Abstract
T-cell progenitors in the embryonic bone marrow express the tyrosine kinase receptor c-kit. RR5, an anti-MHC class II β chain monoclonal antibody, subdivides this c-kit positive population. Intrathymic transfer experiments showed that most of the T-cell progenitors belong to the MHC class II+/c-kit+ bone marrow population in the embryo and young adult. On transplantation, these bone marrow progenitors lose this expression and differentiate into CD4 CD8 T lymphocytes. In contrast, erythroid progenitors are restricted to the MHC class II−/c-kit+ population. The MHC class II+/c-kit+ pro-T cells are metabolically active, because they stain brightly with rhodamin 123. Their cyclin A and B expression level suggests that they are in the mitotic phase of the cell cycle. Thus, we define an easy sorting protocol, which allows enrichment of T-cell progenitors from total bone marrow hemopoietic cells.
Introduction
T-lineage commitment precedes thymus colonization.1,2 In birds, it has recently been shown that T-cell progenitors from embryonic bone marrow (BM), which will migrate to the thymus via the blood stream, expressc-kit.3-5 It is not yet clear at which stage of commitment MHC class II is expressed on hemopoietic progenitors and whether its simultaneous expression with other hemopoietic markers can define irreversibly engaged progenitors. Some in vitro studies with human BM cells show that primitive self-renewing multipotent hemopoietic cells are CD34+ and MHC class II−,6 whereas other studies with human fetal BM cells describe the CD34+, CD38−, and MHC class II+ positive cell population as multipotent.7 Nevertheless, no study refers to the presence of MHC class II molecules on T-cell progenitors. The results presented here define T-cell progenitors as metabolically active cells with a high expression level of the mitotic cyclins A and B with preferential location in the MHC class II/c-kitdouble-positive population of embryonic and adult BM. On in vitro culture, these cells were also able to give rise to myeloid but not to erythroid colonies. These results correlate well with the recent demonstration of the presence of a common precursor able to differentiate into myeloid and T cells.8
Study design
Outbred JA57 and White Leghorn chick embryos were obtained from local facilities. The 2 congenic strains H.B19+ and H.B19−, which can be distinguished by the presence of the ov alloantigen on T-lineage cells in the H.B19+animals, were produced at our animal facilities. The following monoclonal antibodies (mAbs) were used: RR5, a mouse IgG2b, raised in our laboratory (submitted for publication); a mouse antichickenc-kit conjugated to phycoerythrin (PE)3; 11A9, which recognizes the ov epitope expressed on T-lineage cells from the H.B19+ chicken strain9; antichicken CD4(2-6) and CD8(11-39) mAbs directly conjugated to fluorescein isothiocyanate (FITC) or PE, respectively.10,11Antichicken cyclin A (R28) and cyclin B (R18), rabbit polyclonal antibodies, were kind gifts from Prof E. Nigg, University of Geneva, Switzerland.12,13 In some cases, RR5 was used directly coupled to Cy5 using the Fluorolink Cy5 reactive kit from Amersham (Life Science, Arlington Heights, IL). Bone marrow cell suspensions, immunostaining, flow cytometric analysis, and sorting were performed as previously described.3 Three-color fluorescence-activated cell sorter (FACS) analysis was performed on a FACSCalibur (Becton Dickinson, San Jose, CA) using FL1, FL2, and FL4 with appropriate compensations. Antibodies directly labeled with Cy5 or biotinylated antibodies revealed by APC-streptavidin (Molecular Probe, Eugene, OR) were detected on FL4. When necessary, the contribution of dead cells was assessed by adding propidium iodide (PI; Calbiochem, Juro Supply, Lucerne, Switzerland). For cyclin A and B staining, cells were fixed in 4% paraformaldehyde, permeabilized with 0.3% saponin and incubated with rabbit anticyclin polyclonal antibodies or with a control rabbit serum diluted 1:500 in phosphate-buffered saline (PBS) containing 3% bovine serum albumin (BSA) and 0.03% saponin. Goat antirabbit coupled to FITC (Southern Biotechnology Associates) was used as a second step antibody. Cells were incubated with rhodamine 123 (Rh) (Molecular Probes) according to published protocols14,15either directly or after staining with RR5cy5 and c-kitPE. In vitro assay for hemopoietic progenitors in semisolid cultures was performed as previously described.4 In vivo T-cell progenitor assay by intrathymic injection was conducted as already described.4
Results and discussion
The c-kit positive cell population from embryonic and young adult bone marrow contains MHC class II positive cells
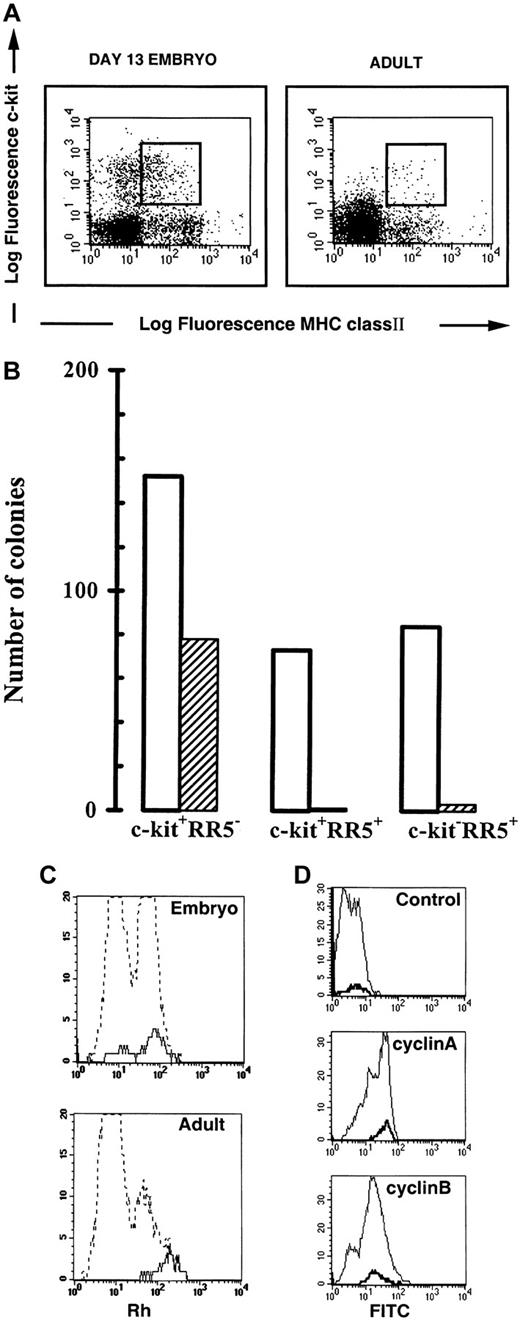
Recent evidence from experiments on mouse and chicken have shown that the T-lineage commitment precede thymus colonization and that the reconstitutive T-cell progenitors were among the c-kitpositive cells.2-5 The anti-MHC class II β-chain mAb RR5 subdivided the c-kit positive population on FACS analysis (Figure 1A). In embryonic BM at day 13.5 (E13.5), c-kit positive cells accounted for more than 15% in the forward side scatter window defined for progenitors and the RR5/c-kit double-positive population represented 3% to 7% of the gated cells (≅1.7% of total BM cells). In the young adult, the c-kit positive cells were less abundant (7%) and the RR5/c-kit double-positive population accounted for less than 2% of the gated cells.
Analysis of bone marrow cells.
(A) Comparative flow cytometric analysis of embryonic and adult bone marrow cells. Embryonic E13.5 and adult BM cells were stained with the RR5 and c-kit mAbs and analyzed by flow cytometry in the FSC/SSC progenitor window. The RR5/c-kitdouble-positive cells shown in the window were sorted for intrathymic injection and in vitro differentiation. (B) Analysis of myeloid and erythroid potential of sorted cells from E13.5 BM. The 1000 cells from each sorted population were cultured in duplicate in erythroid differentiation medium. Results represent the mean number of colonies scored in duplicate cultures from one representative experiment (of 5 experiments). M: macrophage; G: granulocyte; M/G: macrophage/granulocyte; Eb/Ec: erythroblast/erythrocyte colonies. No colonies were obtained from the double negative population. ■ indicates M,G, and M/G; ▨ indicates Eb/Ec. (C) Rhodamine staining of RR5/c-kit double-positive BM cells. Comparative Rh staining of total bone marrow cells (hatched line) and RR5+/c-kit+ cells (continuous line). (D) Cyclin A and B staining of RR5/c-kit double-positive BM cells. Cyclin staining of embryonic total bone marrow cells (thin line) and RR5+/c-kit+ cells (bold line). Control: normal rabbit serum; cyclin A: polyclonal rabbit antichicken cyclin A serum; cyclin B: polyclonal rabbit antichicken cyclin B serum. Rabbit antibodies were detected by a goat antirabbit antibody conjugated to FITC.
Analysis of bone marrow cells.
(A) Comparative flow cytometric analysis of embryonic and adult bone marrow cells. Embryonic E13.5 and adult BM cells were stained with the RR5 and c-kit mAbs and analyzed by flow cytometry in the FSC/SSC progenitor window. The RR5/c-kitdouble-positive cells shown in the window were sorted for intrathymic injection and in vitro differentiation. (B) Analysis of myeloid and erythroid potential of sorted cells from E13.5 BM. The 1000 cells from each sorted population were cultured in duplicate in erythroid differentiation medium. Results represent the mean number of colonies scored in duplicate cultures from one representative experiment (of 5 experiments). M: macrophage; G: granulocyte; M/G: macrophage/granulocyte; Eb/Ec: erythroblast/erythrocyte colonies. No colonies were obtained from the double negative population. ■ indicates M,G, and M/G; ▨ indicates Eb/Ec. (C) Rhodamine staining of RR5/c-kit double-positive BM cells. Comparative Rh staining of total bone marrow cells (hatched line) and RR5+/c-kit+ cells (continuous line). (D) Cyclin A and B staining of RR5/c-kit double-positive BM cells. Cyclin staining of embryonic total bone marrow cells (thin line) and RR5+/c-kit+ cells (bold line). Control: normal rabbit serum; cyclin A: polyclonal rabbit antichicken cyclin A serum; cyclin B: polyclonal rabbit antichicken cyclin B serum. Rabbit antibodies were detected by a goat antirabbit antibody conjugated to FITC.
RR5+/c-kit+bone marrow cells harbor myelomonocytic, T-cell, but no erythroid progenitors
Semisolid cultures of sorted embryonic BM cells showed that, under appropriate conditions, erythroid colonies developed almost exclusively from RR5−/c-kit+ cells (Figure 1B). By contrast, myeloid colonies were recorded in similar numbers from the RR5+/c-kit+ double-positive as well as from the RR5−/c-kit+ and RR5+/c-kit− cells (Figure 1B). The T-cell differentiation potential as judged by intrathymic injection into ov− congenic animals, suggested that T-cell progenitors were enriched in the RR5+/c-kit+ BM population of the embryo when compared with RR5−/c-kit+ cells (Table1). In the young adult, the injection of 1000 double-positive sorted cells resulted in a thymic chimerism of 7.6% ± 2% (mean ± SEM). The in vitro studies showed that the isolated progenitors were not multipotent because they have lost their erythroid potential (Figure 1B). Most of the thymocytes recovered 14 days after grafting were CD4/CD8 double-positive with very few mature single positive cells (data not shown). This proportion was similar to that found in age-matched uninjected ov+ control chickens. Analysis of MHC class II expression revealed that most of the transferred T-cell progenitors lose their expression during T-cell differentiation (data not shown).
Percentage of chimerism after intrathymic injection of sorted embryonic bone marrow cells
| Injected cells per thymic lobe . | Number of animals . | % of chimerism in host thymus . |
|---|---|---|
| Unselected BM cells | ||
| 6 × 103 | 3 | 0 |
| 104 | 2 | 0 |
| 2 × 104 | 4 | 4 ± 2 |
| Cells stained with RR5 and c-kit* | ||
| 103c-kit+/RR5+ | 12 | 13.7 ± 1.8 |
| 102 | 8 | 2.4 ± 0.8 |
| 30 | 8 | 0.9 ± 0.2 |
| 10 | 4 | 1.7 ± 0.8 |
| 3 | 4 | 0.6 ± 0.3 |
| 103c-kit+/RR5− | 5 | 2.3 ± 1 |
| Cells stained with either c-kit or RR5† | ||
| 103Rhhi/c-kit+ | 3 | 6.6 ± 2.5 |
| 103Rhhi/c-kit− | 3 | 0.6 ± 0.4 |
| 103Rhhi/RR5+ | 3 | 15.0 ± 8.2 |
| 103Rhhi/RR5− | 3 | 2.7 ± 1.2 |
| Injected cells per thymic lobe . | Number of animals . | % of chimerism in host thymus . |
|---|---|---|
| Unselected BM cells | ||
| 6 × 103 | 3 | 0 |
| 104 | 2 | 0 |
| 2 × 104 | 4 | 4 ± 2 |
| Cells stained with RR5 and c-kit* | ||
| 103c-kit+/RR5+ | 12 | 13.7 ± 1.8 |
| 102 | 8 | 2.4 ± 0.8 |
| 30 | 8 | 0.9 ± 0.2 |
| 10 | 4 | 1.7 ± 0.8 |
| 3 | 4 | 0.6 ± 0.3 |
| 103c-kit+/RR5− | 5 | 2.3 ± 1 |
| Cells stained with either c-kit or RR5† | ||
| 103Rhhi/c-kit+ | 3 | 6.6 ± 2.5 |
| 103Rhhi/c-kit− | 3 | 0.6 ± 0.4 |
| 103Rhhi/RR5+ | 3 | 15.0 ± 8.2 |
| 103Rhhi/RR5− | 3 | 2.7 ± 1.2 |
BM = bone marrow.
Cells were sorted by fluorescence activated cell sorter (FACS), diluted according to FACS cell count, and 10 μL were injected per thymic lobe (8 lobes injected per chick). At concentrations below 100 cells per 10 μL, 1000 double negative cells were added as “carriers.” Control experiments with 4000 double negative cells resulted in a 0.14% chimerism, which is within the range of the accuracy of the method.
Cells were subsequently incubated with rhodamine 123 (Rh), FACS sorted, and injected intrathymically. Chimerism of the host thymus (in a pool of 4 lobes per chick) was determined 14 days later after staining for the ov marker and exclusion of dead cells by propidium iodide.
RR5/c-kit double-positive T-cell progenitors are metabolically active and express cyclin A and B
Rh 123 staining showed that in embryonic BM, 70% of the double-positive cells were Rhhi, whereas in the adult BM, these cells were exclusively in the Rhhi compartment (Figure 1C, continuous lines). Injection of 1000 Rhloembryonic cells resulted in chimerism of 1.8% showing that the contribution of this population to the T-cell potential of the double-positive cells is minimal. Thus, the majority of the T-cell progenitors in the RR5+/c-kit+population were metabolically active and belonged to the fraction of nonquiescent hemopoietic cells, which, after total body irradiation, have been shown to mediate transient short-term reconstitution.14,15 In the adult mouse, the prothymocytes able to rapidly repopulate the thymus after intravenous injection were in the c-kit+, Lin−, Ly6A/E+, and Rhhi fraction of the BM. The corresponding Rhlo cell population had a delayed thymic repopulation ability, and it was suggested that this delay was due to the inability of these cells to seed the thymus. Seeding could only take place through further maturation of these Rhlo cells into more mature Rhhi cells, presumably in the BM.16 These results confirmed previous data obtained by single Rh staining and sorting experiments.14 To investigate in the embryo the correlation of the Rh staining with the cell cycle status of these cells, we studied the expression of cyclin A and B by flow cytometry.17 Interestingly, all the RR5+/c-kit+ double-positive cells, which included both the Rhlo and hi cells from embryonic BM, were among the cyclin A and B high-expressing cells (Figure 1D, bold lines). This indicated that the RR5+/c-kit+ cells were engaged in the mitotic phase of the cell cycle. To directly evaluate the T-cell potential of the Rhhi population of embryonic BM, we sortedc-kit– or RR5-labeled cells from the Rhhiwindow to perform intrathymic injections. The results showed that progenitors were preferentially located in the Rhhi window of either c-kit or RR5 positive populations (Table 1). Thus, MHC class II, a molecule that has always been considered as a hallmark of definitive differentiation toward dendritic cells, B-cells, or macrophages, is also present on T-cell progenitors and subsequently disappears when the progenitors further differentiate in the thymic environment. The molecule is coexpressed with c-kit on short-term committed progenitors unable to give rise to erythroid colonies in vitro (Figure 1B). The progenitors are in a cycling status characterized by high Rh retention (Figure 1C) and a high level of expression of the so-called mitotic cyclins (Figure 1D).
Acknowledgments
We are indebted to Dominique Wohlwend, Viktor Hasler, Raija Raulimo, Pierre Vaigot, and Suzanne Bissat for excellent technical assistance, Jean-Claude Rumbeli and Etienne Denkinger for photographic work, and Fabrice Bonaccorsi for computer assistance. Special thanks goes to Caroline Johnson-Leger for critical reading and improvement of the manuscript.
Supported by the Swiss National Science Foundation grant no. 21-49 241.96, Association pour la Recherche contre le Cancer (ARC-9122) and (ARC- 9738), Human Frontier HFSP-RG 366/96 and the Academy of Finland (grant no. 4293).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
B. A. Imhof, Department of Pathology, Centre Médical Universitaire (CMU), CH-1211Geneva 4, Switzerland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal