCs, the circulation half-time was 40, 80, and 180 hours, respectively. The percentage of HbF (measured in all 3 subjects) and of F cells (measured in 2 subjects) in labeled RBCs increased with time after re-infusion, indicating that HDE F cells have longer in vivo survival than HDE non-F cells. The percentage of PS+, biotin-labeled HDE cells showed no consistent increase or decrease with time after re-infusion. These data provide evidence that HDE sickle cells, especially those that do not contain HbF, have a very short in vivo survival, and that the percentage of PS+ cells in a re-infused HDE population does not change in a consistent manner as these cells age in the circulation.
Introduction
Sickle red blood cells (RBCs) tend to become potassium depleted and thus dehydrated and abnormally dense. The higher concentration of sickle cell hemoglobin (HbS) leads to more polymerization and sickling, and contributes to both hemolysis and vasocclusion. Several interacting cation transport pathways mediate dehydration, including (1) a nonselective increase in cation permeability related to morphological sickling, (2) a calcium-dependent K channel (Gardos) that most likely depends on calcium influx through the nonselective sickling-dependent pathway, and (3) KCl cotransport, a normal pathway in immature erythroid cells that is very active in sickle RBCs, owing at least in part to their young age distribution but perhaps also to a specific activation related to the presence of HbS.1,2 Administration of clotrimazole,3 an inhibitor of the Ca++-dependent K channel, or magnesium,4 which inhibits KCl cotransport, leads to partial normalization of sickle RBC hydration.
When sickle RBCs are separated by density, there are many cells with normal density, but also a variable number of light and dense cells. The number of dense RBCs varies widely among patients but in the absence of painful episodes is relatively constant for each patient.5 Young sickle cells, including reticulocytes and the even less mature cells displaying transferrin receptor, are seen not only in the light fractions as expected, but also in the dense fractions. Both the lightest and densest cells contain less fetal hemoglobin (HbF) than cells with moderate density,6 and there is evidence that only cells lacking HbF can become extremely dehydrated soon after leaving the marrow.7 Previous studies have used several direct and indirect techniques to show that sickle cells containing HbF survive better in the circulation.6 8-10
In general, dense sickle cells tend to have more severe membrane abnormalities when compared with cells with normal hydration.11 Recently, it has been shown that a higher percentage of cells in the dense fraction have lost normal phospholipid asymmetry and display phosphatidylserine (PS) on the outer membrane leaflet.12 It has been postulated that these cells, owing to the exposure of PS, may be thrombogenic and readily recognized and removed by macrophages.
In the current studies, high-density–enriched (HDE) sickle RBCs were labeled with biotin, re-infused, and analyzed in subsequent blood samples. The biotin label has recently been shown by us10and others13 to be equivalent to a 51Cr label in the measurement of human RBC survival. In addition to survival characteristics, the current studies also examined postinfusion changes in the hydration state of HDE cells, differences between the behavior of HDE F cells and non-F cells, and changes in PS exposure as the cells aged in vivo.
Materials and methods
Approximately 90 mL blood was drawn into heparinized (15 U/mL) syringes with informed consent. Dense RBC enrichment, biotin labeling, and re-infusion were performed within approximately 6 hours. All studies were autologous, and the subjects had neither been transfused for at least 3 months nor had a recent painful episode.
Red cells were washed 3 times with at least 2 volumes of Dulbecco's phosphate-buffered saline (D-PBS; Gibco BRL, Grand Island, NY). After the last centrifugation, the packed RBCs were transferred to gas-sterilized, polypropylene, high-speed centrifuge tubes (16 × 98 mm) and spun (18 000g maximum, 22°C) for 90 minutes. The top 85% of the red cell column was carefully removed and discarded. The bottom 15% of the red cell column (HDE cells, 2 to 3 mL) was labeled with biotin as previously described,10with the use of 3 μg/mL NHS-biotin. Prior to re-infusion, a small aliquot of the labeled RBCs was reacted with streptavidin-phycoerythrin (SA-PE) and analyzed by flow cytometry10 to ensure that all the cells were labeled and that there was baseline separation of labeled and unlabeled cells on the flow cytometric histogram. The remaining biotin-labeled RBCs were re-infused through an 18-μ blood filter (Hem-O-Nate; Bard, Salt Lake City, UT).
Postinfusion blood samples were typically obtained at 7.5, 10, 12.5, 15, and 20 minutes; 1, 2, 4, 8, 16, and 24 hours; 2 and 3 days, and as required thereafter. To facilitate blood sampling, the subjects were admitted for 1 day to the Clinical Research Center at the University of Cincinnati Medical Center. The percentage of labeled RBCs was measured at each time point,10 and the survival of the HDE cells determined. For selected postinfusion time points, the RBCs were separated into 6 density fractions as described,10 and the number of labeled cells in each fraction was determined. For the same selected samples, labeled RBCs were isolated by means of streptavidin-coated magnetic beads (Dynal, Lake Success, NY), and the HbF content was determined by high-performance liquid chromatograhy (HPLC) as previously described.10 In addition, these samples were analyzed with 2-color flow cytometry for biotin and HbF. After incubation with SA-PE, the cells were fixed, permeabilized, and incubated with a fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody to HbF (Wallac; Akron, OH).14 A 2-color flow cytometric protocol was used to determine the percentage of F cells both in the unlabeled cells, which represent the entire circulating cell population, and in the labeled cells, which represent the re-infused cells as they age in the circulation.
To investigate changes in externalized PS as the labeled HDE cells aged, blood samples were incubated first with SA-PE and then with Annexin V-FITC (R&D Systems; Minneapolis, MN) according to the manufacturer's protocol, with the use of 1 μg/mL Annexin V-FITC and 108 RBCs per milliliter. The cells were analyzed by 2-color flow cytometry, allowing determination of the percentage of PS+ cells in the labeled and unlabeled populations.
At selected postinfusion time points, RBCs from density fraction 2 (1.073 < ρ < 1.083) were incubated with valinomycin, a potassium-specific ionophore. Cells with a normal high concentration of potassium will become dehydrated when incubated in the presence of valinomycin, whereas those containing sodium as the dominant monovalent cation will remain hydrated. Valinomycin exposure was as described by Bookchin et al.15 16 Fraction-2 RBCs were washed once with buffer (125 mmol/L NaCl, 20 mmol/L Hepes, 15 mmol/L KCl, 1 mmol/L Na2HPO4, 1 mmol/L MgCl2, and 10 mmol/L glucose, pH 7.6 at room temperature) and brought to a 1% suspension in the same buffer. Valinomycin was added (2 μL of a 1 mmol/L stock solution in absolute alcohol to each milliliter of suspension) and the mixture incubated at 37°C for 30 minutes. After one wash and resuspension to 1 mL in the same buffer, the cells were density fractionated with the use of the same gradient and centrifugation conditions as the primary gradient. In this secondary gradient, the valinomycin-sensitive RBCs fall to the bottom, whereas the resistant cells remain near the top. The number of resistant and sensitive cells were counted, and the number of biotin-labeled RBCs in each group quantitated by reaction with SA-PE and flow cytometry as described above.
Results
In vivo survival of density-enriched sickle cells
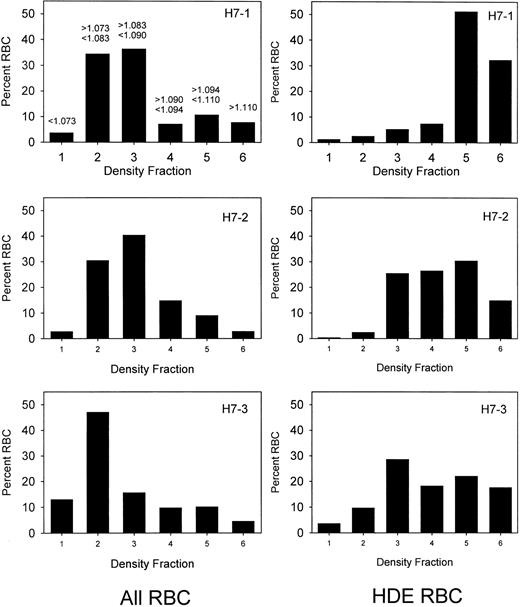
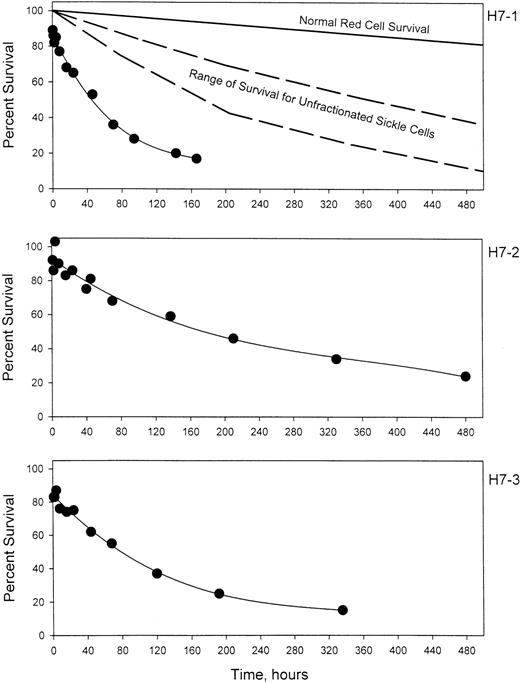
Prior to labeling, sickle cells were centrifuged at high speed in a packed cell column to obtain a preparation enriched in dense cells. This method introduces no material other than D-PBS and allows the subsequent re-infusion of the cells. Figure1 shows the density distribution of the cells prior to and after enrichment of the dense cells for 3 sickle cell patients. Prior to enrichment, 24% to 26% of the cells were in the densest 3 fractions, corresponding to cells with density greater than 1.090 g per cubic centimeter. After enrichment, 58% to 91% of the cells were in these fractions. The density distributions before and after the isolation procedure show a wide degree of variability among the 3 patients. Patient H7-1 in this series had the best isolation of dense cells with more than 80% in fractions 5 and 6. For comparison, in this analytical gradient most of the normal cells are in fraction 3, but also are found in fractions 4 and 5, with fewer than 10% in fraction 5.10 There are essentially no normal cells in fraction 6. The results in patient H7-1 therefore represent cells that are denser than normal. In the other 2 patients, the isolation could be characterized as light cell depletion, since the major effect was removal of cells in fraction 2, which are lighter than about 90% of normal cells.10 Further heterogeneity is present since patient H7-2 has a much higher level of HbF than the other 2 patients. Figure 2 shows the survival of the density-enriched (HDE)–labeled cells. The survival of normal red cells and unfractionated sickle cells from a previous study10 are shown in the top panel. For patients H7-1 and H7-3, both of whom have typical HbF levels, the survival of biotin-labeled HDE cells is much shorter than unfractionated sickle cells. Comparison of H7-2 and H7-3, both of whom had similar density distributions after isolation, shows that the presence of a higher level of HbF in patient H7-2 is associated with much longer survival of HDE cells.
Sickle cell density distributions before and after isolation of dense cells.
The 3 panels on the left represent the red cell density distributions for 3 patients (H7-1, H7-2, and H7-3) prior to isolation of dense cells. On the right are shown the corresponding density distributions of the biotin-labeled HDE cells at the time of re-infusion. The density range for each fraction is shown in the upper left panel.
Sickle cell density distributions before and after isolation of dense cells.
The 3 panels on the left represent the red cell density distributions for 3 patients (H7-1, H7-2, and H7-3) prior to isolation of dense cells. On the right are shown the corresponding density distributions of the biotin-labeled HDE cells at the time of re-infusion. The density range for each fraction is shown in the upper left panel.
The survival of biotin-labeled HDE cells.
For 3 patients with sickle cell disease, a high-density fraction was isolated, labeled with biotin, and re-infused. The average number of labeled cells present in blood samples collected between 7.5 and 20 minutes after re-infusion was taken as 100%. The top panel also shows the expected survival of normal RBCs and the range of survival for unfractionated sickle cells from Franco et al.10
The survival of biotin-labeled HDE cells.
For 3 patients with sickle cell disease, a high-density fraction was isolated, labeled with biotin, and re-infused. The average number of labeled cells present in blood samples collected between 7.5 and 20 minutes after re-infusion was taken as 100%. The top panel also shows the expected survival of normal RBCs and the range of survival for unfractionated sickle cells from Franco et al.10
Longer survival of F cells in the HDE population
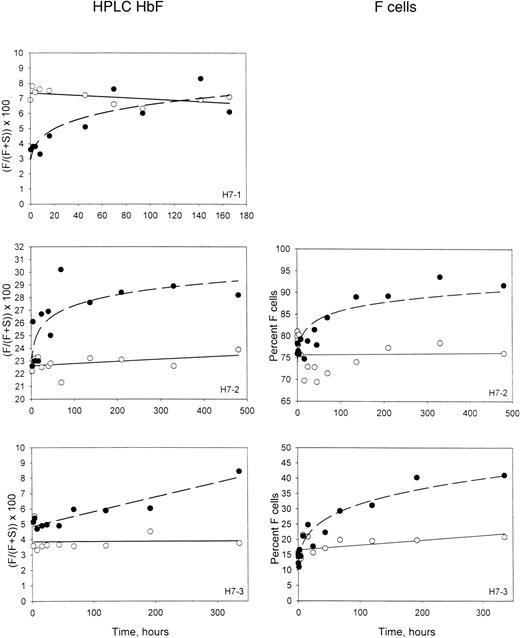
The percentages of HbF in the magnetically isolated biotin-labeled RBCs and the unlabeled RBCs are shown in the left-hand panels of Figure3. As expected in these patients at steady state, the percentage of HbF in unlabeled circulating cells was stable and represents equilibrium between production and removal. In the labeled cells, which are aging in the circulation and undergoing selective removal, the percentage of HbF increases. This must be due to a longer survival of the cells that contain HbF. It is not surprising that this selection would take place in the denser cells, since the inhibitory effect of HbF on polymerization and sickling is expected to be most important in this population. For patient H7-1, the initial HbF content of the labeled HDE cells was less than the unlabeled cells (Figure 3), reflecting the lower HbF content of dense cells.6,9 In the other 2 experiments, in which there were more intermediate-density–labeled cells, the initial HbF contents of the labeled and the unlabeled cells were about the same. In all cases, however, the HbF content of the labeled HDE cells increased with time in the circulation. In 2 subjects, the corresponding 2-color flow cytometric F-cell quantitation is available. Examples of this analysis (Figure 4) show that the fixation and permeabilization method used14 preserves the biotin on the labeled cells. The F-cell data for patients H7-2 and H7-3 are shown in the right-hand panels of Figure 3 for comparison with the HbF in the isolated labeled cells, and demonstrate a similar increase for the percentage of F cells in the labeled population.
Time-dependent changes in the HbF content of labeled HDE cells.
Panels on the left show the fraction of HbF, determined by HPLC, in unlabeled cells (open symbols) and magnetically isolated biotin-labeled cells (closed symbols) at each time point after re-infusion. Panels on the right show similar data, except that the percentage of F cells was determined by flow cytometry for unlabeled and biotin-labeled cells.
Time-dependent changes in the HbF content of labeled HDE cells.
Panels on the left show the fraction of HbF, determined by HPLC, in unlabeled cells (open symbols) and magnetically isolated biotin-labeled cells (closed symbols) at each time point after re-infusion. Panels on the right show similar data, except that the percentage of F cells was determined by flow cytometry for unlabeled and biotin-labeled cells.
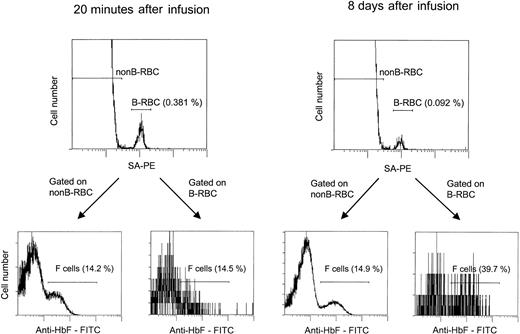
The flow cytometric assay for unlabeled and biotin-labeled F cells.
Cells were incubated with SA-PE, fixed, permeabilized, incubated with anti-HbF–FITC, and analyzed by 2-color flow cytometry. The percentage of labeled cells (B-RBCs) was consistent with values obtained in nonpermeabilized samples. F-cell analyses were gated on the unlabeled (non-B-RBCs) and labeled (B-RBCs) cells. Two time points are shown: 20 minutes, with the unlabeled and labeled cells having approximately the same percentage F cells (14.2% and 14.5%), and 8 days, with the unlabeled cells unchanged (14.9%) and the labeled cells increasing to 39.7%.
The flow cytometric assay for unlabeled and biotin-labeled F cells.
Cells were incubated with SA-PE, fixed, permeabilized, incubated with anti-HbF–FITC, and analyzed by 2-color flow cytometry. The percentage of labeled cells (B-RBCs) was consistent with values obtained in nonpermeabilized samples. F-cell analyses were gated on the unlabeled (non-B-RBCs) and labeled (B-RBCs) cells. Two time points are shown: 20 minutes, with the unlabeled and labeled cells having approximately the same percentage F cells (14.2% and 14.5%), and 8 days, with the unlabeled cells unchanged (14.9%) and the labeled cells increasing to 39.7%.
The amount of F per F cell can be estimated either directly or indirectly. Dover et al8 used a direct microscopic immunodiffusion method to show that sickle erythrocytes had 10.9 ± 3.2 (1 SD) pg of HbF per F cell. Horiuchi et al17 used a direct fluorescent image analysis technique to determine a range of 2.7 to 12.8 in 5 patients with sickle cell disease. In the indirect method, 2 measurements are made: the percentage of HbF in all the cells and the percentage of F cells. It is assumed that all the HbF is in the F cells, and the HbF per F cell is calculated as follows: HbF/Fcell = (% HbF/% F cells) · MCH, where MCH is mean corpuscular hemoglobin. When this formula is applied to the data of Dover et al8 with a value of 31 for MCH, an average of 12 pg HbF per F cell is obtained, which agrees well with the directly measured value.
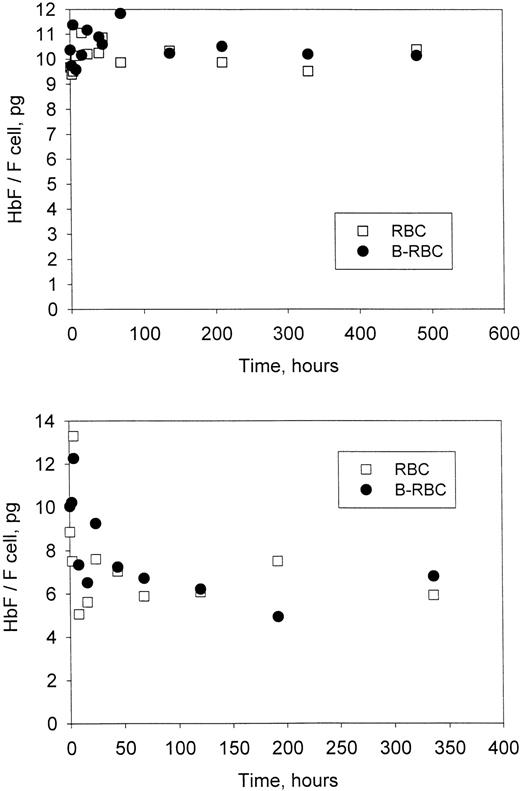
For each time point in Figure 3 (patients H7-2 and H7-3), the HbF per F cell was calculated as described above for the unlabeled and labeled cells. The values for HbF per F cell (Figure5) show no change with time in the labeled HDE cells. In fact, at all times, HbF per F cell was essentially the same for the labeled HDE cells and the unlabeled cells, which include cells with all density values. Values of HbF per F cell represent averages for the population measured, but presumably there is a range of values within each population. If there is selection within the F-cell population (ie, if F cells with higher values of HbF per F cell survive longer than F cells with lower values), then HbF per F cell for the biotin-labeled HDE cells would most likely be higher (since they are older on average) and would increase further as the cells aged in the circulation. These differences were not observed, implying a lack of selection within the F-cell population.
Time-dependent changes in Hb F per F cell.
The average amount of HbF in each F cell was calculated from the data in Figure 3 for each time point as described in “Materials and methods.” Unlabeled (RBC) and biotin-labeled (B-RBC) cells are shown. Top panel, patient H7-2; bottom panel, patient H7-3.
Time-dependent changes in Hb F per F cell.
The average amount of HbF in each F cell was calculated from the data in Figure 3 for each time point as described in “Materials and methods.” Unlabeled (RBC) and biotin-labeled (B-RBC) cells are shown. Top panel, patient H7-2; bottom panel, patient H7-3.
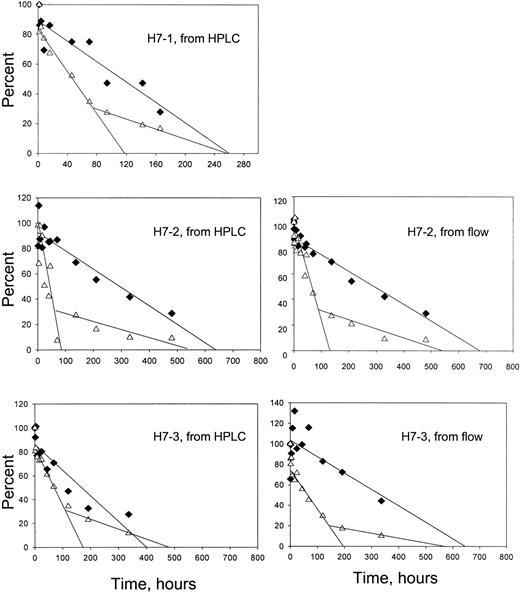
The time-dependent enrichment of F cells in the circulation indicates that HDE F cells survive longer than HDE non-F cells. As shown previously,10 individual survival curves for these 2 cell types can be derived from the overall survival data and the percentages of F and non-F cells at each time point. These percentages may be taken directly from the flow cytometric assays or calculated from the percentage of HbF (by HPLC) and the HbF per F cell for each patient. Figure 6 shows the results of this analysis. For all subjects, the non-F cells had a shorter survival and appeared to have a biphasic disappearance, whereas the F cells had a longer survival and a linear disappearance.
Survival of F and non-F cells.
♦, F cells; ▵, non-F cells. For the panels on the left, the survival for each cell type was derived from the overall survival, the percentage of HbF in the magnetically isolated labeled cells, and the HbF per F cell for each patient. For the panels on the right, the overall survival and the flow cytometric quantitation of the percentage of labeled F cells were used in a similar manner. Formulas and sample calculations are given in Franco et al.10
Survival of F and non-F cells.
♦, F cells; ▵, non-F cells. For the panels on the left, the survival for each cell type was derived from the overall survival, the percentage of HbF in the magnetically isolated labeled cells, and the HbF per F cell for each patient. For the panels on the right, the overall survival and the flow cytometric quantitation of the percentage of labeled F cells were used in a similar manner. Formulas and sample calculations are given in Franco et al.10
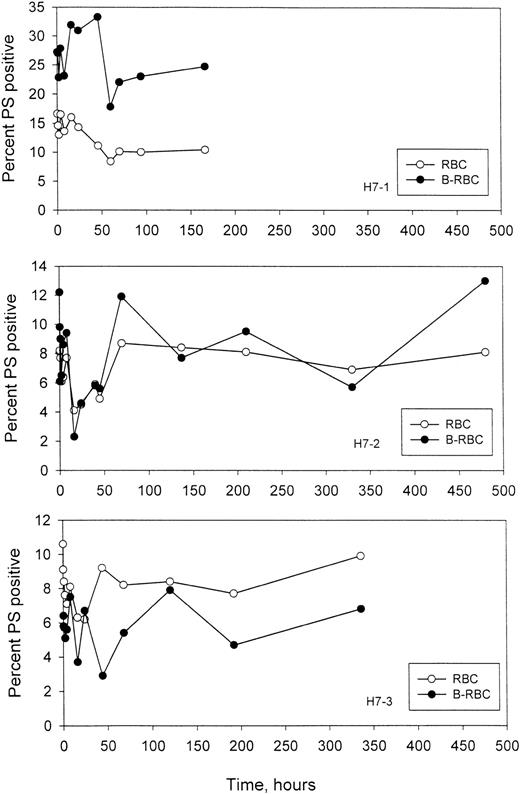
Time-dependent changes in the percentage of PS+ sickle RBCs
It has been postulated that display of PS on the outer leaflet of the RBCs increases the probability of removal from the circulation. In these experiments, the labeled HDE cells were followed as they aged in vivo, and the percentage of PS+ cells was quantitated, along with the percentage of PS+ cells in the unlabeled circulating cells. Examples of the 2-color flow cytometric analysis for labeled and unlabeled PS+ cells are given in Figure 7, and the time-dependent changes for 3 subjects are shown in Figure8. As expected, in H7-1 there was a higher percentage of PS+ cells in the re-infused, labeled population compared with unlabeled cells, since these were essentially all dense cells. In patients H7-2 and H7-3, the percentages were similar for the labeled and unlabeled cells. It is clear from these studies that for labeled HDE cells, the percentage of PS+ cells does not change consistently, either up or down, as the cells age.
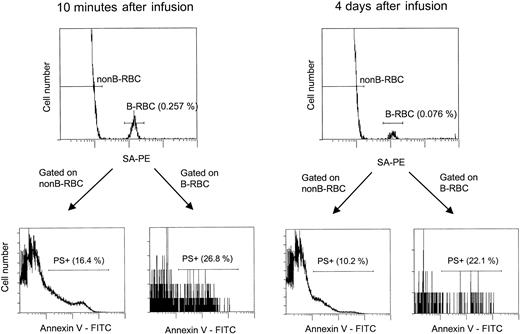
The flow cytometric assay for unlabeled and biotin-labeled PS+ cells.
This analysis is similar to that shown in Figure 4, except that after incubation with SA-PE, the cells were incubated with Annexin V-FITC as described in “Materials and methods.” For patient H7-1, 2 time points are shown: 10 minutes and 4 days after infusion, with the labeled HDE cells consistently having a higher percentage of PS+ cells.
The flow cytometric assay for unlabeled and biotin-labeled PS+ cells.
This analysis is similar to that shown in Figure 4, except that after incubation with SA-PE, the cells were incubated with Annexin V-FITC as described in “Materials and methods.” For patient H7-1, 2 time points are shown: 10 minutes and 4 days after infusion, with the labeled HDE cells consistently having a higher percentage of PS+ cells.
Time-dependent changes in the percentage of PS+ cells.
Unlabeled (RBC) and labeled (B-RBC) cells were assayed as described in Figure 7.
Time-dependent changes in the percentage of PS+ cells.
Unlabeled (RBC) and labeled (B-RBC) cells were assayed as described in Figure 7.
Valinomycin resistance of the older, light, labeled cells
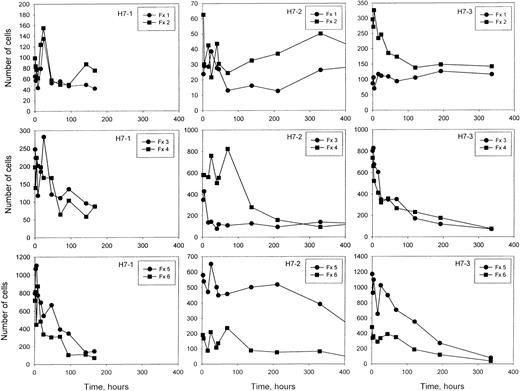
After re-infusion of HDE cells or unfractionated cells,10 a consistent finding was the presence of labeled cells in the light fractions 1 and 2 that, after some initial changes in the first 2 days, remained remarkably constant in number for long periods of time after re-infusion (Figure9). In contrast, the number of labeled cells in the denser fractions decreased with time as expected. The labeled cells must be at least as old as the time since re-infusion, which was up to 20 days in this study with HDE cells and longer in the study with unfractionated cells.10 Bookchin et al15,16 have described a population of valinomycin-resistant, sodium-loaded, nonreticulocyte sickle cells in the light fraction and suggested that they represent a terminal stage. We have previously hypothesized10 that the older, light, labeled cells are continuously renewed from the denser fractions owing to loss of volume regulation, and they may represent the same population described by Bookchin et al.15,16 Therefore, we measured the percentage of valinomycin-resistant cells in fraction 2, both labeled and unlabeled, at selected times after re-infusion. Experiments included one with no enrichment of dense cells for which survival characteristics were previously published (patient S7 in Franco et al10), and 2 of the HDE populations from the current series (H7-2, H7-3).
The number of labeled cells in each density fraction as a function of time after re-infusion.
At each time shown, a blood sample was separated into 6 density fractions, and the number of total cells and the percentage of labeled cells in each fraction was determined. From these values, the number of labeled cells in each fraction was calculated, on the basis of a total of 1 million cells in the original blood sample. Note that the y-axis scales vary for the 3 studies.
The number of labeled cells in each density fraction as a function of time after re-infusion.
At each time shown, a blood sample was separated into 6 density fractions, and the number of total cells and the percentage of labeled cells in each fraction was determined. From these values, the number of labeled cells in each fraction was calculated, on the basis of a total of 1 million cells in the original blood sample. Note that the y-axis scales vary for the 3 studies.
A large majority of older, light, labeled RBCs are valinomycin-resistant (Table 1), consistent with Na+ rather than K+ as the dominant monovalent cation. From these data, it may be concluded that the majority of older cells in the light fraction are resistant to the dehydrating effect of valinomycin.
Resistance to valinomycin-mediated dehydration of labeled and unlabeled fraction-2 RBCs
| Experiment . | Days after infusion . | Valinomycin resistant (%) . | |
|---|---|---|---|
| Unlabeled . | Labeled . | ||
| S7* | 15 | 17 | 95 |
| 20 | 25 | 94 | |
| 27 | 8 | 98 | |
| H7-2 | 20 | 9 | 71 |
| H7-3 | 8 | 8 | 83 |
| Experiment . | Days after infusion . | Valinomycin resistant (%) . | |
|---|---|---|---|
| Unlabeled . | Labeled . | ||
| S7* | 15 | 17 | 95 |
| 20 | 25 | 94 | |
| 27 | 8 | 98 | |
| H7-2 | 20 | 9 | 71 |
| H7-3 | 8 | 8 | 83 |
The survival characteristics of this experiment with unfractionated sickle cells are given in Franco et al.10
Discussion
The studies presented here examine the time-dependent in vivo behavior of a subpopulation of denser sickle cells. The focus was on factors either known to influence cellular survival characteristics (dehydration, HbF) or suspected of doing so (PS exposure). Understanding the relationships between RBC survival, PS externalization, HbF content, and dehydration is a difficult task, which has become increasingly important as therapeutic agents that modify one or more of these cellular properties become available. The clinical use of hydroxyurea to increase HbF and the emergence of experimental agents that change in vivo sickle RBC hydration state3,4 have underscored the gaps in understanding sickle cell pathophysiology. The studies presented here apply cell-by-cell flow cytometric analysis techniques to study the changes that occur in HDE cells as they age in the circulation. These longitudinal studies give another dimension to the study of sickle cells, since they allow the study of time-dependent processes in vivo. When interpreting these studies, one should keep in mind that the labeled HDE cells are highly selected, both in vivo prior to the study and in vitro as part of the study. The rate of dehydration undoubtedly varies among sickle cells, but there is evidence that most cells become dehydrated early in their lifespan.6 10 Since the young light cells are removed during the isolation of HDE cells, changes that occur early in the life of a sickle cell would most likely not be apparent in these studies.
The short survival of dense sickle cells in one patient was first described by Bertles and Milner6 in 1968 and is confirmed in the current studies. The time for removal of 50% of the HDE cells ranged from 40 to 180 hours. For non-F cells, removal from the circulation appeared to occur in 2 phases, with the first phase (approximately 70% of non-F cells) having a very short 50% survival of 40 to 80 hours. The remaining non-F cells (30%) survived about the same amount of time as F cells for each subject, which ranged from approximately 120 (H7-1) to 330 (H7-2 and H7-3) hours. These results are summarized in Table2. The shorter F-cell survival in subject H7-1 may be due to the greater percentage of HDE cells in the dense fractions 5 and 6. These survival times are much shorter than for unfractionated sickle cells, which had corresponding 50% survival values of 240 to 400 hours for all cells, 120 to 170 hours for non-F cells, and 350 to 600 hours for F cells.10
Survival of HDE sickle cells
| Patient . | All cells . | Non-F cells* . | F cells . | ||
|---|---|---|---|---|---|
| HPLC . | Flow cytometry . | HPLC . | Flow cytometry . | ||
| H7-1 | 40 | 45 | NA | 120 | NA |
| H7-2 | 180 | 40 | 65 | 290 | 290 |
| H7-3 | 80 | 80 | 60 | 170 | 330 |
| Patient . | All cells . | Non-F cells* . | F cells . | ||
|---|---|---|---|---|---|
| HPLC . | Flow cytometry . | HPLC . | Flow cytometry . | ||
| H7-1 | 40 | 45 | NA | 120 | NA |
| H7-2 | 180 | 40 | 65 | 290 | 290 |
| H7-3 | 80 | 80 | 60 | 170 | 330 |
All data are given as the time in hours for removal of 50% of the cells. HPLC indicates high-performance liquid chromatography.
These data are for the first phase of removal of the non-F cells.
The 2-phase disappearance of non-F cells was a consistent finding in these patients and was present in both methods of HbF analysis. Examination of the analogous survival curves for unfractionated cells10 shows that there was some evidence of a 2-phase removal but that it was less striking. One possibility is that the 2 phases are a reflection of the different behavior of the intermediate and dense cells present in the labeled population. However, this is not supported by patient H7-1, who had a much purer population of dense cells but the same 2-phase survival behavior.
The remarkably stable number of light, labeled RBCs in fractions 1 and 2 are of considerable interest. These cells do not behave as expected for a typical, young light cell that was labeled and re-infused along with the HDE cells. First, a young light cell would be expected to quickly move to the denser fractions. Indeed, in studies with unfractionated cells,10 the number of labeled light cells declined markedly in the first 2 days after re-infusion. Second, after any initial changes the number of light cells was stable as long as the labeled HDE cells remained in the circulation in these studies and for at least 35 days with unfractionated cells.10 If these cells were light at the time of labeling and remained light, some decrease in their number would be expected at later times, even if they had a relatively long survival. We hypothesize that the most likely explanation for these cells is a steady conversion of dense cells to light cells prior to removal from the circulation. An unequivocal test of this hypothesis, however, will require the re-infusion of a pure population of dense cells, so that the appearance of light cells unambiguously demonstrates their formation from denser cells.
Most of the older (at least 8 days old), labeled light cells were resistant to dehydration by the potassium ionophore valinomycin, while only a low percentage of the unlabeled cells in the same fraction were resistant. Bookchin et al15,16 showed that a small fraction of light sickle cells that were not reticulocytes resisted the dehydrating effect of valinomycin and contained high sodium and low potassium. In the current work, we show that older cells (well beyond the reticulocyte stage) are present in the light fraction at an essentially constant number after re-infusion of labeled cells. These older, labeled, light cells were also shown to be resistant to valinomycin, although cation content was not directly measured. Valinomycin resistance does not prove that the older light cells contain high sodium and low potassium, since other possibilities (eg, severe inhibition of conductive chloride transport) may exist. However, it seems likely that the labeled, older, valinomycin-resistant cells would show the same sodium loading and potassium depletion as the unlabeled, nonreticulocyte, valinomycin-resistant cells demonstrated in the studies of Bookchin et al.15 16 High sodium content in older light cells implies a loss of volume regulation, confirming that they are not typical light cells and supporting the concept that they represent a terminal state.
HbF in the labeled cells was measured in 2 ways as they aged in the circulation. In the first method, biotinylated cells were isolated magnetically and HbF was determined by HPLC. In the second method, the percentage of HbF-containing cells in the biotin-positive population was determined by flow cytometry. These 2 methods of analysis of HbF-containing cells are equivalent only if there is no change in the amount of HbF per F cell as the cells age and are removed from the circulation. That is, if there is a range of HbF in F cells, and if the cells with higher F per F cell survive longer, then the F per F cell of the remaining F cells will be higher, and the amount of HbF in the magnetic bead samples will increase faster than the percentage of F cells. On the other hand, if the F-cell population is essentially uniform, at least with respect to survival, then the ratio of HbF content and percentage of F cells would remain constant, as observed in these experiments. Nevertheless, these experiments with HDE cells do not rule out the possibility that such selection takes place at a very young cell age, eg, during the reticulocyte stage or soon afterward. The data of Dover et al8 showed lower HbF per F cell in sickle reticulocytes compared with mature sickle cells, supporting a survival advantage for F cells with higher levels of HbF per F cell. Our data suggest that this selection does not extend to older, denser cells.
It has previously been shown that the percentage of PS+cells in the very light and dense fractions is higher than the percentage in the middle fractions.12 The presence of older and perhaps damaged cells in the light fractions, as discussed above, could contribute to the higher percentage of PS+cells in those fractions. The day-to-day variability observed for the unlabeled cells in the current studies is consistent with the data of Wood et al,18 who showed that individual patients often have marked variation in the percentage of PS+ cells. Analysis of the survival of PS+ cells with the use of the biotin label is made more difficult by the possibility that labeled cells that were PS− at the time of re-infusion could become PS+ as they age in the circulation. However, the data do put some limits on the survival characteristics of PS+ cells. If a discrete population of HDE cells experiences irreversible loss of asymmetry prior to labeling, and if these cells have a very short survival in the circulation, then we would expect the percentage of biotin-labeled PS+ HDE cells to decrease markedly shortly after re-infusion. This was not observed. On the other hand, if PS positivity is a more dynamic process, with cells regaining asymmetry, or with a balanced removal and formation of PS+ cells during the aging process, then the percentage of PS+ cells would be more stable, as seen in these experiments. However, even in this instance, the circulation time of PS+ cells could not be extremely short, since the replacement of labeled PS+ cells by conversion of labeled PS− cells would rapidly deplete the entire population of labeled cells. This is most evident in patient H7-1, for whom the labeled HDE cells were approximately 25% PS+. If the survival of PS+ cells was extremely short (eg, 1 hour) and the relatively constant percentage with time (Figure 8) was due to conversion of PS− cells to PS+ cells, then most of the labeled HDE cells would be removed within 3 or 4 hours. As noted above, this was not observed.
In summary, these studies show that HDE sickle cells have a very short survival in the circulation, and that HbF provides a significant advantage in this dehydrated population of cells. Furthermore, HDE cells show no consistent change in PS positivity as they age and are selected in the circulation, implying that the survival of PS+ is not extremely short. Finally, an older population of light, labeled cells was detected, supporting the hypothesis that these cells are derived from the dense cell population and represent a terminal state.
Supported by National Institutes of Health grants RO1 HL51174, RO1 HL57614, and P60 HL58421.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert S. Franco, University of Cincinnati College of Medicine, Vontz Center for Molecular Studies, Hematology/Oncology Division ML#508, 3125 Eden Ave, Cincinnati, OH 45267-0508; e-mail:robert.franco@uc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal