Abstract
Signaling by vascular endothelial growth factors (VEGFs) through VEGF receptors (VEGFRs) plays important roles in vascular development and hematopoiesis. The authors analyzed the function of VEGF-C signaling through both VEGFR-2 and VEGFR-3 in vasculoangiogenesis and hematopoiesis using a coculture of para-aortic splanchnopleural mesoderm (P-Sp) explants from mouse embryos with stromal cells (OP9). Vasculogenesis and angiogenesis were evaluated by the extent of vascular bed and network formation, respectively. Addition of VEGF-C to the P-Sp culture enhanced vascular bed formation and suppressed definitive hematopoiesis. Both vascular bed and network formations were completely suppressed by addition of soluble VEGFR-1–Fc competitor protein. Formation of vascular beds but not networks could be rescued by VEGF-C in the presence of the competitor, while both were rescued by VEGF-A. VEGFR-3–deficient embryos show the abnormal vasculature and severe anemia. Consistent with these in vivo findings, vascular bed formation in the P-Sp from the VEGFR-3–deficient embryos was enhanced to that in wild-type or heterozygous embryos, and hematopoiesis was severely suppressed. When VEGFR-3–Fc chimeric protein was added to trap endogenous VEGF-C in the P-Sp culture of the VEGFR-3–deficient embryos, vascular bed formation was suppressed and hematopoiesis was partially rescued. These results demonstrate that because VEGF-C signaling through VEGFR-2 works synergistically with VEGF-A, the binding of VEGF-C to VEGFR-3 consequently regulates VEGFR-2 signaling. In VEGFR-3–deficient embryos, an excess of VEGF-C signals through VEGFR-2 induced the disturbance of vasculogenesis and hematopoiesis during embryogenesis. This indicates that elaborated control through VEGFR-3 signaling is critical in vasculoangiogenesis and hematopoiesis.
Introduction
Embryonic development of blood vessels from endothelial cells consists of vasculogenesis and angiogenesis. Vasculogenesis is a process by which vascular endothelial cell precursors, angioblasts, proliferate and differentiate to form primitive blood vessels. Vascular network formation, or angiogenesis, subsequently occurs as a result of sprouting or branching from pre-existing vessels.1,2 Vasculoangiogenesis is regulated by paracrine signals, many of which are protein ligands that bind to and modulate the activity of transmembrane receptor tyrosine kinases.3-5 Among the vasculoangiogenic factors, vascular endothelial growth factor (VEGF) family members and their receptors are known to be critical for vasculoangiogenesis.6
The VEGF receptor (VEGFR) family consists of 3 members: VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4). At least 5 ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E) and placenta growth factor (PlGF) bind to 1 or 2 of these receptors. VEGF-A stimulates vascular endothelial cells through both VEGFR-1 and VEGFR-23; however, the roles of VEGFR-1 and VEGFR-2 differ significantly. VEGFR-1–deficient mice show aberrant vascular development,7 whereas VEGFR-2–deficient mice show severe defects in both vasculogenesis and hematopoiesis.8 VEGF-A is also indispensable for development of blood vessels: mice hemizygous for VEGF-A die from abnormal vasculature.9,10 VEGF-B binds to only VEGFR-1 and may be involved in regulation of extracellular matrix degradation, cell adhesion, and migration.11 VEGF-C and VEGF-D do not bind to VEGFR-1, although both are ligands for VEGFR-2 and VEGFR-3. Both VEGF-C and VEGF-D primarily affect development of lymphatic vasculature through activation of VEGFR-3.12-14 VEGF-E, which is a ligand for VEGFR-2, has been identified as a gene encoded by the parapoxvirus Orf virus. VEGF-E is expressed as the native protein in mammalian cells. VEGF-E and VEGF-A possess similar bioactivities: both factors stimulate the release of tissue factor and enhance the proliferation, chemotaxis, and sprouting of cultured vascular endothelial cells in vitro and in vivo.15
VEGF-C, which was identified as a ligand for VEGFR-3 and VEGFR-2,12 is expressed in endothelial cell precursors on embryonic day 8.5 (E8.5) in mouse. VEGFR-3 is later expressed in the lymphatic endothelium,16 indicating that it may play a role in the lymphatic vasculature.13 Transgenic mice overexpressing VEGF-C in the skin show atrophic changes in the dermis, where connective tissue is replaced by large lymphatic vessels.17 Thus, endothelial proliferation induced by VEGF-C leads to hyperplasia of the superficial lymphatic network but does not appear to induce sprouting of new vessels after birth. On the other hand, VEGFR-3–deficient mice die by E10.5. Although vasculogenesis and angiogenesis occur, large vessels are abnormally organized with defective lumens before the emergence of the lymphatic vessels.18 These results suggest that VEGF-C plays a critical role in proliferation and differentiation of vascular endothelial cells at an early embryonic stage. It is not known whether the biologic function of VEGF-C is the same as that of VEGF-A; nor has it been shown whether VEGFR-2 and VEGFR-3 transduce the same signals upon binding of VEGF-C.
We have recently established a coculture system consisting of para-aortic splanchnopleural mesoderm (P-Sp) explants and OP9 stromal cells.19 This culture system is used to assay both vasculoangiogenesis and hematopoiesis. In this study, we analyzed the biologic function of VEGF-C signaling through VEGFR-2 and VEGFR-3 using the P-Sp cultures from either wild-type or VEGFR-3–deficient mice in various conditions. Using this system, functional differences in VEGF-C signaling through VEGFR-2 and VEGFR-3 were detected.
Materials and methods
Reagents and animals
Recombinant human VEGF-A was purchased from Pepro Tech EC (London, England). A recombinant mature form of human VEGF-C was purified as described elsewhere.20 C57BL/6 mice, which were purchased from Japan SLC (Shizuoka, Japan), and VEGFR-3 heterozygous mutant mice were housed in environmentally controlled rooms of our facility of Kumamoto University Medical School under the guidelines of Kumamoto University for animal and recombinant DNA experiments. Embryos were genotyped by Southern blot analysis as described previously.18
LacZ and immunohistochemistry staining
Embryos and yolk sacs were removed and fixed in cold 4% paraformaldehyde/phophate-buffered saline (PBS) for 10 minutes, rinsed twice with PBS, and stained from 2 hours to overnight at 37°C in X-Gal buffer (5 mmol/L potassium ferrocyanide, 5 mmol/L potassium ferricyanide, 2 mmol/L MgCl2, and 1 mg/mL X-Gal in PBS [pH 7.2]). Procedures for whole-mount immunohistochemistry were described previously.19 Briefly, yolk sacs and embryos were fixed in 4% paraformaldehyde in PBS for 2 hours. The fixed samples were treated with methanol/30% hydrogen peroxide (5:1) at 4°C and dehydrated in phosphate-buffered Tween (PBST: PBS plus 0.5% Tween 20) for 30 minutes. The dehydrated embryos were incubated with anti–Flk-1/VEGFR-2 (AVAS12, rat antimouse monoclonal; gifts from Dr S-I. Nishikawa, Kyoto University, Kyoto, Japan)21 and anti–PECAM-1/CD31 (MEC13.3, rat antimouse monoclonal; Pharmingen, San Diego, CA) at 4°C overnight in PBSMT (PBS plus 5% skim milk [Difco Laboratories, Detroit, MI] and 0.1% Triton X-100). The following day, embryos and yolk sacs were washed with PBSMT buffer at 4°C 5 times (1 hour each) and incubated overnight at 4°C with 1 μg/mL horseradish peroxidase–conjugated antirat immunoglobulin (Ig) antibody (Biosource, Camarillo, CA). Embryos were then washed with PBSMT at 4°C 5 times (1 hour each) and PBS for 6 minutes 3 times at room temperature. Samples were then incubated with PBS containing 0.3 mg/mL diaminobenzidine (Dojin Chem, Kumamoto, Japan) in the presence of 0.05% NiCl2 for 10 to 30 minutes at room temperature, at which time hydrogen peroxide was added to 0.01% for the enzymatic reaction. The best signal-to-background ratio was typically achieved by a 5- to 10-minute incubation. The staining reaction was stopped by rinsing in PBS followed by a postfixation in 0.1% glutaraldehyde in PBS at 4°C overnight. For the detection of embryonic hemoglobin, rabbit antiembryonic polyclonal antibody (a gift from Dr T. Atsumi, Tsukuba Life Science Center, Ibaraki, Japan) was used.19 The primary antibody was developed with biotinylated antirabbit Ig antibody (Biosource) and, subsequently, horseradish peroxidase–conjugated streptavidin (Dako, Glostrup, Denmark). Then, 3-amino-9-ethyl carbazole was used for the detection for the color reaction.
Production of recombinant fusion protein
We constructed recombinant fusion genes containing the extracellular domain of murine VEGFR-1 and VEGFR-3 and the Fc part of human immunoglobulin as previously described.22,23VEGFR-1–Fc and VEGFR-3–Fc fusion proteins were obtained from the serum-free conditioned medium of COS7 cells transfected with these genes24 and purified over protein-A columns (Affi-Gel protein A; Bio-Rad, Hercules, CA). Coomassie brilliant blue staining of sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to assess purity and disulfide-linked dimerization.
FACS-gal staining
Fluorescence-activated cell sorter (FACS)-gal staining was carried out essentially as described.25 To reduce background fluorescence after dissection, tissues were incubated in Dulbecco modified Eagle medium (DMEM)/fetal calf serum (FCS) containing 3 mmol/L chloroquine for 30 minutes at 37°C, with 5% CO2. Following 2 rinses in PBS, tissues were incubated with dispase I (Boehringer Mannheim, Mannheim, Germany) for 10 minutes at room temperature. Dispersed embryo tissue was drawn through a 23G needle and treated to produce a single-cell suspension. After washing once in DMEM/FCS, cells were counted using a hemocytometer. The cells were then loaded with fluorescein di-β-d-galactopyranoside (FDG) (Molecular Probes, Eugene, OR) by osmotic shock. After the cells had been allowed to equilibrate at 37°C for 10 minutes, an equal volume of prewarmed 2 mmol/L FDG in sterile water was rapidly mixed with the cell suspension. After 1 minute of incubation at 37°C, FDG loading was stopped by adding 2 mL ice-cold staining media, and the tube was placed on ice for 2 hours to allow enzymatic conversion of FDG to fluorescein. After conversion, the cells were stained with monoclonal antibodies. The monoclonal antibodies used in imunofluorescence were anti–Flk-1/VEGFR-2 (AVAS12) and anti–PECAM-1/CD31 (MEC13.3). These monoclonal antibodies were purified and conjugated with phycoerythrin or biotin. Biotinylated antibodies were visualized with allophycocyanin-conjugated streptavidin (Caltag Laboratories, Burlingame, CA). The stained cells were analyzed by a FACSCalibur or a FACSvantage (Becton Dickinson, San Jose, CA).
In vitro culture of P-Sp and hematopoietic progenitors
The stromal cell line, OP9,26 was maintained in α-modified minimum essential media (Gibco BRL, Gaithersburg, MD) supplemented with 20% FCS (JRH Bioscience, Lenexa, KS). P-Sp culture conditions were as described.19 In brief, P-Sp explants of E9.5 embryos were cultured on OP9 stromal cells in RPMI1640 (Gibco BRL) with 10% FCS and 10−5-mol/L 2ME (Sigma, St Louis, MO) supplemented with interleukin (IL)-6 (20 ng/mL), IL-7 (20 ng/mL) (gifts from Dr T. Sudo, Toray Industries, Kamakura, Japan), stem cell factor (50 ng/mL) (a gift from Chemo-Sero-Therapeutic, Kumamoto, Japan), and erythropoietin (EPO, 2 U/mL) (a gift from Snow-Brand Milk Product, Tochigi, Japan) at 37°C in humidified 5%-CO2 air. The in vitro colony assay for hematopoietic progenitors was performed in a methylcellulose-containing semisolid medium as described previously.19
Quantitative analysis of vascular bed areas
After CD31 immunohistochemical staining, images were integrated using a color chilled 3CCD camera (Hamamatsu Photonics, Shizuoka, Japan). Image-processing software (NIH image 1.62/Power Macintosh G3, National Institutes of Health, Bethesda, MD) was used to determine alterations in the size of vascular beds. Four vascular beds from each P-Sp explant were measured under different culture conditions. The values of all parameters are shown as the mean ± SD. P values were calculated by 2-tailed Studentt test analysis.
RT-PCR analysis
RNA was extracted at E9.5 from P-Sp regions, yolk sacs, total embryos, and OP9 stromal cells using RNeasy Mini kit (Qiagen, Hilden, Germany). RNA was reverse transcribed using an Advantage RT-for-PCR Kit (Clontech, Palo Alto, CA). The complementary DNAs (cDNAs) were amplified with Advantage cDNA PCR Kit (Clontech) in a Gene AmpPCR System 9700 (Perkin-Elmer, Norwalk, CT) for 35 cycles. Sequences of specific primer for reverse transcription–polymerase chain reaction (RT-PCR) were as follows: VEGFR-1; sense: 5′-GAGAGCATCTATAAGGCAGCGGATT-3′; antisense: 5′-CACGTTTACAATGAGAGTGGCAGTG-3′; VEGFR-2; sense: 5′-TACACAATTCAGAGCGATGTGTGGT-3′; antisense: 5′-CTGGTTCCTCCAATGGGATATCTTC-3′; VEGFR-3; sense: 5′-AGATGCAGCCGGGCGCTGCGCT-3′; antisense: 5′-TAGGCTGTCCCCGGTGTCAATC-3′; VEGF-A; sense: 5′-AAGGAGAGCAGAAGTCCCATGAAGT-3′; antisense: 5′-TTCACATCTGCTGTGCTGTAGGAAG-3′; VEGF-C; sense: 5′-GCTTCTTGTCTCTGGCGTGTTC-3′; antisense: 5′-AAACTGATTGTGACTGGTTTGG-3′; β-actin; sense: 5′-TCGTGCGTGACATCAAAGAG-3′; antisense: 5′-TGGACAGTGAGGCCAAGATG-3′.
Each cycle consisted of 15 seconds of denaturation at 94°C and 3 minutes of annealing and extension at 68°C. PCR products were separated on a 1.5% agarose gel and stained with ethidium bromide.
Results
Localization of VEGFR-2 and VEGFR-3 in early embryogenesis
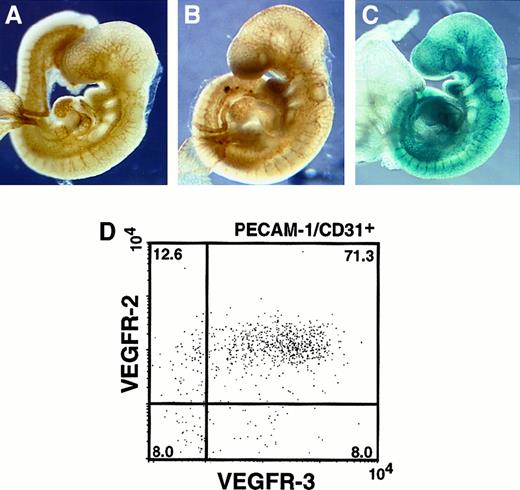
Although VEGFR-2 and VEGFR-3 are VEGF-C receptors, the distinct signaling pathways of VEGF-C through VEGFR-2 and VEGFR-3 have not been clarified. We first analyzed the expression pattern of VEGFR-2 and VEGFR-3 by whole-mount immunohistochemistry. A monoclonal antibody recognizing an endothelial marker, PECAM-1/CD31, was used to specifically visualize the vascular endothelium. The vascular network undergoes angiogenesis and remodeling to form a branched and intricate network in the head region of the E9.5 embryo (Figure1A). We examined the expression pattern of VEGFR-2 using an anti–VEGFR-2 monoclonal antibody. VEGFR-2 was expressed in vascular endothelial cells (Figure 1B). VEGFR-3 expression was examined in whole-mount by LacZ staining using VEGFR-3+/LacZ mice at E9.5.18 VEGFR-3 expression was clearly visible in the major vessels of the trunk where VEGFR-2 was also expressed (Figure 1C). We further analyzed VEGFR-2 and VEGFR-3 expression by FACS analysis. Cells from VEGFR-3+/LacZ mice at E9.5 were stained with FACS-gal and labeled with monoclonal antibodies directed against VEGFR-2 and CD31 (Figure 1D). Consistent with the immunohistochemical staining, flow cytometric analyses revealed that VEGFR-2+VEGFR-3+ cells constituted a major proportion (71%) of the CD31+ fraction at E9.5. Approximately 13% and 8% of all cells expressed only VEGFR-2 or VEGFR-3, respectively.
Localization of VEGFR-2 and VEGFR-3.
(A) E9.5 embryos were stained with anti-CD31 antibody to visualize all endothelial cells. (B) Embryos stained with anti–VEGFR-2 antibody. The pattern of VEGFR-2 expression was similar to that seen in panel A. (C) LacZ staining in VEGFR-3+/LacZ embryos at E9.5. VEGFR-3 expression was detected mainly in large vessels. (D) Flow cytometric analysis of VEGFR-2 and VEGFR-3 in PECAM-1/CD31+cells. After FACS-gal staining, these cells were stained with VEGFR-2 and CD31 antibodies (see “Materials and methods”). The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is representative of 3 independent experiments.
Localization of VEGFR-2 and VEGFR-3.
(A) E9.5 embryos were stained with anti-CD31 antibody to visualize all endothelial cells. (B) Embryos stained with anti–VEGFR-2 antibody. The pattern of VEGFR-2 expression was similar to that seen in panel A. (C) LacZ staining in VEGFR-3+/LacZ embryos at E9.5. VEGFR-3 expression was detected mainly in large vessels. (D) Flow cytometric analysis of VEGFR-2 and VEGFR-3 in PECAM-1/CD31+cells. After FACS-gal staining, these cells were stained with VEGFR-2 and CD31 antibodies (see “Materials and methods”). The numbers indicate the percentage of cells that appeared in each quadrant. The result shown is representative of 3 independent experiments.
Blood vessel defects in the yolk sac of a VEGFR-3–deficient embryo
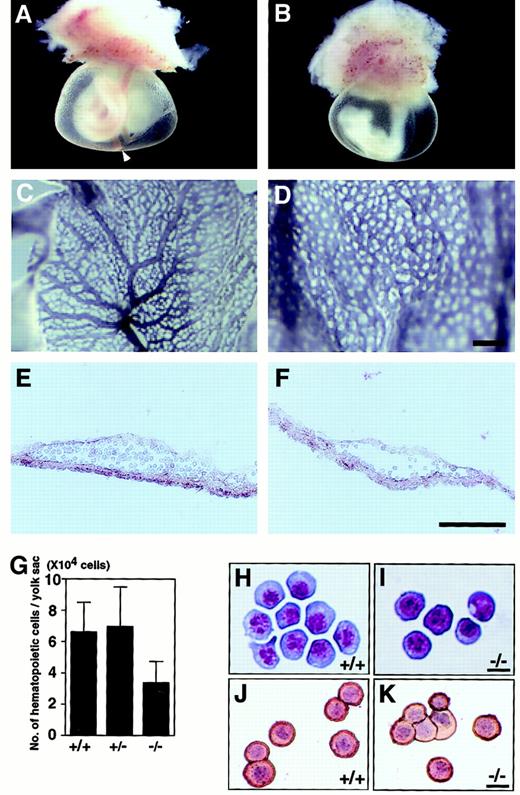
A VEGFR-3–deficient embryo at E9.5 was obviously pale compared with the wild type, allowing unequivocal identification of deficient mutants (Figure 2A-B). To determine the role of VEGFR-3 in vasculogenesis, we examined the vasculature of yolk sacs by immunohistochemical staining with the anti-CD31 antibody. The endothelial cells observed in the yolk sacs of VEGFR-3–deficient embryos expressed CD31 normally. However, we found abnormal vascular network formation and no caliber changes (Figure 2C-D). The main trunk of the vitelline artery was not seen in yolk sacs of VEGFR-3–deficient embryos (Figure 2A-B). Histologic examination of yolk sac sections (E9.5) revealed hematopoietic cells within normal blood vessels but few cells in the vessels of a VEGFR-3–deficient embryo (Figure 2E-F). The total cell number of blood cells from the yolk sacs in VEGFR-3–deficient mutants was reduced to 50% of controls (Figure 2G), and hematopoietic cells stained with May-Grünwald-Giemsa from a wild-type (Figure 2H) and a VEGFR-3–deficient (Figure 2I) embryo were revealed to be mature primitive erythroid cells, which were reactive to antiembryonic hemoglobin antibody (Figure 2J-K). Despite normal maturation of red blood cells, VEGFR-3–deficient embryos show marked anemia. To determine how development of primitive hematopoiesis is disturbed, we examined the colony-forming capacity of yolk sac cells of E9.5 VEGFR-3–deficient embryos. Erythroid and granulocyte-macrophage (GM) colony-forming cells (CFCs) did not show a significant difference (Table 1).
Vasculature and hematopoiesis of yolk sacs from wild-type and VEGFR-3–deficient mice at E9.5.
(A) Wild-type embryo shows a well-developed vasculature at E9.5. The arrowhead in panel A indicates the vitelline artery. (B) A yolk sac from VEGFR-3–deficient mutant shows severe anemia and defective large vessels. Yolk sacs from wild-type (C) and VEGFR-3–deficient mutant (D) at E9.5 stained in whole-mount with anti-CD31 antibody. Note the abnormality of vascular network formation and the lack of large vessels in the VEGFR-3–deficient yolk sac. Section of the yolk sac from wild-type (E) and VEGFR-3–deficient (F) embryo at E9.5. Note the smaller numbers of hematopoietic cells in the yolk sac derived from VEGFR-3–deficient embryos. (G) Number of hematopoietic cells present in the yolk sac from embryos at E9.5. Error bars indicate SEM (n = 4). E9.5 yolk sac stained with May-Grünwald-Giemsa from wild-type (H) and VEGFR-3–deficient (I) embryos. Staining blood cells from a wild-type (J) and VEGFR-3–deficient mutant (K) yolk sac were stained with antiembryonic hemoglobin polyclonal antibody. Hemoglobin expression is visualized as a red reaction product. The nucleus was counterstained with hematoxylin. Scale bars indicate 200 μm (C,D), 100 μm (E,F), and 10 μm (H-K).
Vasculature and hematopoiesis of yolk sacs from wild-type and VEGFR-3–deficient mice at E9.5.
(A) Wild-type embryo shows a well-developed vasculature at E9.5. The arrowhead in panel A indicates the vitelline artery. (B) A yolk sac from VEGFR-3–deficient mutant shows severe anemia and defective large vessels. Yolk sacs from wild-type (C) and VEGFR-3–deficient mutant (D) at E9.5 stained in whole-mount with anti-CD31 antibody. Note the abnormality of vascular network formation and the lack of large vessels in the VEGFR-3–deficient yolk sac. Section of the yolk sac from wild-type (E) and VEGFR-3–deficient (F) embryo at E9.5. Note the smaller numbers of hematopoietic cells in the yolk sac derived from VEGFR-3–deficient embryos. (G) Number of hematopoietic cells present in the yolk sac from embryos at E9.5. Error bars indicate SEM (n = 4). E9.5 yolk sac stained with May-Grünwald-Giemsa from wild-type (H) and VEGFR-3–deficient (I) embryos. Staining blood cells from a wild-type (J) and VEGFR-3–deficient mutant (K) yolk sac were stained with antiembryonic hemoglobin polyclonal antibody. Hemoglobin expression is visualized as a red reaction product. The nucleus was counterstained with hematoxylin. Scale bars indicate 200 μm (C,D), 100 μm (E,F), and 10 μm (H-K).
Yolk sac hematopoietic progenitors in E9.5 embryos
| Colonies per yolk sac . | |||||
|---|---|---|---|---|---|
| Wild-type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 98 ± 26 | 127 ± 25 | 135 ± 38 | 105 ± 31 | 84 ± 16 | 87 ± 22 |
| Colonies per yolk sac . | |||||
|---|---|---|---|---|---|
| Wild-type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 98 ± 26 | 127 ± 25 | 135 ± 38 | 105 ± 31 | 84 ± 16 | 87 ± 22 |
Hematopoietic colony assays from total yolk sac cells of VEGFR-3–deficient embryos and wild-type littermates at E9.5. Number of hematopoietic colonies were scored after 7 days of culture of yolk sac cells. Total cell numbers of colonies (>40 cells) per one yolk sac were obtained from 3 independent experiments and expressed as the means ± SEM (n = 3). Methylcellulose culture was performed in the presence of IL-3 (200 U/mL), stem cell factor (50 ng/mL), and EPO (2 U/mL).
Development of definitive hematopoietic cells in VEGFR-3–deficient embryos
VEGFR-3–deficient embryos display early embryonic lethality due to defects in the organization of large vessels prior to the emergence of lymphatic vessels.18 In addition, VEGFR-3–deficient embryos showed severe anemia (Figure 2A-B) and cardiac effusion, as reported by Dumont et al.18 Immunohistologic analysis of the embryo section showed abnormal vasculature and decreased numbers of blood cells in the dorsal aorta (data not shown).
To examine definitive hematopoiesis of the VEGFR-3–deficient embryos, we performed coculture of E9.5 P-Sp on OP9 stromal cells, which can support hematopoietic cell development.19 After 10 days of the culturing, we counted the total cell number per explant of P-Sp. It was found that the cell number decreased to 15% of the wild-type and heterozygous in VEGFR-3–homozygous mutant embryos, as described below. Staining with May-Grünwald-Giemsa showed that the blood cells from a VEGFR-3–deficient embryo contained very few hematopoietic cells, including erythroblasts, mature neutrophils, and macrophages (data not shown). CFCs in nonadherent cells were examined in a methylcellulose medium. As shown in Table2, the CFCs in P-Sp cells from VEGFR-3–deficient embryos were not detected on days 7 and 10. Moreover, FACS analyses also revealed less than 50% of erythroid cells (CD45+Ter119+ cells) in VEGFR-3–deficient embryos (data not shown). These findings demonstrated that total cell numbers of erythroid cells and definitive hematopoietic progenitors deceased in the VEGFR-3–deficient embryos.
Hematopoietic progenitors in P-Sp explants
| Colonies per P-Sp explant . | |||||
|---|---|---|---|---|---|
| Wild-type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 122 ± 53 | 887 ± 53 | 108 ± 47 | 705 ± 45 | 0 | 0 |
| Colonies per P-Sp explant . | |||||
|---|---|---|---|---|---|
| Wild-type . | Heterozygote . | Homozygote . | |||
| Erythroid . | GM . | Erythroid . | GM . | Erythroid . | GM . |
| 122 ± 53 | 887 ± 53 | 108 ± 47 | 705 ± 45 | 0 | 0 |
P-Sp of E9.5 VEGFR-3–deficient embryos and wild-type littermates were cultured on OP9 cells for 10 days. Nonadherent cells were then assayed for hematopoietic colonies in a methylcellulose medium. The number of hematopoietic colonies were scored after 7 days of culture. Total cell numbers of colonies (>40 cells) per one explant were obtained from 3 independent experiments and expressed as the means ± SEM (n = 4). Methylcellulose culture was performed in the presence of IL-3 (200 U/mL), stem cell factor (50 ng/mL), and EPO (2 U/mL).
VEGF-C is a regulator in differentiation into both endothelial and hematopoietic cells
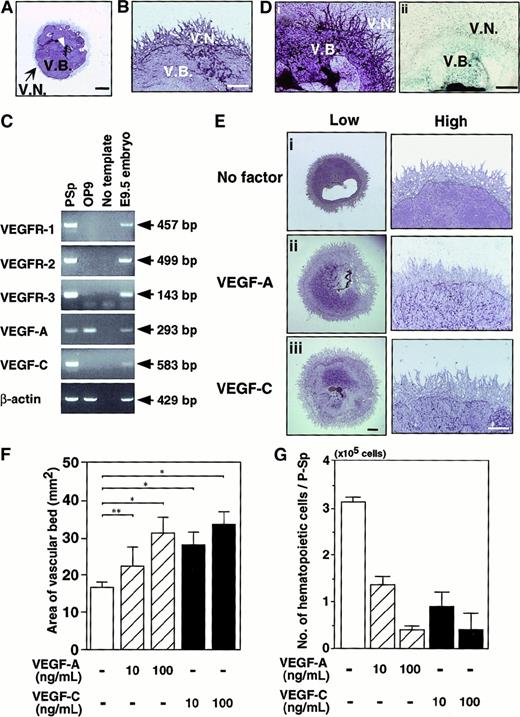
We investigated VEGF-C signaling through VEGFR-2 and VEGFR-3 using a P-Sp explant coculture system with OP9 stromal cells. The formation of vascular beds and networks from P-Sp explants was observed (Figure3A). Vascular beds are dense sheets of endothelial cells formed by proliferation of endothelial cells. Formation of a vascular bed in an explant is regarded as an indicator of vasculogenesis. Subsequent sprouting or branching of endothelial cells from vascular beds induced networks, a process similar to angiogenesis (Figure 3B). To investigate the endogenous expression of VEGFs and VEGFRs in this culture system, we examined the messenger RNA (mRNA) expression of VEGF-A, VEGF-C, and their specific receptors (VEGFR-1, VEGFR-2, and VEGFR-3) in P-Sp explants and OP9 stromal cells by RT-PCR. Messenger RNA encoding VEGFR-1, VEGFR-2, VEGFR-3, VEGF-A, and VEGF-C were detected in P-Sp explants, whereas VEGF-A mRNA was detected in OP9 cells (Figure 3C). We further histochemically examined the expression of VEGFR-2 and VEGFR-3 in the cultured vasculature of P-Sp. VEGFR-2 expression was clearly visible in a vascular bed and vascular networks, whereas VEGFR-3 expression was visualized only in a vascular bed (Figure 3D).
Effects of VEGF-A and VEGF-C on vasculoangiogenesis.
Representative vascular beds and networks stained with the anti-CD31 antibody in the para-aortic splanchnopleural mesoderm (P-Sp) coculturing system. Culture conditions are described in “Materials and methods.” Vascular bed formation (V.B.) proliferated around the P-Sp explant (arrowhead). Subsequently, endothelial cells sprouted on OP9 cells to form a vascular network (V.N.) after 14 days of culturing. (B) High magnification shows that vascular networks formed around vascular beds. (C) RNA was isolated from P-Sp explant, OP9 stromal cells, and whole embryos at E9.5, and the indicated transcripts were detected by RT-PCR using gene-specific primers described in “Materials and methods.” The PCR products were electrophoresed on 1.5% agarose gels. Bands were visualized by ethidium bromide staining. Messenger RNA from whole embryos was used as a positive control. (D) Staining profile of the cultured P-Sp explant. Plates were fixed after 10 days of culturing and then stained with anti–VEGFR-2 antibody (i). VEGFR-3 expression was visualized by LacZ staining using VEGFR-3+/LacZ P-Sp (ii). VEGFR-2 was expressed in vascular beds and networks. VEGFR-3 expression was only in vascular beds. (E) VEGF-A (100 ng/mL) or VEGF-C (100 ng/mL) was added to the culture of P-Sp explants. After 14 days of coculturing, the vascular cells were stained with the anti-CD31 antibody. Enhanced vascular bed formation is seen in the presence of VEGF-A (Eii) and VEGF-C (Eiii) compared with no factor (Ei) (low). Vascular network formation is not changed in the presence of VEGF-A and VEGF-C at higher magnification (high). (F) Quantitative analysis of vascular bed areas was performed with the NIH image computer analyzing system. The vascular bed area from P-Sp explants was measured. Error bars indicate SEM (n = 4). *P < .001, **P < .01. (G) The development of hematopoietic cells in the presence of VEGF-A and VEGF-C. The number of cells in the presence of VEGF-A and VEGF-C were reduced in a dose-dependent manner. Error bars indicate SEM (n = 4). Scale bar indicates 1 mm (A,E; low magnification,), 0.5 mm (B,D,E; high magnification).
Effects of VEGF-A and VEGF-C on vasculoangiogenesis.
Representative vascular beds and networks stained with the anti-CD31 antibody in the para-aortic splanchnopleural mesoderm (P-Sp) coculturing system. Culture conditions are described in “Materials and methods.” Vascular bed formation (V.B.) proliferated around the P-Sp explant (arrowhead). Subsequently, endothelial cells sprouted on OP9 cells to form a vascular network (V.N.) after 14 days of culturing. (B) High magnification shows that vascular networks formed around vascular beds. (C) RNA was isolated from P-Sp explant, OP9 stromal cells, and whole embryos at E9.5, and the indicated transcripts were detected by RT-PCR using gene-specific primers described in “Materials and methods.” The PCR products were electrophoresed on 1.5% agarose gels. Bands were visualized by ethidium bromide staining. Messenger RNA from whole embryos was used as a positive control. (D) Staining profile of the cultured P-Sp explant. Plates were fixed after 10 days of culturing and then stained with anti–VEGFR-2 antibody (i). VEGFR-3 expression was visualized by LacZ staining using VEGFR-3+/LacZ P-Sp (ii). VEGFR-2 was expressed in vascular beds and networks. VEGFR-3 expression was only in vascular beds. (E) VEGF-A (100 ng/mL) or VEGF-C (100 ng/mL) was added to the culture of P-Sp explants. After 14 days of coculturing, the vascular cells were stained with the anti-CD31 antibody. Enhanced vascular bed formation is seen in the presence of VEGF-A (Eii) and VEGF-C (Eiii) compared with no factor (Ei) (low). Vascular network formation is not changed in the presence of VEGF-A and VEGF-C at higher magnification (high). (F) Quantitative analysis of vascular bed areas was performed with the NIH image computer analyzing system. The vascular bed area from P-Sp explants was measured. Error bars indicate SEM (n = 4). *P < .001, **P < .01. (G) The development of hematopoietic cells in the presence of VEGF-A and VEGF-C. The number of cells in the presence of VEGF-A and VEGF-C were reduced in a dose-dependent manner. Error bars indicate SEM (n = 4). Scale bar indicates 1 mm (A,E; low magnification,), 0.5 mm (B,D,E; high magnification).
To clarify the effect of VEGF-A and VEGF-C, we added these factors to the culture of P-Sp explants. VEGF-A and VEGF-C enhanced the vascular bed formation but not the vascular network formation (Figure 3E). Quantitative analyses revealed that VEGF-A and VEGF-C evoked an increase in the area of the vascular bed dose-dependently (Figure 3F). On the other hand, VEGF-A and VEGF-C suppressed the generation of hematopoietic cells from P-Sp explants dose-dependently (Figure 3G). These results show that VEGF-C stimulates the vasculogenesis and suppresses the hematopoiesis in a dose-dependent manner like VEGF-A (Figure 3F-G). They also suggest that VEGF-C signaling through VEGFR-2 and VEGFR-3 is critical to the proliferation and differentiation of vascular endothelial cells and hematopoietic cells at an early embryonic stage. However, it is not clear whether the biologic function of VEGF-C is the same as that of VEGF-A; nor has it been shown whether VEGFR-2 and VEGFR-3 transduce the same signals upon binding to VEGF-C.
VEGF-C rescued vascular bed formation of the P-Sp explants in the absence of VEGF-A
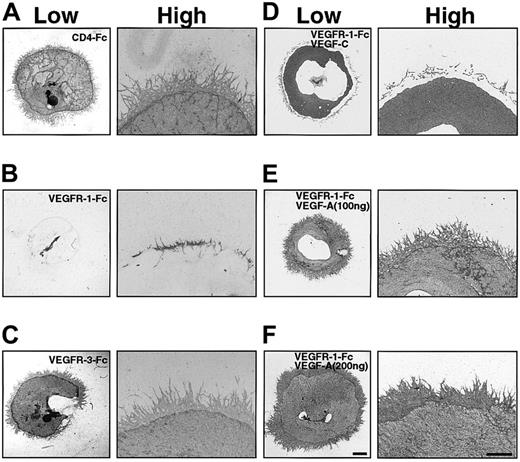
To assay the function of VEGF-A and VEGF-C in P-Sp explants, we prepared chimeric proteins containing the extracellular domain of either VEGFR-1 or VEGFR-3, which contain the ligand binding site, fused to human IgG Fc (VEGFR-1–Fc and VEGFR-3–Fc). These soluble chimeric proteins function as competitors by binding their respective ligands and preventing them from binding to receptors. Addition of 20 μg/mL of VEGFR-1–Fc to explant cultures suppressed formation of vascular beds and networks compared with a control, 20 μg/mL of CD4-Fc (Figure4A-B). By contrast, the formation of vascular beds and networks was unchanged by addition of 20 μg/mL of VEGFR-3–Fc (Figure 4A,C). In the presence of VEGFR-1–Fc, we next added VEGF-C to this P-Sp culture system. The addition of 100 ng/mL of VEGF-C rescued the formation of vascular beds completely but not vascular networks (Figure 4D), whereas VEGF-A rescued the formation of vascular beds and networks in a dose-dependent manner (Figure 4E-F). These results indicate that VEGF-C promotes independently the vascular bed formation through VEGFR-2 and VEGFR-3.
VEGFR-1–Fc inhibits vascular bed and vascular network formation.
Effects of chimeric proteins containing the extracellular domain of VEGFR-1 or VEGFR-3 in the OP9 coculturing system. Chimeric proteins (20 μg/mL) of the extracellular domain of VEGFR-1 and VEGFR-3 fused to the Fc of human Ig (VEGFR-1–Fc and VEGFR-3–Fc) were added to explant cultures. After 14 days, explants and OP9 cells were stained with the anti-CD31 antibody. CD4-Fc (20 μg/mL) was added as a control (A). Formation of vascular beds and networks was suppressed by 20 μg/mL VEGFR-1–Fc (B) but not by 20 μg/mL VEGFR-3–Fc (C). Addition of VEGF-C (100 ng/mL) rescued only the vascular bed formation of P-Sp explant suppressed by VEGFR-1–Fc (D). Addition of VEGF-A (100 ng/mL) (E) and (200 ng/mL) (F) rescued both vascular beds and network formation of P-Sp explants in the presence of VEGFR-1–Fc. Scale bar indicates 1 mm (low), 0.5 mm (high).
VEGFR-1–Fc inhibits vascular bed and vascular network formation.
Effects of chimeric proteins containing the extracellular domain of VEGFR-1 or VEGFR-3 in the OP9 coculturing system. Chimeric proteins (20 μg/mL) of the extracellular domain of VEGFR-1 and VEGFR-3 fused to the Fc of human Ig (VEGFR-1–Fc and VEGFR-3–Fc) were added to explant cultures. After 14 days, explants and OP9 cells were stained with the anti-CD31 antibody. CD4-Fc (20 μg/mL) was added as a control (A). Formation of vascular beds and networks was suppressed by 20 μg/mL VEGFR-1–Fc (B) but not by 20 μg/mL VEGFR-3–Fc (C). Addition of VEGF-C (100 ng/mL) rescued only the vascular bed formation of P-Sp explant suppressed by VEGFR-1–Fc (D). Addition of VEGF-A (100 ng/mL) (E) and (200 ng/mL) (F) rescued both vascular beds and network formation of P-Sp explants in the presence of VEGFR-1–Fc. Scale bar indicates 1 mm (low), 0.5 mm (high).
Enlarged vascular bed formation of P-Sp explants from VEGFR-3–deficient embryos
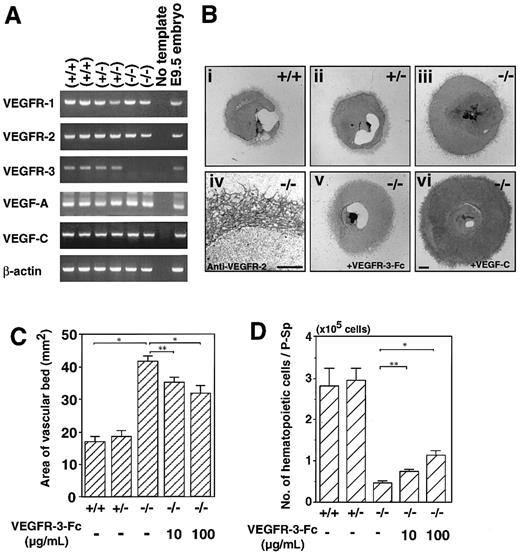
To investigate the differences in VEGF-C signaling through VEGFR-2 and VEGFR-3, we examined vascular bed and network formation from P-Sp explants of VEGFR-3–deficient embryos. First, we confirmed the mRNA expression of VEGF-related genes and receptors in wild-type, heterozygous, and homozygous mutant embryos by RT-PCR. The expression of VEGFR-1, VEGFR-2, VEGF-A, and VEGF-C was unchanged in VEGFR-3 homozygote mutant embryos (Figure5A).
Enlarged vascular bed formation in VEGFR-3–deficient embryos.
(A) RT-PCR analysis of gene expression in wild-type (+/+), heterozygous mutant (+/−), and VEGFR-3 homozygous mutant (−/−) embryos at E9.5. PCR primers and animal genotypes are described in “Materials and methods.” Data are from 2 independent littermates. PCR products were electrophoresed on agarose gels. VEGFR-3 message was not detected in the VEGFR-3 homozygous mutant embryos, and message levels for the remaining VEGFRs and VEGFs were unchanged among the 3 genotypes. (B) P-Sp explants derived from E9.5 wild-type (+/+) (Bi), VEGFR-3 heterozygous mutants (+/−) (Bii), VEGFR-3 homozygous mutant (−/−) embryos (Biii), and VEGFR-3 homozygous mutant embryos (−/−) in the presence of VEGFR-3–Fc (100 μg/mL) (Bv) were cultured on OP9 cells. Note that vascular bed formation was enhanced in VEGFR-3 homozygous mutant embryo explants (Biii) compared with in wild-type (Bi) and heterozygous (Bii) littermates. The vascular beds and networks from VEGFR-3–deficient embryo P-Sp expressed VEGFR-2 (Biv). VEGFR-3–Fc inhibited the vascular bed formation from VEGFR-3 homozygous mutant embryo explant (Bv). On the other hand, VEGF-C enhanced the formation of the vascular bed in VEGFR-3–deficient embryo explants (Bvi). (C) Quantitative analysis of the vascular bed area. The vascular areas from P-Sp explants were measured. Each column represents the mean area of the vascular bed. Error bars indicate SEM (n = 4). (D) Addition of VEGFR3–Fc rescued the suppression of hematopoietic cells from VEGFR-3 homozygous mutant P-Sp in a dose-dependent manner. Error bars indicate SEM (n = 3). *P < .001, **P < .01. Scale bar indicates 1 mm (Bi-iii, v, vi), 0.5 mm (Biv).
Enlarged vascular bed formation in VEGFR-3–deficient embryos.
(A) RT-PCR analysis of gene expression in wild-type (+/+), heterozygous mutant (+/−), and VEGFR-3 homozygous mutant (−/−) embryos at E9.5. PCR primers and animal genotypes are described in “Materials and methods.” Data are from 2 independent littermates. PCR products were electrophoresed on agarose gels. VEGFR-3 message was not detected in the VEGFR-3 homozygous mutant embryos, and message levels for the remaining VEGFRs and VEGFs were unchanged among the 3 genotypes. (B) P-Sp explants derived from E9.5 wild-type (+/+) (Bi), VEGFR-3 heterozygous mutants (+/−) (Bii), VEGFR-3 homozygous mutant (−/−) embryos (Biii), and VEGFR-3 homozygous mutant embryos (−/−) in the presence of VEGFR-3–Fc (100 μg/mL) (Bv) were cultured on OP9 cells. Note that vascular bed formation was enhanced in VEGFR-3 homozygous mutant embryo explants (Biii) compared with in wild-type (Bi) and heterozygous (Bii) littermates. The vascular beds and networks from VEGFR-3–deficient embryo P-Sp expressed VEGFR-2 (Biv). VEGFR-3–Fc inhibited the vascular bed formation from VEGFR-3 homozygous mutant embryo explant (Bv). On the other hand, VEGF-C enhanced the formation of the vascular bed in VEGFR-3–deficient embryo explants (Bvi). (C) Quantitative analysis of the vascular bed area. The vascular areas from P-Sp explants were measured. Each column represents the mean area of the vascular bed. Error bars indicate SEM (n = 4). (D) Addition of VEGFR3–Fc rescued the suppression of hematopoietic cells from VEGFR-3 homozygous mutant P-Sp in a dose-dependent manner. Error bars indicate SEM (n = 3). *P < .001, **P < .01. Scale bar indicates 1 mm (Bi-iii, v, vi), 0.5 mm (Biv).
Surprisingly, the vascular beds formed by P-Sp of the VEGFR-3 homozygous mutant embryos were larger than those seen in explants from wild-type and heterozygous mice (Figure 5Bi-iii). In quantitative analyses, the vascular bed from VEGFR-3 homozygous mutant mice was 2.5 times larger than that from control embryos (Figure 5C). VEGFR-2 expression was detected in vascular beds and networks of VEGFR-3–deficient mice (Figure 5Biv). Taking into consideration that VEGF-C signals transduce only through VEGFR-2 in VEGFR-3–deficient mice, these results suggest that VEGF-C signal-stimulated the vasculogenesis through VEGFR-2. To confirm this, VEGFR-3–Fc was added in this culture system. Formation of the vascular bed was significantly suppressed (Figure 5Bv,C), suggesting that VEGFR-3–Fc traps endogenous VEGF-C and decreases the signaling through VEGFR-2. Furthermore, exogenous VEGF-C enhanced the formation of vascular beds in VEGFR-3–deficient mice (Figure 5Bvi). On the other hand, addition of VEGFR-3–Fc significantly increased the number of hematopoietic cells generated from P-Sp of VEGFR-3–deficient mice (Figure5D).
Discussion
In this report, we show that VEGF-C signaling through VEGFR-2 and VEGFR-3 plays essential and distinct roles in embryonic vasculogenesis. First, we examined the expression of VEGFR-2 and VEGFR-3 in mouse embryos. Both receptors show similar immunohistochemical staining patterns on vascular endothelial cells of mouse embryos at E9.5. VEGFR-1 and VEGFR-2 expression is also restricted to the vascular endothelium.27,28 VEGFR-3 is expressed in the vascular endothelium during embryonic development and is subsequently restricted to lymphatic endothelium postnatally.13,16 These results show that the VEGFR-2 and VEGFR-3 expression pattern changes throughout the course of embryogenesis and suggest that both receptors are important for vasculoangiogenesis. FACS analysis showed that 71% of the CD31+ cells were positive for both VEGFR-2 and VEGFR-3. It is interesting to characterize VEGFR-2+ cells (13%) and VEGFR-3+ cells (8%). It is reported that VEGFR-3 is expressed in avian notochord at the gastrulation stage.29VEGFR-2+VEGFR-3+ cells could potentially transduce both VEGF-A and VEGF-C signals. These results pose 2 questions: first, is the biologic function of VEGF-C equivalent to that of VEGF-A in these cells and, second, do VEGFR-2 and VEGFR-3 transduce the same VEGF-C signals to endothelial cells?
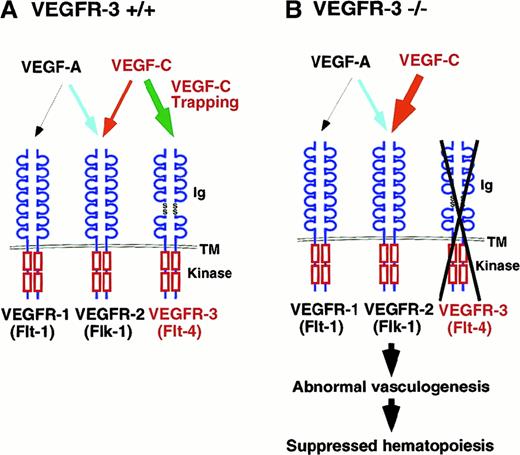
We evaluated the effects of VEGFs on vasculoangiogenesis using mouse embryonic P-Sp explants containing endothelial precursor cells cocultured with OP9 stromal cells.19 We showed that VEGFR-1, VEGFR-2, VEGFR-3, VEGF-A, and VEGF-C are all expressed in this system, although VEGF-A was detected in OP9 cells. This observation indicates that signaling through VEGFR-1, VEGFR-2, and VEGFR-3 potentially mediates endothelial cell growth in the culture system. Furthermore, we observed that VEGFR-2 was expressed in vascular beds and networks, whereas VEGFR-3 was detected in vascular beds. These results show that VEGFR-2 was expressed in all vascular endothelial cells, but VEGFR-3 was restricted to proliferating endothelial cells. In fact, exogenous VEGF-A or VEGF-C enhanced vascular bed formation 3-fold compared with controls. Consistent with this, it has been shown that VEGF-A and VEGF-C promote differentiation of endothelial cells in vitro in chick embryos.29 30 Our findings lead us to several conclusions: (1) VEGF-A/VEGFR-2 is a primary signal of endothelial proliferation, (2) VEGF-C/VEGFR-3 is also involved in vasculogenesis and hematopoiesis and, finally, (3) the binding of VEGF-C to VEGFR-3 regulates VEGFR-2 signaling. Taken together, they suggest the model depicted in Figure 6.
Role of VEGF-C signaling in vasculogenesis and angiogenesis.
(A) In this model, VEGF-C transduced different signals through VEGFR-2 and VEGFR-3 in vasculoangiogenesis. VEGF-C signaling through VEGFR-2 (red arrow) enhances VEGF-A signaling, promoting vasculoangiogenesis. VEGFR-3 works as a trapper of VEGF-C for the inhibition of VEGF-C signaling through VEGFR-2 (green arrow). The extracellular domain of VEGFRs is depicted at the top, and the split kinase domains are shown at the C-terminal to the transmembrane region. Various ligand and receptor combinations are depicted by arrows. Ig indicates immunoglobulin-like domain; Kinase, tyrosine kinase domain; TM, transmembrane domain. (B) Signal disturbance in VEGFR-3–deficient embryos. In VEGFR-3–deficient embryos, vascular endothelial cells show proliferation but, on the other hand, hematopoiesis is suppressed by the disturbance of signaling of VEGF-C.
Role of VEGF-C signaling in vasculogenesis and angiogenesis.
(A) In this model, VEGF-C transduced different signals through VEGFR-2 and VEGFR-3 in vasculoangiogenesis. VEGF-C signaling through VEGFR-2 (red arrow) enhances VEGF-A signaling, promoting vasculoangiogenesis. VEGFR-3 works as a trapper of VEGF-C for the inhibition of VEGF-C signaling through VEGFR-2 (green arrow). The extracellular domain of VEGFRs is depicted at the top, and the split kinase domains are shown at the C-terminal to the transmembrane region. Various ligand and receptor combinations are depicted by arrows. Ig indicates immunoglobulin-like domain; Kinase, tyrosine kinase domain; TM, transmembrane domain. (B) Signal disturbance in VEGFR-3–deficient embryos. In VEGFR-3–deficient embryos, vascular endothelial cells show proliferation but, on the other hand, hematopoiesis is suppressed by the disturbance of signaling of VEGF-C.
Vascular bed and network formation is completely inhibited by addition of a soluble VEGFR-1–Fc chimeric protein to the coculture system, indicating that soluble VEGFR-1–Fc inhibits VEGF-A and VEGF-B binding to VEGFR-1 and VEGFR-2. Endogenous levels of VEGF-C are not sufficient to stimulate formation of vascular beds and networks in the presence of VEGFR-1–Fc. That VEGF-A plays an important role in vasculogenesis is supported by the finding that proliferation of endothelial cells is significantly inhibited in VEGF-A mutant embryos.9,10 Vascular bed and network formation is, however, normal in P-Sp cultures in the presence of VEGFR-3–Fc. Although VEGF-C and VEGF-D signaling is inhibited by VEGFR-3–Fc, VEGF-A signaling through VEGFR-1 and VEGFR-2 independently promotes vasculogenesis. Exogenous VEGF-C rescued vascular bed formation but not network formation in the presence of VEGFR-1–Fc, suggesting that VEGF-C promotes vasculogenesis but not angiogenesis. Even in VEGF-A mutant embryos, some endothelial cells survive,9 and that survival may be promoted by VEGF-C. We are interested in determining whether exogenous VEGF-C will rescue the VEGF-A–deficient phenotype.
The vascular bed formed by VEGFR-3–deficient mice is 2.5 times larger than that formed by wild-type or VEGFR-3 heterozygous embryos in P-Sp culture. This finding is correlated with the phenotypes observed in VEGFR-3–deficient embryos. An immature pattern is seen in the perineural vascular plexus of the developing head region, and large vessels are disorganized with defective lumens.18 In addition, we noted the absence of the large vitelline artery. VEGF-C binding affinity is reported to be about 135 pM for VEGFR-3 and about 410 pM for VEGFR-2.12,31 Excessive VEGF-C signaling through VEGFR-2 may occur in P-Sp from VEGFR-3–deficient embryos because VEGFR-2 would be the sole receptor for VEGF-C. Through VEGFR-2, VEGF-C enhances vasculogenesis synergistically with VEGF-A. VEGFR-3 sequesters VEGF-C, thereby regulating VEGFR-2 signaling. In a similar context, Fong et al reported that VEGFR-1 regulates the amount of free VEGF-A available for vascular development.32 In the absence of VEGFR-1, there may be disregulation of the amount of free VEGF-A, leading to excessive development of hemangioblasts. Although there is no direct evidence that VEGF-C through VEGFR-2 enhances VEGF-A signaling, there is evidence to support our conclusion. Keyt et al showed that there are no homologous regions in the receptor binding sites of VEGF-C and VEGF-A.33 Furthermore, VEGF-C was shown to synergize with VEGF-A in the induction of angiogenesis in collagen gels.34 If VEGF-A and VEGF-C recognize the same binding site in VEGFR-2, these synergistic effects would not be seen. In VEGFR-2–deficient mice, VEGFR-3 expression is detectable by RT-PCR analysis.8 It is indicated that the VEGFR-3 signaling cannot replace the VEGFR-2 signaling during early embryogenesis. However, it is interesting to clarify whether exogenous VEGF-C can rescue the defect of VEGFR-2 knockout mice.
We demonstrated that VEGFR-3 is essential for definitive hematopoietic cell proliferation. Hematopoiesis consists of primitive hematopoiesis in the yolk sac and definitive hematopoiesis, which develops in the P-Sp region, including the vitelline artery.35-37 Primitive hematopoietic cell numbers in the yolk sac of VEGFR-3–deficient mice were reduced, although their precursors did not decrease significantly. On the other hand, definitive hematopoiesis was profoundly suppressed in VEGFR-3–deficient embryos. Severe anemia may be a cause of their death in addition to cardiovascular abnormalities. Because the direct effect of VEGFs on hematopoiesis has not been reported, the defect in definitive hematopoiesis might be caused by vascular abnormalities, including defect of the vitelline artery between the embryo and yolk sac. Hematopoietic cells and endothelial cells are derived from common progenitor cells, hemangioblasts,38 which might be VEGFR-2+ and TIE-2+.39,40 It is not clear whether VEGFR-3 is involved at this level. Endothelial differentiation requires VEGFs, whereas hematopoietic differentiation proceeds in the absence of VEGFs.30 Similarly, we show that VEGF-C suppressed the definitive hematopoiesis and enhanced endothelial cell growth from P-Sp. The VEGFR-3–deficient embryo is a good model that shows abnormal vasculogenesis and suppressed hematopoiesis as a result. The anemia may be a secondary effect due to the defects in vascular cells that produce hematopoietic cytokines and growth factors (Figure 6).
Here we have demonstrated that VEGF-C signaling through VEGFR-3 plays an important role in vasculogenesis and that the levels of free VEGF-C may be critical for this signaling. In addition, we have also shown that VEGF-C signaling through VEGFR-2 enhances VEGF-A activity. These findings may contribute to the development of new therapeutics for ischemic diseases.
Acknowledgments
The authors thank Dr O. Ohneda for valuable discussions and Hiroaki Kodama for providing us with OP9 stromal cells.
Supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshio Suda, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University, 2-2-1 Honjo, Kumamoto 860-0811, Japan; e-mail:sudato@gpo.kumamoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal