Abstract
Thymomas are the only tumors that are proven to generate mature T cells from immature precursors. It is unknown, however, whether intratumorous thymopoiesis has an impact on the peripheral T-cell pool and might thus be related to the high frequency of thymoma-associated myasthenia gravis. This study shows, using fluorescence-activated cell sorting-based analyses and T-cell proliferation assays, that thymopoiesis and T-cell function in thymomas correspond with immunologic alterations in the blood. Specifically, the proportion of circulating CD45RA+CD8+ T cells is significantly increased in patients with thymoma compared with normal controls, in accordance with intratumorous T-cell development that is abnormally skewed toward the CD8+ phenotype. Moreover, it is primarily the proportion of circulating CD45RA+CD8+ T cells that decreases after thymectomy. The results also demonstrate that T cells reactive toward recombinant autoantigens are distributed equally between thymomas and blood, whereas T-cell responses to foreign antigen (ie, tetanus toxoid) are seen only among circulating T cells and not among thymoma-derived T cells. These functional studies support the hypothesis that thymopoiesis occurring within thymomas alters the peripheral T-cell repertoire. Because many thymomas are enriched with autoantigen-specific T cells, a disturbance of circulating T-cell subset composition by export of intratumorous T cells may contribute to paraneoplastic autoimmune disease arising in patients with thymoma.
Introduction
Thymomas are epithelial tumors of the thymus.1 Among human neoplasms, thymomas are associated with the highest frequency of paraneoplastic autoimmune disease, of which myasthenia gravis (MG) is the most common.2Autoantibodies in patients with thymoma are directed mainly against antigens of skeletal muscle and the nervous system.3However, the main autoantigen, acetylcholine receptor (AChR), is usually not present intact within the neoplastic thymic milieu,4,5 and expression of other autoantigens does not always correspond with the presence of MG.6,7 Moreover, autoantibodies are not produced within the thymoma, and autoimmune disease does not always remit after tumor resection.8These findings render difficult the formation of a unifying hypothesis regarding the onset of thymoma-associated autoimmune disease.8 These features are different from those seen in the more common form of MG associated with thymic follicular hyperplasia (TFH). In this form, the intact autoantigen AChR is present within the hyperplastic thymus.9 Autoantigen-specific T and B cells occur within the thymus, and MG frequently remits after thymectomy.8
Thymomas are also unique among human neoplasms in their capacity to generate mature T cells.10 Recently, it has been shown that intratumorous T-cell maturation is abnormal in that the mature CD45RA+ T-cell subset is decreased compared with normal thymus.11 Furthermore, mature intratumorous T cells often have an increased autoantigen-specific potential toward fragments of the α subunit of AChR compared with T cells from normal thymus.7,11,12 Based on these observations, it has been proposed that thymomas may generate autoantigen-specific T cells by a process of abnormal positive or negative T-cell selection.7,8,13 Because there is virtually no interaction between autoantigen-specific T cells and autoantibody-producing B cells within thymomas,14 this hypothesis implies that autoantigen-specific T cells must leave the thymoma to become pathogenically relevant.2,15 Unfortunately, determining whether naive mature T cells in the blood are derived from the thymoma is difficult, because normal thymus adjacent to the tumor also releases mature T cells into the circulating T-cell pool.16 Until now, the question of T-cell export from thymomas has only been addressed in rare cases in which blood lymphocytosis has resolved on resection of the thymoma, as reviewed by de Jong and colleagues.17 Whether these cases are representative of conventional thymoma, however, is unknown.
The present study addresses the question of whether conventional thymomas release mature autoantigen-specific T cells into the periphery. We have used a fluorescence-activated cell sorting (FACS)-based method for quantifying circulating naive (CD45RA+) T cells, previously described as a viable method to assess T-cell export from the thymus, comparable to direct fluorescein isothiocyanate (FITC) labeling of thymocytes in rodent models.18-22 Using this method, we have demonstrated a significant increase in the proportion of circulating CD45RA+CD8+ T cells in patients with thymoma, compared with normal controls. Interestingly, this specific alteration of T-cell subset composition in the blood is reversed by thymectomy. Functional studies demonstrate correspondence between autoantigen-driven T-cell responses in thymomas and similar responses in the blood. Taken together, these findings suggest that abnormal thymopoiesis occurring within thymomas may alter the peripheral T-cell repertoire, thereby generating greater autoantigen-specific potential.
Patients, materials, and methods
Patients, tumors, and cell preparation
Approval for these studies was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki.
The clinical data are summarized in Table1. Not all patients, cells, or tissues were used for every FACS-based analysis. The number of patients studied in each test is indicated in the appropriate figure legends. The diagnosis of MG was based on clinical features, decrement testing on 3-Hz serial stimulation, and the detection of anti-AChR autoantibodies as described previously.11,23 Thymomas were classified according to the recent World Health Organization (WHO) classification1 as either WHO type AB (mixed) or WHO type B2 (cortical). Because mixed and cortical thymomas are the most frequent among MG-associated thymomas, and they exhibit thymus-like features,7,11,23 we specifically selected these thymoma subtypes for study. Blood samples were taken at thymectomy and 3 to 66 months later. At these time points, tumor recurrence was not detected in any case. Hematologic data were available in 13 of 15 patients with thymoma and 9 of 11 patients with TFH for whom peripheral blood lymphocytes (PBL) were characterized by FACS-based analysis (Figures1 and 2). No patient with available hematologic data exhibited leukocytosis (range, 4200-9000/μL; mean, 6450/μL). Blood samples from healthy (nonmyasthenic) individuals matched for sex and age were studied as controls. Other control groups included patients with typical early-onset MG without thymoma but with TFH and patients with medullary thymomas (WHO type A) containing few intratumorous T cells, most of which express a mature immunophenotype. Thymocytes were isolated from normal thymus or thymoma, and PBL from blood, as described previously.11 Cells were used immediately for proliferation assays. Aliquots were cryopreserved in freezing medium containing 90% fetal calf serum and 10% dimethyl sulfoxide (Sigma, Deisenhofen, Germany) for later antibody staining.
Clinical data
| Fig. no. . | Diagnosis . | No. . | Sex F/M . | Age (y) mean (range) . | Tumor stage* (no. of cases) . | Anti-AChR antibody titer (nmol/L)† mean (range) . | Treatment‡ (no. of cases) . | Thymoma subgroups . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT MG+ . | MXT MG+ . | CT MG− . | MXT MG− . | ||||||||
| 1 | Tm | 15 | 9/6 | 55.1 (33-75) | II (12); III (1); IVa (2) | 60.2 (3-342) | None | 8 | 1 | 4 | 2 |
| TFH | 11 | 10/1 | 25.9 (13-36) | — | 24.7 (0.5-87) | None | — | — | — | — | |
| 2 | Tm | 15 | 9/6 | 55.1 (33-75) | II (12); III (1); IVa (2) | 60.2 (3-342) | None | 8 | 1 | 4 | 2 |
| TFH | 7 | 7/0 | 27.2 (13-36) | — | 33.0 (1-87) | None | — | — | — | — | |
| 3A | resT | 9 | 7/2 | 52.4 (33-79) | I (2); II (6); IVa (1) | 25.7 (10-70) | Steroids (2) | 8 | 1 | — | — |
| Tm | 19 | 14/5 | 54.1 (33-79) | I (2); II (13); III (2); IVa (2) | 30.6 (3-71) | Steroids (1) | 10 | 3 | 3 | 3 | |
| TFH | 11 | 10/1 | 25.9 (13-36) | — | 24.7 (0.5-87) | None | — | — | — | — | |
| 3B | Tm | 9 | 6/3 | 57.8 (36-79) | II (7); III (1); IVa (1) | 34.3 (17-67) | Steroids (1) | 2 | 1 | 3 | 3 |
| TFH | 7 | 7/0 | 27.2 (13-36) | — | 33.0 (1-87) | None | — | — | — | — | |
| 4A | Tm | 12 | 7/5 | 54.2 (33-75) | II (10); IVa (2) | 64.6 (2-342) | None | 7 | 1 | 1 | 3 |
| TFH | 3 | 3/0 | 21.7 (13-32) | — | 26.0 (12-44) | None | — | — | — | — | |
| 4B | Tm | 10 | 6/4 | 53.2 (33-75) | II (9); IVa (1) | 72.2 (3-342) | None | 5 | 1 | 1 | 3 |
| 5 | AChR | 20 | 13/7 | 53.6 (29-75) | I (2); II (15); III (2); IVa (1) | 20.8 (3-70) | Steroids (2) | 16 | 4 | — | — |
| NF-M | 16 | 12/4 | 52.3 (29-68) | I (1); II (12); III (2); IVa (1) | 19.0 (3-65) | Steroids (2) | 14 | 2 | — | — | |
| TT | 14 | 10/4 | 57.6 (33-75) | I (2); II (9); III (3) | 18.2 (3-62) | Steroids (2) | 10 | 4 | — | — | |
| Med | 6 | 3/3 | 67.8 (60-74) | I (2); II (4) | ND MG+ (3 cases) | Steroids (3) | — | — | — | — | |
| Fig. no. . | Diagnosis . | No. . | Sex F/M . | Age (y) mean (range) . | Tumor stage* (no. of cases) . | Anti-AChR antibody titer (nmol/L)† mean (range) . | Treatment‡ (no. of cases) . | Thymoma subgroups . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT MG+ . | MXT MG+ . | CT MG− . | MXT MG− . | ||||||||
| 1 | Tm | 15 | 9/6 | 55.1 (33-75) | II (12); III (1); IVa (2) | 60.2 (3-342) | None | 8 | 1 | 4 | 2 |
| TFH | 11 | 10/1 | 25.9 (13-36) | — | 24.7 (0.5-87) | None | — | — | — | — | |
| 2 | Tm | 15 | 9/6 | 55.1 (33-75) | II (12); III (1); IVa (2) | 60.2 (3-342) | None | 8 | 1 | 4 | 2 |
| TFH | 7 | 7/0 | 27.2 (13-36) | — | 33.0 (1-87) | None | — | — | — | — | |
| 3A | resT | 9 | 7/2 | 52.4 (33-79) | I (2); II (6); IVa (1) | 25.7 (10-70) | Steroids (2) | 8 | 1 | — | — |
| Tm | 19 | 14/5 | 54.1 (33-79) | I (2); II (13); III (2); IVa (2) | 30.6 (3-71) | Steroids (1) | 10 | 3 | 3 | 3 | |
| TFH | 11 | 10/1 | 25.9 (13-36) | — | 24.7 (0.5-87) | None | — | — | — | — | |
| 3B | Tm | 9 | 6/3 | 57.8 (36-79) | II (7); III (1); IVa (1) | 34.3 (17-67) | Steroids (1) | 2 | 1 | 3 | 3 |
| TFH | 7 | 7/0 | 27.2 (13-36) | — | 33.0 (1-87) | None | — | — | — | — | |
| 4A | Tm | 12 | 7/5 | 54.2 (33-75) | II (10); IVa (2) | 64.6 (2-342) | None | 7 | 1 | 1 | 3 |
| TFH | 3 | 3/0 | 21.7 (13-32) | — | 26.0 (12-44) | None | — | — | — | — | |
| 4B | Tm | 10 | 6/4 | 53.2 (33-75) | II (9); IVa (1) | 72.2 (3-342) | None | 5 | 1 | 1 | 3 |
| 5 | AChR | 20 | 13/7 | 53.6 (29-75) | I (2); II (15); III (2); IVa (1) | 20.8 (3-70) | Steroids (2) | 16 | 4 | — | — |
| NF-M | 16 | 12/4 | 52.3 (29-68) | I (1); II (12); III (2); IVa (1) | 19.0 (3-65) | Steroids (2) | 14 | 2 | — | — | |
| TT | 14 | 10/4 | 57.6 (33-75) | I (2); II (9); III (3) | 18.2 (3-62) | Steroids (2) | 10 | 4 | — | — | |
| Med | 6 | 3/3 | 67.8 (60-74) | I (2); II (4) | ND MG+ (3 cases) | Steroids (3) | — | — | — | — | |
According to Masaoka and colleagues.37
Refers to titers of myasthenic thymoma patients (all nonmyasthenic patients have titers < 0.5 nmol/L).
Immunosuppressive treatment.
AChR indicates acetylcholine receptor; CT, cortical thymoma; MG, myasthenia gravis; Med, medullary thymoma; MXT, mixed thymoma; ND, not determined; NF-M, midsize neurofilament; resT, residual thymus; TFH, thymic follicular hyperplasia; Tm, thymoma.
Overlap among patients analyzed by FACS and proliferation assay: PBL from 4 of 9 myasthenic thymoma patients, thymocytes from 10 of 13 myasthenic thymoma patients, and PBL from 6 healthy controls were analyzed by FACS and standard T-cell proliferation assays.
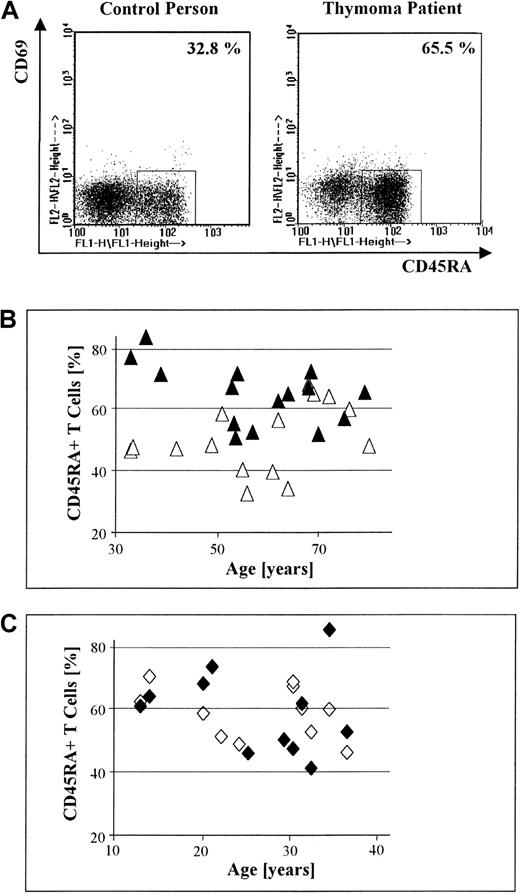
Significant increase in the percentage of peripheral CD45RA+ T cells in patients with thymoma compared with nonthymoma patients.
(A) Dot-plot displays of 3-color FACS-based analyses of CD69 and CD45RA expression by PBL, gated on CD3+ T cells, in a control versus a thymoma patient (both men, 64 years old). The proportion of circulating CD45RA+CD69− T cells is increased in the thymoma patient compared with the healthy control. (B) The percentage of circulating CD45RA+ T cells in 15 patients with thymoma (▴) is significantly higher than the percentage of circulating CD45RA+ T cells in sex- and age-matched healthy controls (▵; P = .01). (C) There is no significant difference in the percentage of circulating CD45RA+ T cells between 11 MG patients without thymoma but with TFH (♦) and normal sex- and age-matched controls (◊; P = .95).
Significant increase in the percentage of peripheral CD45RA+ T cells in patients with thymoma compared with nonthymoma patients.
(A) Dot-plot displays of 3-color FACS-based analyses of CD69 and CD45RA expression by PBL, gated on CD3+ T cells, in a control versus a thymoma patient (both men, 64 years old). The proportion of circulating CD45RA+CD69− T cells is increased in the thymoma patient compared with the healthy control. (B) The percentage of circulating CD45RA+ T cells in 15 patients with thymoma (▴) is significantly higher than the percentage of circulating CD45RA+ T cells in sex- and age-matched healthy controls (▵; P = .01). (C) There is no significant difference in the percentage of circulating CD45RA+ T cells between 11 MG patients without thymoma but with TFH (♦) and normal sex- and age-matched controls (◊; P = .95).
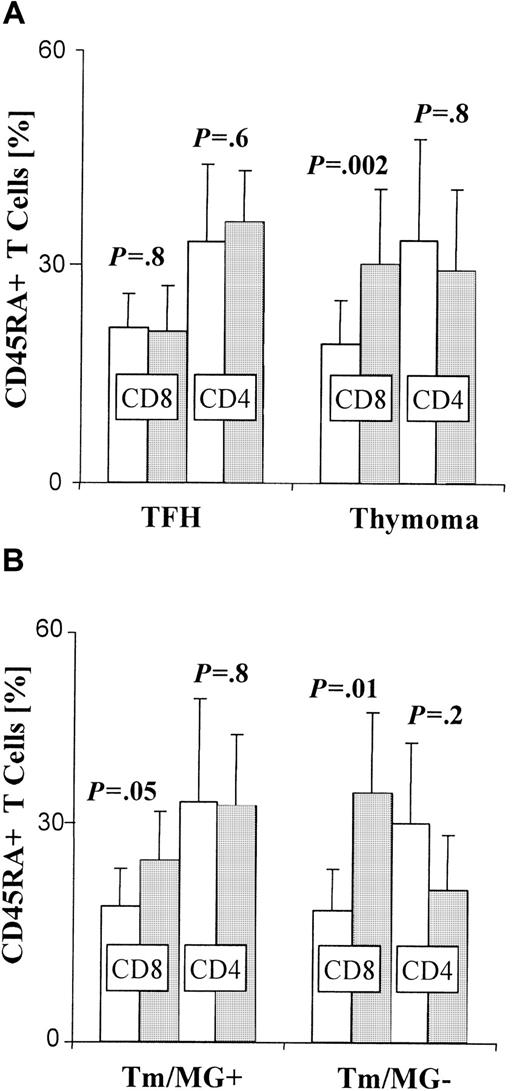
Significant increase in the percentage of peripheral CD45RA+ CD8+ T cells in thymoma patients.
Three-color FACS-based analyses of CD8, CD4, and CD45RA expression by PBL, gated on CD3+ T cells. Error bars depict the SE. Controls, ■; patients, ░. (A) Bars indicate the mean percentage of CD8+ or CD4+ cells among circulating CD45RA+ T cells in patients with thymic follicular hyperplasia (TFH; n = 7), thymoma patients (Tm; n = 15), and corresponding control groups. The CD8+ subset in thymoma patients is significantly increased when compared with controls (P = .002), whereas the percentage of circulating CD45RA+CD8+ cells does not differ significantly between MG patients with TFH and controls (P = .8). In contrast, there is no significant difference in the percentage of CD45RA+CD4+ T cells between either of the 2 patient groups and their respective controls (TFH: P = .6, Tm: P = .8). (B) The difference between the percentage (means indicated by bars) of circulating CD45RA+CD8+ T cells in patients with thymoma and normal controls is more significant when thymoma is not associated with MG (Tm/MG−) (n = 6) (P = .01) than when it is (Tm/MG+) (n = 9) (P = .05).
Significant increase in the percentage of peripheral CD45RA+ CD8+ T cells in thymoma patients.
Three-color FACS-based analyses of CD8, CD4, and CD45RA expression by PBL, gated on CD3+ T cells. Error bars depict the SE. Controls, ■; patients, ░. (A) Bars indicate the mean percentage of CD8+ or CD4+ cells among circulating CD45RA+ T cells in patients with thymic follicular hyperplasia (TFH; n = 7), thymoma patients (Tm; n = 15), and corresponding control groups. The CD8+ subset in thymoma patients is significantly increased when compared with controls (P = .002), whereas the percentage of circulating CD45RA+CD8+ cells does not differ significantly between MG patients with TFH and controls (P = .8). In contrast, there is no significant difference in the percentage of CD45RA+CD4+ T cells between either of the 2 patient groups and their respective controls (TFH: P = .6, Tm: P = .8). (B) The difference between the percentage (means indicated by bars) of circulating CD45RA+CD8+ T cells in patients with thymoma and normal controls is more significant when thymoma is not associated with MG (Tm/MG−) (n = 6) (P = .01) than when it is (Tm/MG+) (n = 9) (P = .05).
The absolute number of PBL per milliliter was available for the majority (75%) of the patients with thymoma and respective controls (n = 10), calculated from the volume of blood drawn and the number of lymphocytes counted after isolation by density gradient centrifugation.11 The exact blood volume used for isolation was not documented in the remainder of cases. The results in steroid-treated patients were not significantly different in any of the assays.
Flow cytometric analysis
Cells (2 × 105) were stained with a panel of surface antigen-directed monoclonal antibodies (mAbs) as described previously.11 The panel of mAbs included anti-CD3 (phycoerythrin [PE]-labeled), anti-CD4 (FITC-labeled), and anti-CD8 (PE-labeled) (Dako, Hamburg, Germany); anti-CD69 (FITC-labeled) and anti-CD69 (PE-labeled) (Becton Dickinson, Heidelberg, Germany); anti-CD45RA and anti-CD45RO (both FITC- and PE-labeled) (Dianova, Hamburg, Germany); and anti-CD3 (Tricolor-labeled) and the isotype control IgG2a (FITC-labeled) (Medac, Hamburg, Germany). Other isotype controls and anti-CD3 (FITC-labeled) were purchased from Sigma.
Sampling and data analyses were performed on a FACScan flow cytometer with Lysis II software (Becton Dickinson) as described.11The CD3+ T-cell subset was gated for all analyses. The proportion of monocytes (CD4+CD14+) in this gate was always less than 1.5%.
Cloning and expression of recombinant proteins
Recombinant glutathione-S-transferase (GST)-fusion proteins of the AChR α subunit (amino acid 301-398), of the midsize neurofilament NF-M (amino acid 459-737), and of a control protein (GST) were prepared as described previously.11
Proliferation assay
Thymocytes and autologous irradiated PBL used as antigen-presenting cells or PBL alone were cultured with either tetanus toxoid (TT) 10 U/mL (Behringer Institut, Marburg, Germany), fusion proteins of NF-M or αAChR, or control protein GST (5 μg/mL); proliferation was measured by 3H-thymidine incorporation as described.11 The proliferation rate was calculated as a stimulation index (SI), representing the ratio of counts in the presence of antigen versus counts in the presence of GST (for fusion proteins) or medium (for TT).12
The Mann-Whitney U test was used for statistical analysis of independent samples; the Wilcoxon rank test was used for dependent values.11
Results
The proportion of CD45RA+ T cells is increased in the blood of patients with thymoma
The absolute number of PBL was calculated for patients with thymoma and healthy sex- and age-matched controls (see “Materials and methods”). Proportions of circulating CD3+ T cells and naive T cells were also evaluated for these groups by staining of PBL with mAbs directed against CD69, CD45RA, and CD3, using FACS-based analysis. T cells expressing both CD45RA and CD69, an immunophenotype corresponding to thymocytes, were not detected in any case. The proportions of naive T cells in the blood of a representative patient and corresponding control are shown in Figure 1A. The absolute number of PBL and the proportion of circulating CD3+ T cells in patients with thymoma did not differ significantly from those values in healthy controls, regardless of thymoma subtype (P = .98 and P = .84, respectively; data not shown). In contrast, the percentage of circulating CD45RA+CD69− T cells in thymoma patients with lymphocyte-rich subtypes, specifically mixed and cortical thymomas (n = 15), was significantly higher (P = .01) compared with healthy controls (Figure 1B). Nonthymoma MG patients with TFH (n = 11) did not demonstrate a higher proportion of CD45RA+ T cells in the blood when compared with controls (P = .95) (Figure 1C). Likewise, we found no increase in the proportion of CD45RA+ T cells in the blood of 6 patients with medullary thymoma, an intrinsically lymphocyte-poor subtype, compared with controls (P = 1.0; data not shown). When patients with cortical thymoma (n = 12) and mixed thymoma (n = 3) were analyzed separately, the proportion of CD45RA+ T cells in the blood of patients with cortical thymoma remained significantly increased (P = .01) over the control group. No significant increase (P = .13) was noted among CD45RA+ T cells in the blood of patients with mixed thymoma, however, which we attribute to the few cases available for analysis.
The CD8+ but not CD4+ subset among CD45RA+ T cells is increased in the blood of patients with thymoma
To differentiate among phenotypic subsets of naive T cells in the blood of thymoma and nonthymoma (TFH) patients, and their respective controls, we isolated PBL from each group, stained with mAbs against CD45RA, CD4, and CD8, and used FACS-based analysis. We found a significant increase (P = .002) in the proportion of CD45RA+CD8+ T cells in the blood of patients with thymoma (n = 15) compared with controls (Figure 2A). A similar increase was also observed in patients with medullary thymoma (n = 6;P = .04; data not shown). In contrast, the proportion of circulating CD45RA+CD8+ T cells was not significantly higher (P = .8) among nonthymoma MG patients with TFH (n = 7) than that found in controls (Figure 2A). When thymoma patients with and without MG were analyzed separately, the increased percentage of circulating CD45RA+CD8+T cells was even more significant (P = .01) in patients without MG (n = 6) than in those with MG (P = .05; n = 9; Figure 2B). In contrast, the CD4+ subset among CD45RA+ T cells was not significantly altered in the blood of TFH and thymoma patients compared with corresponding control groups (Figure 2A-B). When data were analyzed in regard to thymoma subtype, the proportion of circulating CD45RA+CD8+ T cells was significantly increased (P = .005) in patients with cortical thymoma (n = 12) compared with controls. No significant increase over controls (P = .9) was observed in patients with mixed thymoma (n = 3), however.
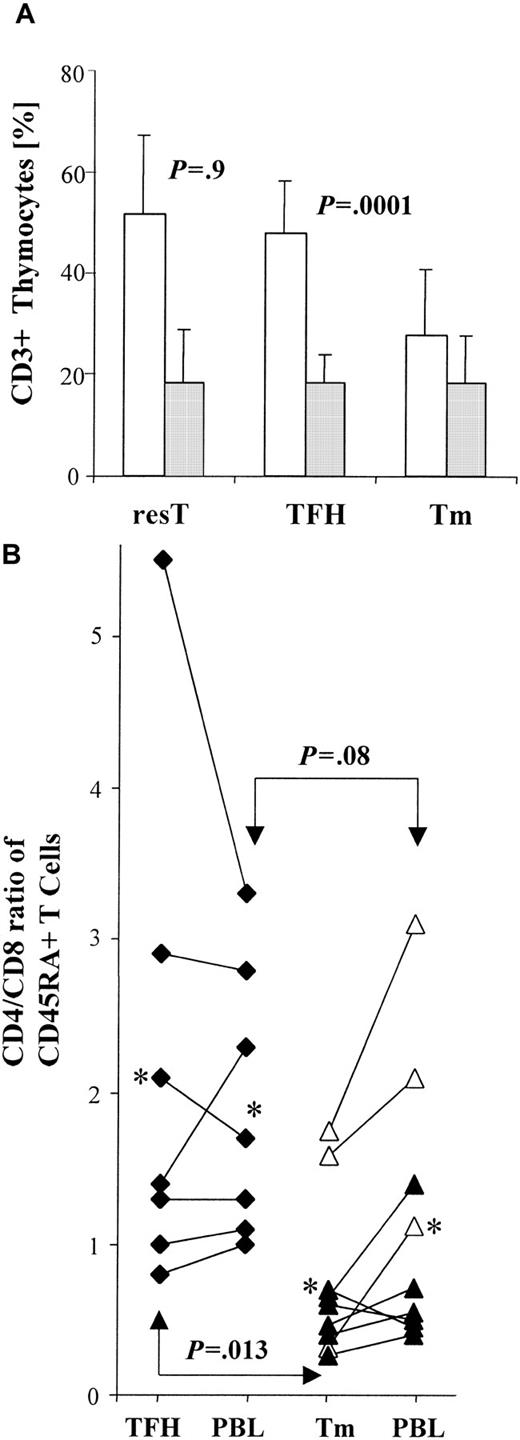
A relative decrease in CD4+ T cells accounts for a lower CD4/CD8 ratio in thymomas
To investigate whether the increase in the proportion of CD45RA+CD8+ T cells in the blood of patients with thymoma might be the result of either a relative overproduction of CD8+ T cells or an abnormally low generation of CD4+ T cells, we determined the proportion of CD8+CD4− cells and of CD4+CD8− cells among CD3+thymocytes, both in thymomas and in nonneoplastic thymuses. The proportion of CD8+ mature T cells was virtually identical in residual thymus (resT) (n = 9) to that seen in TFH (n = 11) and thymoma (n = 19) (Figure 3A). In contrast, we found a significantly lower (P = .0001) proportion of mature CD4+ T cells in thymoma compared with either resT or TFH (Figure 3A). The significantly decreased proportion of CD4+ cells among CD3+ T cells in thymoma compared with TFH (Figure 3A) was paralleled by a significant decrease (P = .013) in the CD4/CD8 ratio among CD45RA+T cells in thymoma when compared with this ratio among CD45RA+ T cells isolated from the thymus in cases of TFH (Figure 3B). For most patients (7 of 9 cases; P = .07) the CD4/CD8 ratio among CD45RA+ T cells isolated from thymoma was lower than the CD4/CD8 ratio among CD45RA+ PBL (Figure3b). In contrast, we found similar CD4/CD8 ratios (P = .83) among CD45RA+ T cells isolated from either thymus or blood in TFH patients (Figure 3B).
The CD4/CD8 ratio is decreased in thymoma.
(A) Bar graph shows the mean percentages of mature CD4+(■) and CD8+ (░) thymocytes in residual thymus (resT; n = 9), thymus with thymic follicular hyperplasia (TFH; n = 11), and thymoma (Tm; n = 19), as determined by FACS-based analyses gated on CD3+ T cells. Error bars indicate the SE. The percentage of mature CD8+ T cells is similar among nonneoplastic thymuses (resT, TFH) and thymomas (P = .9). The percentage of CD4+ T cells is similar between resT and TFH (P = .9), but significantly lower between nonneoplastic thymuses (resT, TFH) and thymomas (P = .0001). (B) Diagram compares CD4/CD8 ratios among CD45RA+ T cells in individual cases. T cells were derived from thymus and blood (PBL) of TFH patients (n = 7) and from thymoma (Tm) and PBL of thymoma patients (n = 9). The proportion of CD4/CD8 CD45RA+ T cells was calculated for thymocytes and PBL from each patient, using FACS-based analyses gated on CD3+ T cells. Each asterisk represents the mean ratio among either TFH or Tm and corresponding PBL, respectively. CD4/CD8 ratios of thymoma-derived CD45RA+ T cells were significantly decreased (P = .013) when compared with those of thymocytes from patients with TFH. CD4/CD8 ratios among CD45RA+ PBL were also decreased in thymoma patients compared with TFH patients, although this represented only a trend (P = .08). In patients with thymoma, CD4/CD8 ratios were elevated (P = .07) among circulating CD45RA+ T cells compared with thymocytes, whereas CD4/CD8 ratios were almost identical among CD45RA+ T cells in the periphery and thymus of TFH patients (P = .83). ▵, Tm/MG+; ▴, Tm/MG −; ♦, TFH/MG+.
The CD4/CD8 ratio is decreased in thymoma.
(A) Bar graph shows the mean percentages of mature CD4+(■) and CD8+ (░) thymocytes in residual thymus (resT; n = 9), thymus with thymic follicular hyperplasia (TFH; n = 11), and thymoma (Tm; n = 19), as determined by FACS-based analyses gated on CD3+ T cells. Error bars indicate the SE. The percentage of mature CD8+ T cells is similar among nonneoplastic thymuses (resT, TFH) and thymomas (P = .9). The percentage of CD4+ T cells is similar between resT and TFH (P = .9), but significantly lower between nonneoplastic thymuses (resT, TFH) and thymomas (P = .0001). (B) Diagram compares CD4/CD8 ratios among CD45RA+ T cells in individual cases. T cells were derived from thymus and blood (PBL) of TFH patients (n = 7) and from thymoma (Tm) and PBL of thymoma patients (n = 9). The proportion of CD4/CD8 CD45RA+ T cells was calculated for thymocytes and PBL from each patient, using FACS-based analyses gated on CD3+ T cells. Each asterisk represents the mean ratio among either TFH or Tm and corresponding PBL, respectively. CD4/CD8 ratios of thymoma-derived CD45RA+ T cells were significantly decreased (P = .013) when compared with those of thymocytes from patients with TFH. CD4/CD8 ratios among CD45RA+ PBL were also decreased in thymoma patients compared with TFH patients, although this represented only a trend (P = .08). In patients with thymoma, CD4/CD8 ratios were elevated (P = .07) among circulating CD45RA+ T cells compared with thymocytes, whereas CD4/CD8 ratios were almost identical among CD45RA+ T cells in the periphery and thymus of TFH patients (P = .83). ▵, Tm/MG+; ▴, Tm/MG −; ♦, TFH/MG+.
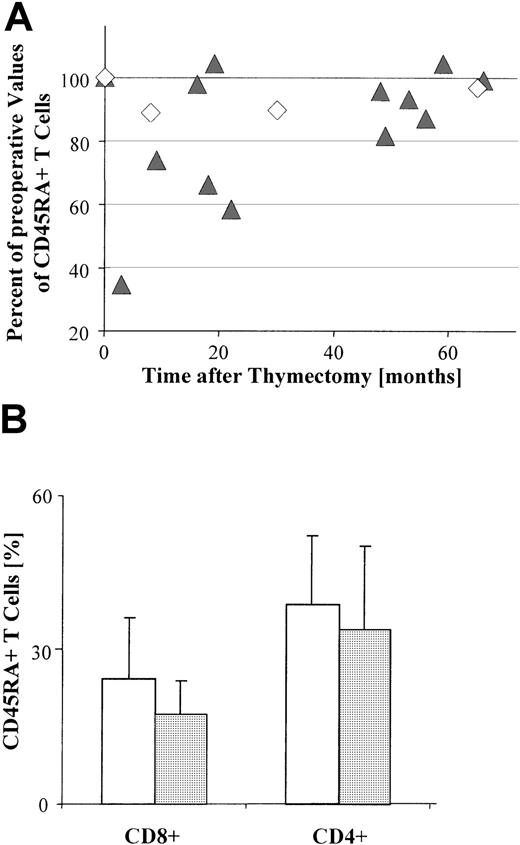
The proportion of peripheral CD45RA+ T cells decreases after thymoma resection
Thymoma resection might be expected to reduce the proportion of naive T cells if the increased proportion of CD45RA+ T cells in the blood of patients with thymoma results from intratumorous thymopoiesis. To investigate this hypothesis, we compared the percentage of circulating CD45RA+ T cells before and after resection (n = 15) and found a significant decrease (P = .005) in the proportion of these cells following resection (Figure 4A). In contrast, there was no significant decrease (P = .88) in the absolute number of PBL following thymectomy compared with the absolute number prior to surgery (data not shown). Similar results were obtained after thymectomy regardless of whether PBL were derived from patients with TFH (n = 3) or from those with thymoma (n = 12;P = .02; Figure 4A). The reduction in CD45RA+T cells was most pronounced within a short interval after thymectomy (1-18 months; Figure 4A).
Decrease in the percentage of peripheral CD45RA+CD8+ T cells after thymoma resection.
(A) The relative percentage of circulating CD45RA+ T cells, as determined by FACS-based analysis of PBL gated on CD3+ T cells, is significantly decreased in most patients (13 of 15) following surgery (P = .005). This decrease is still significant (P = .02) when only thymoma patients (Tm; n = 12) are included. ▴, Tm; ◊, TFH. Each symbol indicates the percentage of circulating CD45RA+ T cells for one patient at a given postoperative interval, and relative to the percentage of circulating CD45RA+ T cells immediately prior to surgery (adjusted to 100%). (B) Bar graph shows the mean percentages of CD4+and CD8+ subsets of circulating CD45RA+ T cells in patients with thymoma (n = 10) preoperatively (■) and postoperatively (░), as determined by FACS-based analyses gated on CD3+ T cells. Error bars indicate the SE. There is no significant difference (P = .8) between the proportion of CD4+ T cells observed either before surgery or after surgery; for CD8+ T cells, however, the preoperative values differ significantly (P = .04) from the postoperative values.
Decrease in the percentage of peripheral CD45RA+CD8+ T cells after thymoma resection.
(A) The relative percentage of circulating CD45RA+ T cells, as determined by FACS-based analysis of PBL gated on CD3+ T cells, is significantly decreased in most patients (13 of 15) following surgery (P = .005). This decrease is still significant (P = .02) when only thymoma patients (Tm; n = 12) are included. ▴, Tm; ◊, TFH. Each symbol indicates the percentage of circulating CD45RA+ T cells for one patient at a given postoperative interval, and relative to the percentage of circulating CD45RA+ T cells immediately prior to surgery (adjusted to 100%). (B) Bar graph shows the mean percentages of CD4+and CD8+ subsets of circulating CD45RA+ T cells in patients with thymoma (n = 10) preoperatively (■) and postoperatively (░), as determined by FACS-based analyses gated on CD3+ T cells. Error bars indicate the SE. There is no significant difference (P = .8) between the proportion of CD4+ T cells observed either before surgery or after surgery; for CD8+ T cells, however, the preoperative values differ significantly (P = .04) from the postoperative values.
When CD4+ and CD8+ subsets in the blood were analyzed separately, the postoperative decrease was significant (P = .04) only for the CD45RA+CD8+T-cell population (Figure 4B).
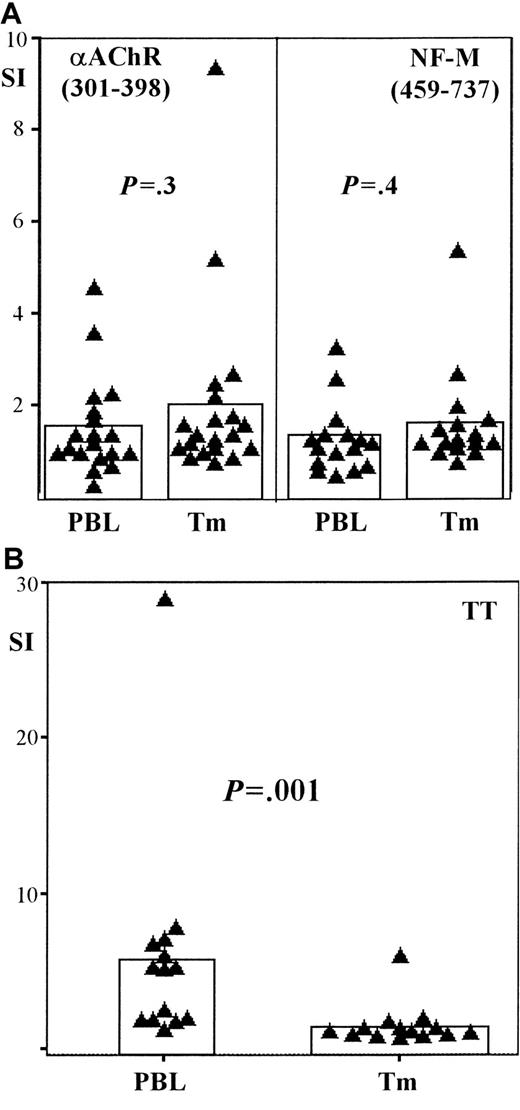
Autoantigen-specific T cells are equally distributed between tumor and blood in patients with thymoma-associated MG
To investigate a possible exchange of autoantigen-specific T cells between thymoma and blood in patients with MG, we performed T-cell proliferation assays using T cells from both compartments. We tested a fragment (301-398) of the main autoantigen αAChR11 and a fragment (459-737) of the NF-M protein, which was recently shown to be a characteristic autoantigen in thymoma patients with MG.7To check for memory T-cell import from the blood into the thymoma, we also stimulated T cells from the blood and thymoma with the recall antigen TT. Similar T-cell proliferation assays were performed using PBL from nonmyasthenic persons (n = 12), as a negative control.
We found no significant differences in anti-αAChR (P = .3) and anti–NF-M (P = .4) T-cell responses between tumor and blood in thymoma patients with MG (Figure5A). In contrast, responses to TT were significantly higher (P = .001) among T cells in the blood of these patients compared to responses among intratumorous T cells (Figure 5B). Anti-αAChR (301-398) responses were significantly higher (P = .05) in the blood of thymoma patients with MG when compared with controls, whereas there was only a trend (P = .08) toward higher anti–NF-M responses in the blood of myasthenic thymoma patients (data not shown). Previous studies show that intratumorous thymocytes from myasthenic patients have elevated responses to αAChR and NF-M when compared with responses of intratumorous thymocytes derived from thymoma patients without MG.7 The similarity between these previous findings and our results using PBL suggests a relationship between PBL autoreactivity and intratumorous thymopoiesis.
Autoantigen-specific T cells are present in the blood of patients with thymoma-associated MG.
Scatter-bar plots showing the proliferative responses of peripheral T cells (PBL) and intratumorous T cells (Tm) harvested from patients with thymoma-associated MG. (A) Recombinant fragments of the acetylcholine receptor α subunit (αAChR, 301-398) and of the neurofilament protein (NF-M, 459-737) are used as stimulatory antigens, whereas tetanus toxoid (TT) is used as the recall antigen in panel B. Proliferative responses are given in terms of a stimulation index (SI) (see “Materials and methods”). Each triangle represents the SI of either PBL or Tm from an individual patient; bars indicate the mean SI among either PBL or Tm for each particular antigen. Autoantigen-specific T-cell responses in PBL and Tm are similar on stimulation with either αAChR (P = .3; n = 19) or NF-M (P = .4; n = 16); TT-specific responses, however, are significantly increased among PBL compared with Tm (P = .001; n = 14).
Autoantigen-specific T cells are present in the blood of patients with thymoma-associated MG.
Scatter-bar plots showing the proliferative responses of peripheral T cells (PBL) and intratumorous T cells (Tm) harvested from patients with thymoma-associated MG. (A) Recombinant fragments of the acetylcholine receptor α subunit (αAChR, 301-398) and of the neurofilament protein (NF-M, 459-737) are used as stimulatory antigens, whereas tetanus toxoid (TT) is used as the recall antigen in panel B. Proliferative responses are given in terms of a stimulation index (SI) (see “Materials and methods”). Each triangle represents the SI of either PBL or Tm from an individual patient; bars indicate the mean SI among either PBL or Tm for each particular antigen. Autoantigen-specific T-cell responses in PBL and Tm are similar on stimulation with either αAChR (P = .3; n = 19) or NF-M (P = .4; n = 16); TT-specific responses, however, are significantly increased among PBL compared with Tm (P = .001; n = 14).
Discussion
Intratumorous T-cell maturation occurs in MG-associated thymomas,10,11 with the formation of T cells autoantigen-specific to MG-related antigens.7,12,24 It has been suggested that these T cells leave the thymoma and contribute to the peripheral autoimmune cascade that leads to myasthenic symptoms of muscle weakness.2,15 This hypothesis is supported by the present study, which demonstrates that the unique subset composition of intratumorous T cells is reflected in the blood of patients with thymoma. Using FACS-based analysis, we have detected a significant increase in the proportion of circulating CD45RA+ T cells in patients with thymoma, compared with normal controls (Figure 1A-B). We conclude that this increase is due to the peripheralization of thymoma-derived mature T cells, because naive T cells and recent thymic emigrants preferentially express the CD45RA+phenotype.25,26 This conclusion is supported by the results of Berzins and colleagues19,20 who have shown that an increase in T-cell pool size caused by thymic lobe engraftment in mice correlates almost exactly with the number of emigrant T cells exported from these grafts. In addition, we have found no increase in the proportion of CD45RA+ T cells in the blood of patients with MG unrelated to thymoma, in whom the hyperplastic thymus is thought to exhibit normal thymopoiesis (Figure 1C).2,11This latter finding also excludes the possibility that alteration of the circulating CD45RA+ T-cell subset is characteristic of MG in general. Further evidence of the influence of intratumorous thymopoiesis on peripheral T-cell subset composition is provided by our finding that patients with medullary thymoma have an unaltered proportion of CD45RA+ T cells in the blood. Medullary thymomas exhibit only minimal intratumorous thymopoiesis and consequently harbor only a low number of mature T cells, mostly of the CD8+ phenotype.27 Moreover, the decrease in the proportion of CD45RA+ T cells immediately after thymectomy (Figure 4A) strongly suggests a priori the export of thymoma-derived T cells into the blood. This finding is also consistent with data provided by Berzins and coworkers showing a decline in peripheral naive T cells following the removal of thymic implants in mice.20
The FACS-based analysis also shows that most cells comprising the expanded pool of circulating CD45RA+ T cells in patients with thymoma express the CD8+ immunophenotype (Figure 2A). This is equally true for cortical, mixed, and medullary thymoma subtypes, suggesting that even minimal intratumoral thymopoiesis with preferential generation of CD8+ T cells27 is sufficient to skew the T-cell subset composition in the blood toward the CD8+ phenotype. An increase in the proportion of circulating CD45RA+CD8+ T cells is highest in nonmyasthenic patients with thymoma (Figure 2B), implying that these thymomas export relatively more CD8+ T cells into the periphery than do MG-associated thymomas. Correspondingly, thymoma resection leads to a significant decrease in the proportion of circulating CD45RA+CD8+ T cells, but has relatively little effect on the proportion of circulating CD45RA+CD4+ T cells (Figure 4B). In fact, the proportion of CD4+ T cells is only slightly decreased, a finding reported previously in patients with MG undergoing thymectomy.28 These alterations in the blood are in accordance with intratumorous T-cell development that is abnormally skewed toward the CD8+ phenotype (Figure 3A) as shown by a significantly decreased CD4/CD8 ratio among CD45RA+ naive T cells (Figure 3B). Again, these findings argue in support of the conclusion that mature T cells leave the thymoma and skew the T-cell repertoire in the periphery. That the decrease in the CD4/CD8 ratio among CD45RA+ T cells is less pronounced in the blood (P = .08) of patients with thymoma than in their thymomas (P = .013; Figure 3B) can be explained by the fact that both residual thymus and thymoma contribute to the peripheral T-cell pool in patients with thymoma.16
Still another argument in favor of the hypothesis that thymomas alter the peripheral T-cell repertoire is provided by our functional studies. Using T-cell proliferation assays, we have demonstrated very similar autoantigen-specific T-cell responses among both intratumorous and peripheral T cells in thymoma patients with MG (Figure 5A). This implies an exchange of autoantigen-specific T cells between these 2 compartments, although it is less clear whether these T cells are generated in the thymoma or whether they are imported from the blood and accumulate within the tumor. In response to this question, we have shown that T cells primed outside the thymus by TT vaccination are generally undetectable among intratumorous T cells, indicating that import of memory T cells from the blood into the thymoma is severely impaired (Figure 5B). Therefore, we assume that the similar responses directed against autoantigens by both intratumorous and circulating T cells are best explained by intratumorous generation of autoantigen-specific T cells, followed by their export into the periphery.
Despite the apparent influence that thymomas exert on circulating T cell subsets, the absolute number of PBL is not significantly changed in our patients with thymoma. Thymomas, though often quite large, usually harbor an absolute number of mature T cells similar to the number of mature T cells present in the residual thymus.11Thymomas may thus function analogously to a single grafted thymus in mice.19 In such mice, the effect of additional T cell input from one graft on peripheral T-cell subset compositionis fairly prominent, but the change in the absolute number of circulating T cells is only marginal.19,20 This latter phenomenon is thought to be due to peripheral homeostatic mechanisms.19 20
The mechanism(s) by which intratumorous autoantigen-specific T cells contribute to the pathogenesis of paraneoplastic MG in patients with thymoma is currently unknown. T-cell export from the thymoma into the periphery is presumed to be a necessary step, however, and required for the interaction between thymoma-derived autoantigen-specific T cells and autoantibody-producing B cells that reside entirely outside the tumor.11,12,14 CD4+ T cells are believed to play a pivotal role in T/B cell interactions during the effector phase of MG pathogenesis.29 The autoimmune reaction, once initiated in the periphery, is thought to be self-sustaining.2,13 This assumption is supported by the lack of a subsequent decline in autoantibody titer following thymectomy and by the clinical observation that thymoma resection is rarely palliative with respect to myasthenic symptoms.8 However, the present investigation cannot directly show that thymomas export CD4+ T cells, because all patients with available hematologic data had normal leukocyte counts (see “Materials and methods”) and normal proportions of naive CD4+ T cells in the blood (Figure 2). It may be that CD4+ T cells are exported from the thymoma together with CD8+ T cells, but in a relatively smaller proportion than from the normal thymus. Enrichment of this CD4+ T-cell subset by autoaggressive T cells might then explain the rather specific association of thymoma with MG.7 12 Conversely, the higher proportion of CD8+ T cells in the blood of nonmyasthenic thymoma patients (Figure 2B) may represent an absolute lower number of CD4+T cells emigrating from the tumor, rather than the output of a greater number of CD8+ T cells. As in various immunodeficiency states, diminished CD4+ T-cell help is suggested by the significantly lower CD4/CD8 ratio in nonmyasthenic patients with thymoma (Figure 2B), and may thus form the basis for impaired production of autoantigen-specific antibodies resulting in failure to develop MG.
Notwithstanding the pivotal role of CD4+ T cells in the final effector phase of MG, the expansion of circulating CD45RA+CD8+ T cells in thymoma patients with MG (Figure 2B) indicates that T-cell export of naive autoantigen-specific CD45RA+CD8+ T cells may also contribute to the initiation of MG within the periphery of thymoma patients.8 Sufficient evidence now suggests that increased numbers of autoantigen-specific CD8+ T cells play a role in several presumably CD4+ T-cell–dependent human organ-specific autoimmune diseases30,31 and in experimental autoimmune myasthenia gravis in the rat.32 A similar role for CD8+ T cells has recently been postulated in the initial pathogenesis of thymoma-associated MG,8,33 given its significant correlation with the HLA-A24 phentotype but not with major histocompatibility complex (MHC) class II alleles.33Although it seems an affront to this hypothesis that the proportion of CD45RA+CD8+ T cells is highest in nonmyasthenic patients with thymoma (Figure 2B), several pathogenic models are nevertheless compatible with a role for CD8+ T cells in the triggering of MG. For example, thymoma-derived CD8+ T cells may be duplicitous: autoantigen-specific in myasthenic thymoma patients and regulatory in nonmyasthenic thymoma patients. One explanation for this duplicity may be the presence of aberrant autoantigen-specific CD4+ T cells in myasthenic patients. Normally, the CD4+ T-cell repertoire is made tolerant to self-antigens and therefore provides no help for autoreactive CD8+ T-cell responses during cross-presentation, resulting in sustained peripheral tolerance.34,35 In myasthenic thymoma patients, however, aberrant autoantigen-specific intratumorous CD4+ T cells may assist in autoreactive CD8+ T cell responses. In support of this pathogenic model, autoantigen-specific CD4+ T cells are reported to be characteristic of myasthenic but not of nonmyasthenic thymomas7,24 36; the present study also suggests that such T cells may leave the thymus (Figure 5).
An alternative model invokes 2 independent steps that mayboth be required for autoimmunization to occur.8 An initial step dependent on CD8+ T cells may be necessary to initiate the autoimmune cascade, thus explaining the correlation between an MHC class I allele (HLA-A24) and the development of MG in patients with thymoma.33 A second step dependent on activated CD4+ T cells may then be required to stimulate B cells for autoantibody production. Again, the presence of aberrant autoimmune CD4+ T cells specifically in myasthenic thymoma patients24 36 supports this scenario. Further analysis of the potential role of CD8+ T cells as mediators of autoimmunity is warranted and could prove essential for a full understanding of the pathogenesis of thymoma-associated autoimmune disease.
Acknowledgments
We thank Mrs A. Homburger and Mrs E. Oswald for technical support and Dr S. Czub and Dr S. Sopter for critical reading of the manuscript.
Supported by BMBF 2000 (IZKF 01 KS 903/C5).
V.H. and A.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alexander Marx, Institute of Pathology, University of Würzburg, Josef-Schneider-Strasse 2, D-97080, Würzburg, Germany; e-mail: alex.marx@mail.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal